

高等学校化学学报 ›› 2021, Vol. 42 ›› Issue (9): 2934.doi: 10.7503/cjcu20210092
李奕川1( ), 朱国富1, 王宇1, 柴永明1, 刘晨光1, 何盛宝1,2
), 朱国富1, 王宇1, 柴永明1, 刘晨光1, 何盛宝1,2
收稿日期:2021-02-08
出版日期:2021-09-10
发布日期:2021-09-08
通讯作者:
李奕川
E-mail:liyichuan@upc.edu.cn
基金资助:
LI Yichuan1( ), ZHU Guofu1, WANG Yu1, CHAI Yongming1, LIU Chenguang1, HE Shengbao1,2
), ZHU Guofu1, WANG Yu1, CHAI Yongming1, LIU Chenguang1, HE Shengbao1,2
Received:2021-02-08
Online:2021-09-10
Published:2021-09-08
Contact:
LI Yichuan
E-mail:liyichuan@upc.edu.cn
Supported by:摘要:
b取向MFI型分子筛膜能够显著促进分子的传输效率, 被广泛应用于混合物分离及催化领域. 虽然传统的原位水热晶化法已较为成熟, 然而仍难以调控膜层的b轴取向生长. 本文以304不锈钢片为基底, 采用经典的原位水热晶化法研究了基底表面物化性质、 前驱液配比及晶化条件对钛硅分子筛膜b轴取向生长的影响. 结果表明, TiO2中间层表面的羟基能够定向诱导分子筛晶粒的吸附, 进而提高钛硅分子筛膜的b轴取向程度. 同时, 前驱液中模板剂和水含量对晶粒的大小及膜层的定向生长具有显著影响, 即仅在合适的碱度下才能形成高b轴取向的钛硅分子筛膜.
中图分类号:
TrendMD:
李奕川, 朱国富, 王宇, 柴永明, 刘晨光, 何盛宝. 基底表面性质与前驱液化学环境对原位定向构筑钛硅分子筛膜的影响. 高等学校化学学报, 2021, 42(9): 2934.
LI Yichuan, ZHU Guofu, WANG Yu, CHAI Yongming, LIU Chenguang, HE Shengbao. Effects of Substrate Surface Properties and Precursor Chemical Environment on In⁃situ Oriented Construction of Titanium Silicalite Zeolite Membranes. Chem. J. Chinese Universities, 2021, 42(9): 2934.
| No. | Substrate | Modification method | Calcination temperature/℃ | KCPO(0k0) (%) |
|---|---|---|---|---|
| M01?1 | 304 Stainless steel slides | — | — | 81.86 |
| M01?2 | α?Al2O3 slides | — | — | 73.54 |
| M02?1 | 304 Stainless steel slides | Supported oxide layer by dip?coating TiO2 sol and calcination | 450 | 88.87 |
| M02?2 | 304 Stainless steel slides | Supported oxide layer by dip?coating SiO2 sol and calcination | 450 | 75.85 |
| M03?1 | 304 Stainless steel slides | Supported oxide layer by dip?coating TiO2 sol and calcination | 300 | 86.14 |
| M03?2 | 304 Stainless steel slides | Supported oxide layer by dip?coating TiO2 sol and calcination | 600 | 87.80 |
