

高等学校化学学报 ›› 2025, Vol. 46 ›› Issue (7): 20240535.doi: 10.7503/cjcu20240535
王智远, 董义, 齐保辉, 魏学洋, 张佳辉, 黄起中, 李积升, 高娜, 邸士莹, 胡玉峰( )
)
收稿日期:2024-12-09
出版日期:2025-07-10
发布日期:2025-01-18
通讯作者:
胡玉峰
E-mail:huyf3581@sina.com
基金资助:
WANG Zhiyuan, DONG Yi, QI Baohui, WEI Xueyang, ZHANG Jiahui, HUANG Qizhong, LI Jisheng, GAO Na, DI Shiying, HU Yufeng( )
)
Received:2024-12-09
Online:2025-07-10
Published:2025-01-18
Contact:
HU Yufeng
E-mail:huyf3581@sina.com
Supported by:摘要:
离子液体的催化活性与其酸度的大小密不可分, 而Hammett酸度函数(H0)是一种表示酸度的重要参数. 本文合成了一系列可用于三聚甲醛合成过程的吡咯烷酮类和咪唑类离子液体, 并对其在水溶液中的H0进行了系统的实验和理论研究, 比较了阴、 阳离子结构和溶剂的选择对酸度的影响规律. 研究了盐效应对1-丙基磺酸-3-甲基咪唑甲烷磺酸盐([C3SMIM][MSA])、 甲烷磺酸、 三氟甲烷磺酸和硫酸的H0的影响. 结果表明, 阴离子对酸度的影响更加显著. 当阴离子相同时, 阳离子取代基的碳链越长, 酸度越强; 当阳离子相同时, 阴离子的电荷密度越小, 酸度越强; 对于同一类型的离子液体, 磺酸功能化的离子液体比未功能化的离子液体酸度要强. 大部分盐类起的是盐析效应, 对酸度起增强作用, 少数盐会减弱酸度, 如对甲苯磺酸钠和1-丙基磺酸-3-甲基咪唑内盐(C3SMIM)等, 这些盐的共同特点是具有较大的离子尺寸, 电荷密度较低.
中图分类号:
TrendMD:
王智远, 董义, 齐保辉, 魏学洋, 张佳辉, 黄起中, 李积升, 高娜, 邸士莹, 胡玉峰. 酸性离子液体H0的测定及盐效应的影响. 高等学校化学学报, 2025, 46(7): 20240535.
WANG Zhiyuan, DONG Yi, QI Baohui, WEI Xueyang, ZHANG Jiahui, HUANG Qizhong, LI Jisheng, GAO Na, DI Shiying, HU Yufeng. Determination of Acidic Ionic Liquid H0 and the Effect of Salt Effect. Chem. J. Chinese Universities, 2025, 46(7): 20240535.
| Cation | Abbreviation | Structure | Anion | Abbreviation | Structure |
|---|---|---|---|---|---|
| 2⁃Pyrrolidone cation | [HNHP]+ | 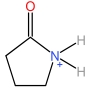 | Hydrogen sulfate anion | [HSO4] - | 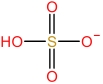 |
| N⁃Methylpyrrolidone cation | [HNMP]+ | 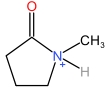 | Dihydrophosphate anion | [H2PO4] - | 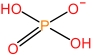 |
| N⁃Octylpyrrolidone cation | [HNOP]+ | 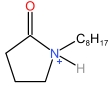 | Methanesulfonate anion | [MSA]- | 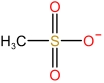 |