| M03?3 | 304 Stainless steel slides | Supported oxide layer by dip?coating TiO2 sol and calcination | 750 | 58.93 |
| M03?4 | 304 Stainless steel slides | Supported oxide layer by dip?coating TiO2 sol and calcination | 900 | 49.70 |
Table 1 Summary of surface modification conditions and crystallographic preferred orientation index of (0k0) plane[KCPO(0k0)] of TS-1 zeolite membranes*
| No. | Substrate | Modification method | Calcination temperature/℃ | KCPO(0k0) (%) |
|---|---|---|---|---|
| M01?1 | 304 Stainless steel slides | — | — | 81.86 |
| M01?2 | α?Al2O3 slides | — | — | 73.54 |
| M02?1 | 304 Stainless steel slides | Supported oxide layer by dip?coating TiO2 sol and calcination | 450 | 88.87 |
| M02?2 | 304 Stainless steel slides | Supported oxide layer by dip?coating SiO2 sol and calcination | 450 | 75.85 |
| M03?1 | 304 Stainless steel slides | Supported oxide layer by dip?coating TiO2 sol and calcination | 300 | 86.14 |
| M03?2 | 304 Stainless steel slides | Supported oxide layer by dip?coating TiO2 sol and calcination | 600 | 87.80 |
| M03?3 | 304 Stainless steel slides | Supported oxide layer by dip?coating TiO2 sol and calcination | 750 | 58.93 |
| M03?4 | 304 Stainless steel slides | Supported oxide layer by dip?coating TiO2 sol and calcination | 900 | 49.70 |
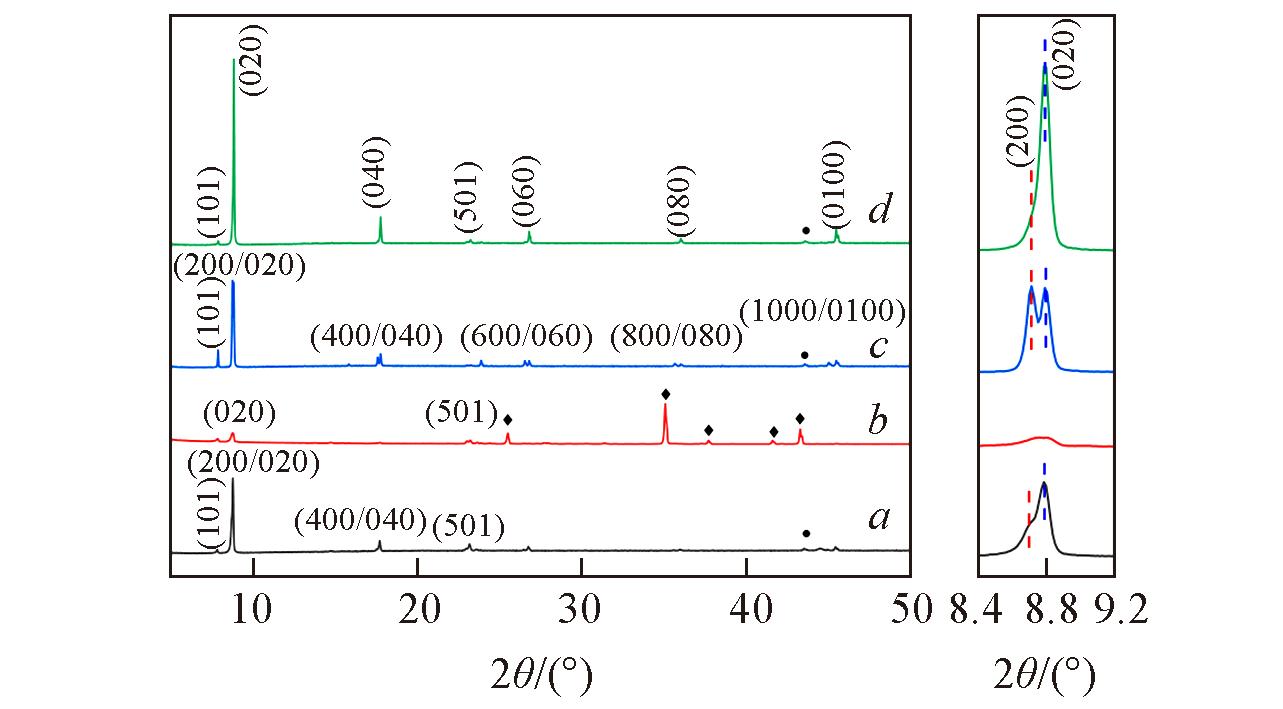
Fig.1 XRD patterns of TS?1 zeolite membranes prepared on different substrate surfaces● and ? in the left figure represent diffraction peaks of 304 stainless steel and alumina, respectively. The right figure is an enlarged diagram of the main diffraction peak near 8.8°. a. 304 Stainless steel; b. alumina sheet; c. 304 stainless steel(supported SiO2 oxide layer); d. 304 stainless steel(supported TiO2 oxide layer).
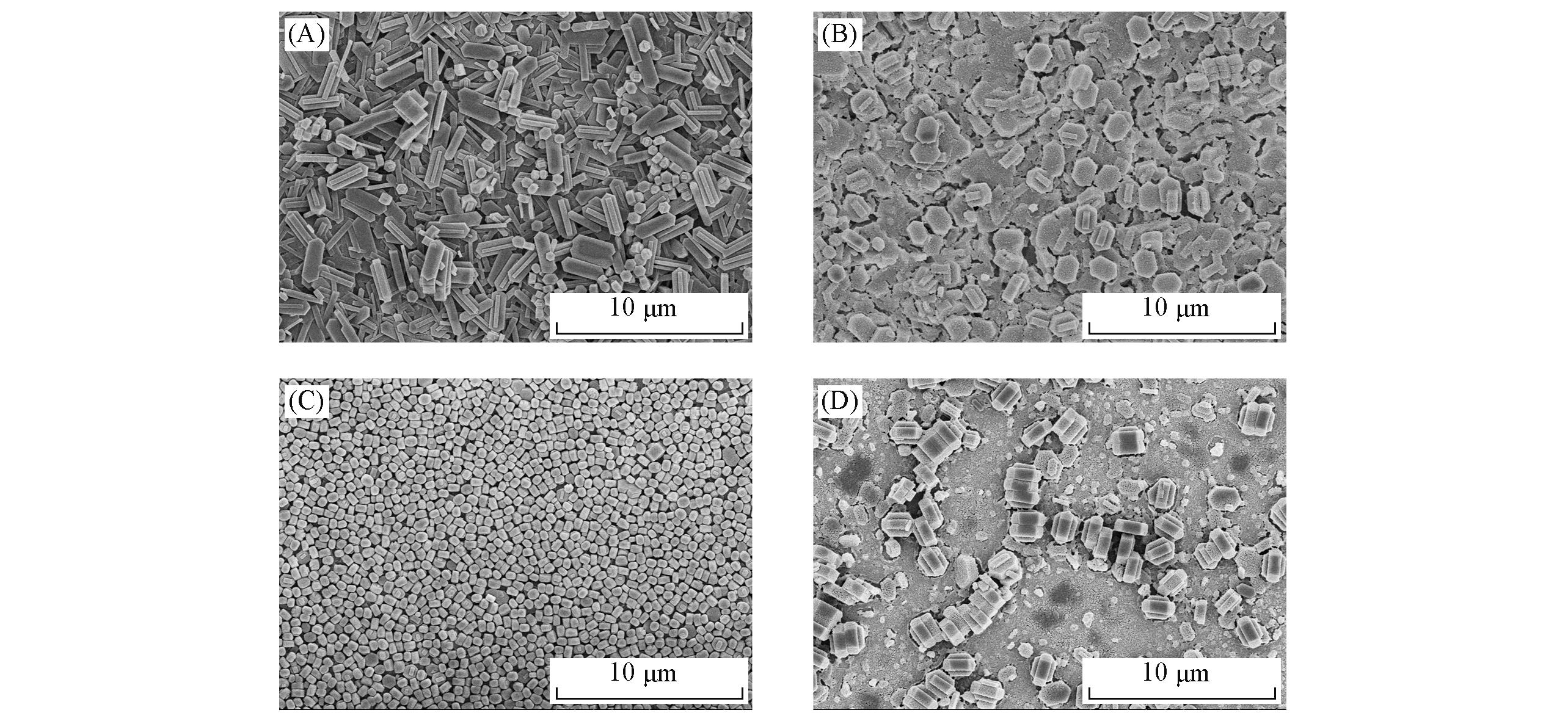
Fig.2 SEM images of TS?1 zeolite membranes grown on 304 stainless steel(A), alumina sheet(B), and 304 stainless steel sheet modified by TiO2(C) and SiO2(D)(A) M01-1; (B) M01-2; (C) M02-1; (D) M02-2.