| N⁃Cyclohexylpyrrolidonecation | [HNCYP]+ | 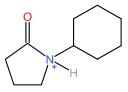 | Trifluoromethane sulfonate anion | [TFO]- | 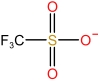 |
| 1⁃Methylimidazole cation | [MIM]+ | 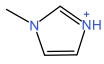 | p⁃Toluenesulfonic acid anion | [p⁃TSA]- | 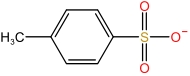 |
| 1⁃Propylsulfonic acid⁃3⁃methylimidazole cation | [C3SMIM]+ | 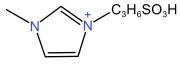 | p⁃Chlorophenylsulfonate anion | [p⁃ClBSA]- | 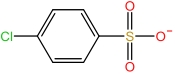 |
Table 1 Cations and anions of the ionic liquid used in this study
| Cation | Abbreviation | Structure | Anion | Abbreviation | Structure |
|---|---|---|---|---|---|
| 2⁃Pyrrolidone cation | [HNHP]+ |  | Hydrogen sulfate anion | [HSO4] - |  |
| N⁃Methylpyrrolidone cation | [HNMP]+ |  | Dihydrophosphate anion | [H2PO4] - |  |
| N⁃Octylpyrrolidone cation | [HNOP]+ |  | Methanesulfonate anion | [MSA]- |  |
| N⁃Cyclohexylpyrrolidonecation | [HNCYP]+ |  | Trifluoromethane sulfonate anion | [TFO]- |  |
| 1⁃Methylimidazole cation | [MIM]+ |  | p⁃Toluenesulfonic acid anion | [p⁃TSA]- |  |
| 1⁃Propylsulfonic acid⁃3⁃methylimidazole cation | [C3SMIM]+ |  | p⁃Chlorophenylsulfonate anion | [p⁃ClBSA]- |  |
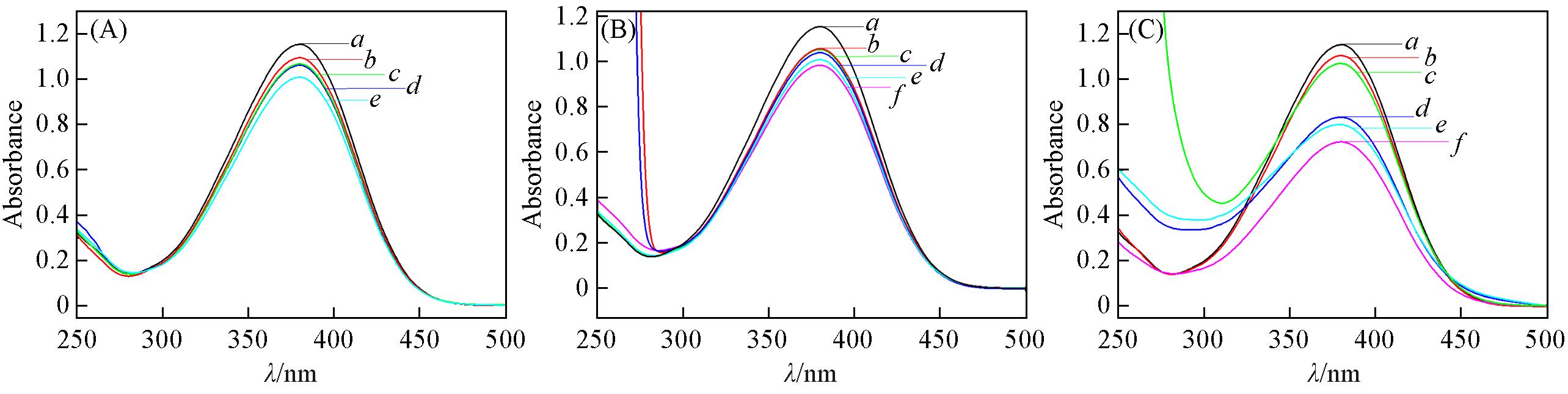
Fig.2 UV⁃Vis absorption spectra of pyrrolidone ionic liquids(A), N⁃methylpyrrolidone ionic liquids(B), N⁃cyclohexyl⁃2⁃pyrrolidone and N⁃octylpyrrolidone ionic liquids(C)(A) a. Blank; b.[HNHP][H2PO4]; c. [HNHP][MSA]; d.[HNHP][TFO]; e. [HNHP][HSO4]. (B) a. Blank; b. [HNMP][MSA]; c. [HNMP][p-TSA]; d.[HNMP][p-ClBSA]; e. [HNMP][TFO]; f. [HNMP][HSO4]. (C) a. Blank; b. [HNCYP][p-TSA]; c. [HNCYP][TFO]; d.[HNOP][H2PO4]; e. [HNOP][TFO]; f. [HNOP][HSO4].
| Ionic liquid | Amax | B fraction(%) | BH+ fraction(%) | H0 |
|---|---|---|---|---|
| p⁃Nitroaniline | 1.154 | 100.00 | 0 | — |
| [HNHP][H2PO4] | 1.095 | 94.89 | 5.11 | 2.26 |
| [HNHP][MSA] | 1.067 | 92.46 | 7.54 | 2.08 |
| [HNHP][TFO] | 1.061 | 91.94 | 8.06 | 2.05 |
| [HNHP][HSO4] | 1.008 | 87.35 | 12.65 | 1.83 |
| [HNMP][MSA] | 1.056 | 91.51 | 8.49 | 2.02 |
| [HNMP][p⁃TSA] | 1.053 | 91.25 | 8.75 | 2.01 |
| [HNMP][p⁃ClBSA] | 1.039 | 90.03 | 9.97 | 1.95 |
| [HNMP][TFO] | 1.010 | 87.52 | 12.48 | 1.84 |
| [HNMP][HSO4] | 0.983 | 85.18 | 14.82 | 1.75 |
| [HNOP][H2PO4] | 0.834 | 72.27 | 27.73 | 1.41 |
| [HNOP][TFO] | 0.801 | 69.41 | 30.59 | 1.35 |
| [HNOP][HSO4] | 0.725 | 62.82 | 37.18 | 1.22 |
| [HNCYP][TFO] | 1.071 | 92.81 | 7.19 | 2.10 |
| [HNCYP][p⁃TSA] | 1.106 | 95.84 | 4.16 | 2.35 |
Table 2 H0 of pyrrolidone, N-methyl pyrrolidone, N-octylpyrrolidone and N-cyclohexyl-2-pyrrolidone ionic liquids
| Ionic liquid | Amax | B fraction(%) | BH+ fraction(%) | H0 |
|---|---|---|---|---|
| p⁃Nitroaniline | 1.154 | 100.00 | 0 | — |
| [HNHP][H2PO4] | 1.095 | 94.89 | 5.11 | 2.26 |
| [HNHP][MSA] | 1.067 | 92.46 | 7.54 | 2.08 |
| [HNHP][TFO] | 1.061 | 91.94 | 8.06 | 2.05 |
| [HNHP][HSO4] | 1.008 | 87.35 | 12.65 | 1.83 |
| [HNMP][MSA] | 1.056 | 91.51 | 8.49 | 2.02 |
| [HNMP][p⁃TSA] | 1.053 | 91.25 | 8.75 | 2.01 |
| [HNMP][p⁃ClBSA] | 1.039 | 90.03 | 9.97 | 1.95 |
| [HNMP][TFO] | 1.010 | 87.52 | 12.48 | 1.84 |
| [HNMP][HSO4] | 0.983 | 85.18 | 14.82 | 1.75 |
| [HNOP][H2PO4] | 0.834 | 72.27 | 27.73 | 1.41 |
| [HNOP][TFO] | 0.801 | 69.41 | 30.59 | 1.35 |
| [HNOP][HSO4] | 0.725 | 62.82 | 37.18 | 1.22 |
| [HNCYP][TFO] | 1.071 | 92.81 | 7.19 | 2.10 |
| [HNCYP][p⁃TSA] | 1.106 | 95.84 | 4.16 | 2.35 |
| Ionic liquid | Amax | B fraction(%) | BH+ fraction(%) | H0 |
|---|---|---|---|---|
| m⁃Nitroaniline | 1.519 | 100.00 | 0 | — |
| [MIM][H2PO4] | 1.504 | 98.99 | 1.01 | 4.49 |
| [MIM][MSA] | 1.488 | 97.96 | 2.04 | 4.24 |
| [MIM][TFO] | 1.489 | 98.00 | 2.00 | 4.19 |
| [MIM][HSO4] | 1.447 | 95.23 | 4.77 | 3.86 |
| p⁃Nitroaniline | 1.061 | 100.00 | 0 | — |
| [C3SMIM][MSA] | 0.981 | 92.46 | 7.54 | 2.08 |
| [C3SMIM][TFO] | 0.974 | 91.80 | 8.20 | 2.04 |
| [C3SMIM][p⁃TSA] | 0.915 | 86.24 | 13.76 | 1.79 |
| [C3SMIM][HSO4] | 0.914 | 86.15 | 13.85 | 1.78 |
Table 3 H0 of imidazolium-based ionic liquids
| Ionic liquid | Amax | B fraction(%) | BH+ fraction(%) | H0 |
|---|---|---|---|---|
| m⁃Nitroaniline | 1.519 | 100.00 | 0 | — |
| [MIM][H2PO4] | 1.504 | 98.99 | 1.01 | 4.49 |
| [MIM][MSA] | 1.488 | 97.96 | 2.04 | 4.24 |
| [MIM][TFO] | 1.489 | 98.00 | 2.00 | 4.19 |
| [MIM][HSO4] | 1.447 | 95.23 | 4.77 | 3.86 |
| p⁃Nitroaniline | 1.061 | 100.00 | 0 | — |
| [C3SMIM][MSA] | 0.981 | 92.46 | 7.54 | 2.08 |
| [C3SMIM][TFO] | 0.974 | 91.80 | 8.20 | 2.04 |
| [C3SMIM][p⁃TSA] | 0.915 | 86.24 | 13.76 | 1.79 |
| [C3SMIM][HSO4] | 0.914 | 86.15 | 13.85 | 1.78 |
| Ionic liquid | H0 in aqueoussolution | H0 in dichloromethanesolution | Ionic liquid | H0 in aqueoussolution | H0 in dichloromethanesolution |
|---|---|---|---|---|---|
| [MIM][H2PO4] | 4.49 | 2.55 | [C3SMIM][HSO4] | 1.78 | 0.34 |
| [MIM][HSO4] | 3.86 | 0.73 | [HNMP][p⁃ClBSA] | 1.95 | 0.71 |
| [C3SMIM][TFO] | 2.04 | 0.01 | [HNMP][HSO4] | 1.75 | 0.52 |
Table 4 H0 of acidic ionic liquids in aqueous solution and dichloromethane solution
| Ionic liquid | H0 in aqueoussolution | H0 in dichloromethanesolution | Ionic liquid | H0 in aqueoussolution | H0 in dichloromethanesolution |
|---|---|---|---|---|---|
| [MIM][H2PO4] | 4.49 | 2.55 | [C3SMIM][HSO4] | 1.78 | 0.34 |
| [MIM][HSO4] | 3.86 | 0.73 | [HNMP][p⁃ClBSA] | 1.95 | 0.71 |
| [C3SMIM][TFO] | 2.04 | 0.01 | [HNMP][HSO4] | 1.75 | 0.52 |
| System | Amax | B fraction(%) | BH+ fraction(%) | H0 |
|---|---|---|---|---|
| p⁃Nitroaniline | 0.776 | 100.00 | 0 | — |
| CH3SO3H | 0.330 | 42.57 | 57.43 | 0.860 |
| CH3SO3H+NaCl | 0.319 | 41.12 | 58.88 | 0.834 |
| CH3SO3H+MgCl2 | 0.299 | 38.58 | 61.42 | 0.788 |
| CH3SO3H+C3SMIM | 0.414 | 53.39 | 46.61 | 1.049 |
| CH3SO3H+CF3SO3Na | 0.311 | 40.12 | 59.88 | 0.816 |
| CH3SO3H+CH3SO3Na | 0.322 | 41.45 | 58.55 | 0.840 |
| CH3SO3H+C3SMIM+CH3SO3Na | 0.361 | 46.49 | 53.51 | 0.929 |
| CH3SO3H+C3SMIM+CF3SO3Na | 0.335 | 43.14 | 56.86 | 0.870 |
| CF3SO3H | 0.325 | 41.90 | 58.10 | 0.848 |
| CF3SO3H+C3SMIM | 0.354 | 45.64 | 54.36 | 0.914 |
| CF3SO3H+CF3SO3Na | 0.306 | 39.40 | 60.60 | 0.803 |
| CF3SO3H+CF3SO3Na+C3SMIM | 0.332 | 42.74 | 57.26 | 0.863 |
Table 5 H0 of aqueous solution systems of CH3SO3H/CF3SO3H+salt
| System | Amax | B fraction(%) | BH+ fraction(%) | H0 |
|---|---|---|---|---|
| p⁃Nitroaniline | 0.776 | 100.00 | 0 | — |
| CH3SO3H | 0.330 | 42.57 | 57.43 | 0.860 |
| CH3SO3H+NaCl | 0.319 | 41.12 | 58.88 | 0.834 |
| CH3SO3H+MgCl2 | 0.299 | 38.58 | 61.42 | 0.788 |
| CH3SO3H+C3SMIM | 0.414 | 53.39 | 46.61 | 1.049 |
| CH3SO3H+CF3SO3Na | 0.311 | 40.12 | 59.88 | 0.816 |
| CH3SO3H+CH3SO3Na | 0.322 | 41.45 | 58.55 | 0.840 |
| CH3SO3H+C3SMIM+CH3SO3Na | 0.361 | 46.49 | 53.51 | 0.929 |
| CH3SO3H+C3SMIM+CF3SO3Na | 0.335 | 43.14 | 56.86 | 0.870 |
| CF3SO3H | 0.325 | 41.90 | 58.10 | 0.848 |
| CF3SO3H+C3SMIM | 0.354 | 45.64 | 54.36 | 0.914 |
| CF3SO3H+CF3SO3Na | 0.306 | 39.40 | 60.60 | 0.803 |
| CF3SO3H+CF3SO3Na+C3SMIM | 0.332 | 42.74 | 57.26 | 0.863 |
| System | Amax | B fraction(%) | BH+ fraction(%) | H0 |
|---|---|---|---|---|
| o⁃Nitroaniline | 2.828 | 100.00 | 0 | — |
| H2SO4+Na2HPO4 | 2.797 | 98.90 | 1.10 | 1.665 |
| H2SO4+NaH2PO4 | 2.646 | 93.56 | 6.44 | 0.873 |
| H2SO4+Na2SO4 | 2.384 | 84.30 | 15.70 | 0.440 |
| H2SO4 | 2.152 | 76.10 | 23.90 | 0.213 |
| H2SO4+KCl | 1.902 | 67.26 | 32.74 | 0.023 |
| H2SO4+NaCl | 1.799 | 63.61 | 36.39 | -0.047 |
| H2SO4+LiCl | 1.763 | 62.34 | 37.66 | -0.071 |
| H2SO4+ZnCl2 | 1.682 | 59.48 | 40.52 | -0.123 |
| H2SO4+NaHSO4 | 1.484 | 52.48 | 47.52 | -0.247 |
| H2SO4+MgCl2 | 1.346 | 47.60 | 52.40 | -0.332 |
Table 6 H0 of aqueous solution system of sulfuric acid+salt
| System | Amax | B fraction(%) | BH+ fraction(%) | H0 |
|---|---|---|---|---|
| o⁃Nitroaniline | 2.828 | 100.00 | 0 | — |
| H2SO4+Na2HPO4 | 2.797 | 98.90 | 1.10 | 1.665 |
| H2SO4+NaH2PO4 | 2.646 | 93.56 | 6.44 | 0.873 |
| H2SO4+Na2SO4 | 2.384 | 84.30 | 15.70 | 0.440 |
| H2SO4 | 2.152 | 76.10 | 23.90 | 0.213 |
| H2SO4+KCl | 1.902 | 67.26 | 32.74 | 0.023 |
| H2SO4+NaCl | 1.799 | 63.61 | 36.39 | -0.047 |
| H2SO4+LiCl | 1.763 | 62.34 | 37.66 | -0.071 |
| H2SO4+ZnCl2 | 1.682 | 59.48 | 40.52 | -0.123 |
| H2SO4+NaHSO4 | 1.484 | 52.48 | 47.52 | -0.247 |
| H2SO4+MgCl2 | 1.346 | 47.60 | 52.40 | -0.332 |
| System | Amax | B fraction(%) | BH+ fraction(%) | H0 |
|---|---|---|---|---|
| p⁃Nitroaniline | 0.776 | 100.00 | 0 | — |
| H2SO4 | 0.250 | 32.22 | 67.78 | 0.667 |
| H2SO4+[MIM][MSA] | 0.279 | 35.94 | 64.06 | 0.739 |
| H2SO4+[MIM][TFO] | 0.254 | 32.72 | 67.28 | 0.677 |
| H2SO4+[MIM][HSO4] | 0.238 | 30.68 | 69.32 | 0.636 |
| H2SO4+[MIM][H2PO4] | 0.436 | 56.19 | 43.81 | 1.098 |
Table 7 H0 of aqueous solution systems of sulfuric acid+ionic liquids
| System | Amax | B fraction(%) | BH+ fraction(%) | H0 |
|---|---|---|---|---|
| p⁃Nitroaniline | 0.776 | 100.00 | 0 | — |
| H2SO4 | 0.250 | 32.22 | 67.78 | 0.667 |
| H2SO4+[MIM][MSA] | 0.279 | 35.94 | 64.06 | 0.739 |
| H2SO4+[MIM][TFO] | 0.254 | 32.72 | 67.28 | 0.677 |
| H2SO4+[MIM][HSO4] | 0.238 | 30.68 | 69.32 | 0.636 |
| H2SO4+[MIM][H2PO4] | 0.436 | 56.19 | 43.81 | 1.098 |
| [1] | Wang Z. Y., Su Z. Y., Xu Y., Qi J. G., Qi B. H., Wei X. Y., Chen X. J., Hu Y. F., Liu Z. C., Guo X., ACS Sustain. Chem. Eng., 2024, 12(37), 14087—14098 |
| [2] | Yuan K., Zhang T., Lv L., Wang Y., Zou Z. P., Tang S. W., Ind. Eng. Chem. Res., 2024, 63(28), 12440—12451 |
| [3] | Li F., Zhang T., Lv L., Tang W. X., Wang Y., Tang S. W., Chinese J. Chem. Eng., 2024, 73, 42—50 |
| [4] | Wang Z. Y., Wei X. Y., Qi B. H., Chen X. J., Pi J. J., Yang Y., Zhang J. H., Wang N. N., Jiang S. Q., Huang Q. Z., Gao N., Hu Y. F., Liu Z. C., Guo X., J. Chem. Eng. Data, 2024, 69(12), 4430—4437 |
| [5] | Grützner T., Hasse H., Lang N., Siegert M., Ströfer E., Chem. Eng. Sci., 2007, 62(18—20), 5613—5620 |
| [6] | Yang W. F., Inner Mongolia Petrochemical Industry, 2021, 47(3), 32—35 |
| 杨文峰. 内蒙古石油化工, 2021, 47(3), 32—35 | |
| [7] | Sood K., Saini Y., Thakur K. K., Mater. Todays: Proc., 2023, 81, 739—744 |
| [8] | Wang D. L., Li D., Guangzhou Chemical Industry, 2022, 50(5), 81—84 |
| 王大六, 李丹. 广州化工, 2022, 50(5), 81—84 | |
| [9] | Ren C. X., Li J. S., Wang J. J., Jiang S. Q., Guo X., Qi J. G., Jiao C. Z., Wang Y. C., Hu Y. F., Liu Z. C., J. Chem. Technol. Biot., 2022, 97(5), 1275—1279 |
| [10] | Gao N., Yang Y., Wang Z. Y., Guo X., Jiang S. Q., Li J. S., Hu Y. F., Liu Z. C., Xu C. M., Chem. Rev., 2024, 124(1), 27—123 |
| [11] | Zhou T., Gui C. M., Sun L. G., Hu Y. X., Lyu H., Wang Z. H., Song Z., Yu G. Q., Chem. Rev., 2023, 123(21), 12170—12253 |
| [12] | Tang X., Lv S. Y., Jiang K., Zhou G. H., Liu X. M., J. Power Sources, 2022, 542, 231792 |
| [13] | Zheng D. X., Li D., Huang W. J., Wu X. H., Nie N., Renew. Sust. Energ. Rev., 2014, 37, 47—68 |
| [14] | Ma C. Y., Shukla S. K., Samilkannu R., Mikkola J. P., Ji X. Y., ACS Sustain. Chem. Eng., 2020, 8(1), 415—426 |
| [15] | Liu C. Z., Wang F., Stiles A. R., Guo C., Appl. Energ., 2012, 92, 406—414 |
| [16] | Schneider S., Hawkins T., Rosander M., Vaghjiani G., Chambreau S., Drake G., Energy Fuels, 2008, 22(4), 2871—2872 |
| [17] | Zhou Q., Zhao Y. Y., Guo L. Y., Shi Y. F., Zheng R. R., Chem. J. Chinese Universities, 2024, 45(5), 20230488 |
| 周俏, 赵圆圆, 郭立颖, 史亚飞, 郑荣荣. 高等学校化学学报, 2024, 45(5), 20230488 | |
| [18] | Zhang S., Zhang T., Tang S. W., J. Chem. Eng. Data, 2016, 61(6), 2088—2097 |
| [19] | Ivanenko T. Y., Kondrasenko A. A., Rubaylo A. I., J. Mol. Liq., 2023, 391, 123438 |
| [20] | Long F. A., Mclntyre D., J. Am. Chem. Soc., 1954, 76(12), 3243—3247 |
| [21] | Paul M. A., J. Am. Chem. Soc., 1954, 76(12), 3236—3239 |
| [22] | Harbottle G., J. Am. Chem. Soc., 1951, 73(8), 4024—4025 |
| [23] | Ling S., Experiment and Theory Study on Physicochemical Properties of Pyrrolidonium Ionic Liquids and Its Multicomponent Aqueous Solutions, China University of Petroleum (Beijing), Beijing, 2012 |
| 凌山. 吡咯烷酮类离子液体及其多元水溶液物性的实验和理论研究, 北京: 中国石油大学(北京), 2012 | |
| [24] | Wang Z. X., Experiment and Theory Study on Physicochemical Properties of Novel Ionic Liquids and Synthesis of Trioxane by Formaldehyde with These Ionic Liquid Catalysts, China University of Petroleum (Beijing), Beijing, 2013 |
| 王智鑫. 新型离子液体的物性及催化甲醛合成三聚甲醛反应的理论和实验研究, 北京: 中国石油大学(北京), 2013 | |
| [25] | Huang H. Z., Experiment and Theory Study on Physicochemical Properties of Novel Ionic Liquids and Synthesis of Trioxane by Formaldehyde with These Ionic Liquid Catalyst Systems, China University of Petroleum (Beijing), Beijing, 2015 |
| 黄和志. 新型离子液体的物性及催化合成三聚甲醛反应的理论和实验研究, 北京: 中国石油大学(北京), 2015 | |
| [26] | Guo X., Wang Z. Y., Yang Y., Zhang J. H., Liu Y. D., Mu Z. Y., Jiang S. Q., Ren C. X., Lv D., Hu Y. F., Liu Z. C., Green Chem. Eng., 2024, 5(1), 108—118 |
| [27] | Zhou F., Zhang Y., Zhang T., Liang B., Tang S. W., Natural Gas Chemical Industry, 2013, 38(6), 87—91 |
| 周飞, 张圆, 张涛, 梁斌, 唐盛伟. 天然气化工(C1化学与化工), 2013, 38(6), 87—91 | |
| [28] | Cindioglu A., Ibrahim A. S. I., Sonmez O., Fuel, 2025, 382, 133791 |
| [29] | Guo H., Li H. N., Cao X. Y., Wang Z. Y., Zhang Q., Zhang G. B., Green Process. Synth., 2020, 9(1), 554—558 |
| [30] | Han B. Y., Jiang J. H., Zhang W. D., Yin F., Liu S. Q., Zhao X. L., Liu J., Wang C. M., Yang H., Energ. Source. Part A, 2019, 41(20), 2448—2459 |
| [31] | Fang J. H., Wang L., Chen Z. Y., Wang S., Yuan L., Saeed A., Hussain I., Zhao J. W., Liu R. X., Miao Q. Q., ACS Appl. Mater. Interfaces, 2024, 16(18), 23443—23451 |
| [32] | Gu Y. L., Zhang J., Duan Z. Y., Deng Y. Q., Adv. Synth. Catal., 2005, 347(4), 512—516 |
| [33] | Li Z., Zhao Y. W., Han F., Yang L., Song H. Y., Chen J., Xia C. G., Scientia Sinica Chimica, 2012, 42(4), 502—524 |
| 李臻, 赵应伟, 韩峰, 杨磊, 宋河远, 陈静, 夏春谷. 中国科学: 化学, 2012, 42(4), 502—524 | |
| [34] | Qi J. G., Hu Y. F., Ma W. T., Wang H. Y., Jiang S. Q., Yin L. Y., Zhang X. M., Yang Z. Y., Wang Y. C., Chem. Eng. J., 2018, 331, 311—316 |
| [35] | Yin L. Y., Hu Y. F., Zhang X. M., Qi J. G., Ma W. T., RSC Adv., 2015, 5, 37697—37702 |
| [1] | 洪扬, 李丹丹, 张景顺, 章子旺, 高国华. 多孔聚离子液体催化二氧化碳辅助环氧乙烷水合反应[J]. 高等学校化学学报, 2025, 46(5): 20240570. |
| [2] | 王璐. 离子液体辅助水热合成1T-MoS2及其锌离子储存性能[J]. 高等学校化学学报, 2024, 45(9): 20240145. |
| [3] | 周俏, 赵园园, 郭立颖, 史亚飞, 郑荣荣. 酸性离子液体的制备及在生物基聚2,5-呋喃二甲酸乙二醇酯合成中的催化性能[J]. 高等学校化学学报, 2024, 45(5): 20230488. |
| [4] | 张璐, 刘杰, 娄生辉, 江惠, 王松, 李三喜, 唐涛, 张爱玲. 季膦萘磺酸盐离子液体与甲基膦酸二甲酯协同阻燃环氧树脂[J]. 高等学校化学学报, 2024, 45(4): 20230467. |
| [5] | 杨思卿, 许神剑, 唐卿涵, 缪涵, 杨溪, 林绍梁. 木质素基聚离子液体膜的制备与应用[J]. 高等学校化学学报, 2024, 45(10): 20240248. |
| [6] | 张孟佳, 邹南, 罗佳美, 钟雄辉, 李玲. Zr-MOF固载聚离子液体对CO2环加成反应的催化性能[J]. 高等学校化学学报, 2024, 45(10): 20240113. |
| [7] | 秦海敬, 贺乾军, 徐敏敏, 袁亚仙, 姚建林. 离子液体中PMBA脱羧反应及界面水影响的电化学SERS研究[J]. 高等学校化学学报, 2024, 45(1): 20230349. |
| [8] | 崔伟, 赵德银, 白文轩, 张晓东, 余江. CO2在非质子溶剂与铁基离子液体复合体系中的吸收[J]. 高等学校化学学报, 2022, 43(8): 20220120. |
| [9] | 彭奎霖, 李桂林, 江重阳, 曾少娟, 张香平. 电解液调控CO2电催化还原性能微观机制的研究进展[J]. 高等学校化学学报, 2022, 43(7): 20220238. |
| [10] | 季双琦, 靳钊, 观文娜, 潘翔宇, 关彤. 双阳离子型离子液体和十八烷基修饰的混合模式硅胶固定相的制备及色谱性能[J]. 高等学校化学学报, 2022, 43(6): 20220008. |
| [11] | 常斯惠, 陈涛, 赵黎明, 邱勇隽. 离子液体增塑生物基聚丁内酰胺的热分解机理[J]. 高等学校化学学报, 2022, 43(11): 20220353. |
| [12] | 王蔓, 王鑫, 周静, 高国华. 聚离子液体催化碳酸乙烯酯与甲醇的酯交换反应[J]. 高等学校化学学报, 2021, 42(12): 3701. |
| [13] | 万仁, 宋璠, 彭昌军, 刘洪来. 水溶液中非常规离子无限稀释摩尔电导率的基团贡献法[J]. 高等学校化学学报, 2021, 42(12): 3672. |
| [14] | 周墨林, 蒋欣, 易婷, 杨向光, 张一波. 硫化物固态电解质Li10GeP2S12与锂金属间界面稳定性的改善研究[J]. 高等学校化学学报, 2020, 41(8): 1810. |
| [15] | 高崇,于凤丽,解从霞,于世涛. 氨基醇杂多酸类离子液体催化环酮的Baeyer-Villiger氧化反应[J]. 高等学校化学学报, 2020, 41(5): 1101. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||