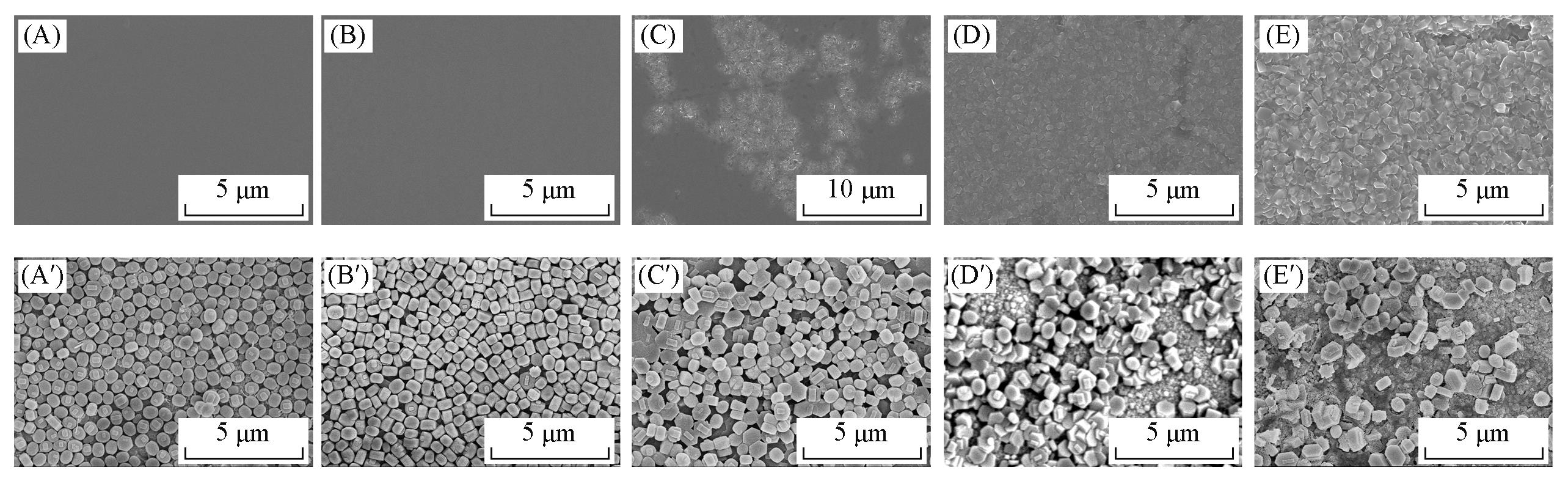
Fig.4 SEM images of TiO2 oxide layers(A—E) and TS?1 zeolite membranes grown on TiO2 oxide layers(A′—E′)Calcination temperature of TiO2 layers/℃: (A, A′) 300; (B, B′) 450; (C, C′), 600; (D, D′) 750; (E, E′) 900.
| No. | n(TEOS)∶n(TBOT)∶n(TPAOH)∶n(H2O) | Crystallization time and temperature | pH | KCPO(0k0)(%) |
|---|---|---|---|---|
| M04?1 | 1∶0.010∶0.10∶40 | 72 h/180 ℃ | 10.68 | 77.01 |
| M04?2 | 1∶0.010∶0.20∶40 | 72 h/180 ℃ | 11.52 | 74.28 |
| M05?1 | 1∶0.010∶0.15∶20 | 72 h/180 ℃ | 11.64 | 77.27 |
| M05?2 | 1∶0.010∶0.15∶100 | 72 h/180 ℃ | 10.80 | 80.56 |
| M06?1 | 1∶0.010∶0.15∶40 | 72 h/170 ℃ | 11.33 | 84.29 |
| M06?2 | 1∶0.010∶0.15∶40 | 72 h/190 ℃ | 11.33 | 85.49 |
| M07?1 | 1∶0.010∶0.15∶40 | 12 h/180 ℃ | 11.31 | 87.15 |
| M07?2 | 1∶0.010∶0.15∶40 | 96 h/180 ℃ | 11.31 | 85.30 |
Table 2 Summary of chemical environment of precursor solution and crystallographic preferred orientation index of (0k0) plane[KCPO(0k0)] of TS-1 zeolite membranes*
| No. | n(TEOS)∶n(TBOT)∶n(TPAOH)∶n(H2O) | Crystallization time and temperature | pH | KCPO(0k0)(%) |
|---|---|---|---|---|
| M04?1 | 1∶0.010∶0.10∶40 | 72 h/180 ℃ | 10.68 | 77.01 |
| M04?2 | 1∶0.010∶0.20∶40 | 72 h/180 ℃ | 11.52 | 74.28 |
| M05?1 | 1∶0.010∶0.15∶20 | 72 h/180 ℃ | 11.64 | 77.27 |
| M05?2 | 1∶0.010∶0.15∶100 | 72 h/180 ℃ | 10.80 | 80.56 |
| M06?1 | 1∶0.010∶0.15∶40 | 72 h/170 ℃ | 11.33 | 84.29 |
| M06?2 | 1∶0.010∶0.15∶40 | 72 h/190 ℃ | 11.33 | 85.49 |
| M07?1 | 1∶0.010∶0.15∶40 | 12 h/180 ℃ | 11.31 | 87.15 |
| M07?2 | 1∶0.010∶0.15∶40 | 96 h/180 ℃ | 11.31 | 85.30 |
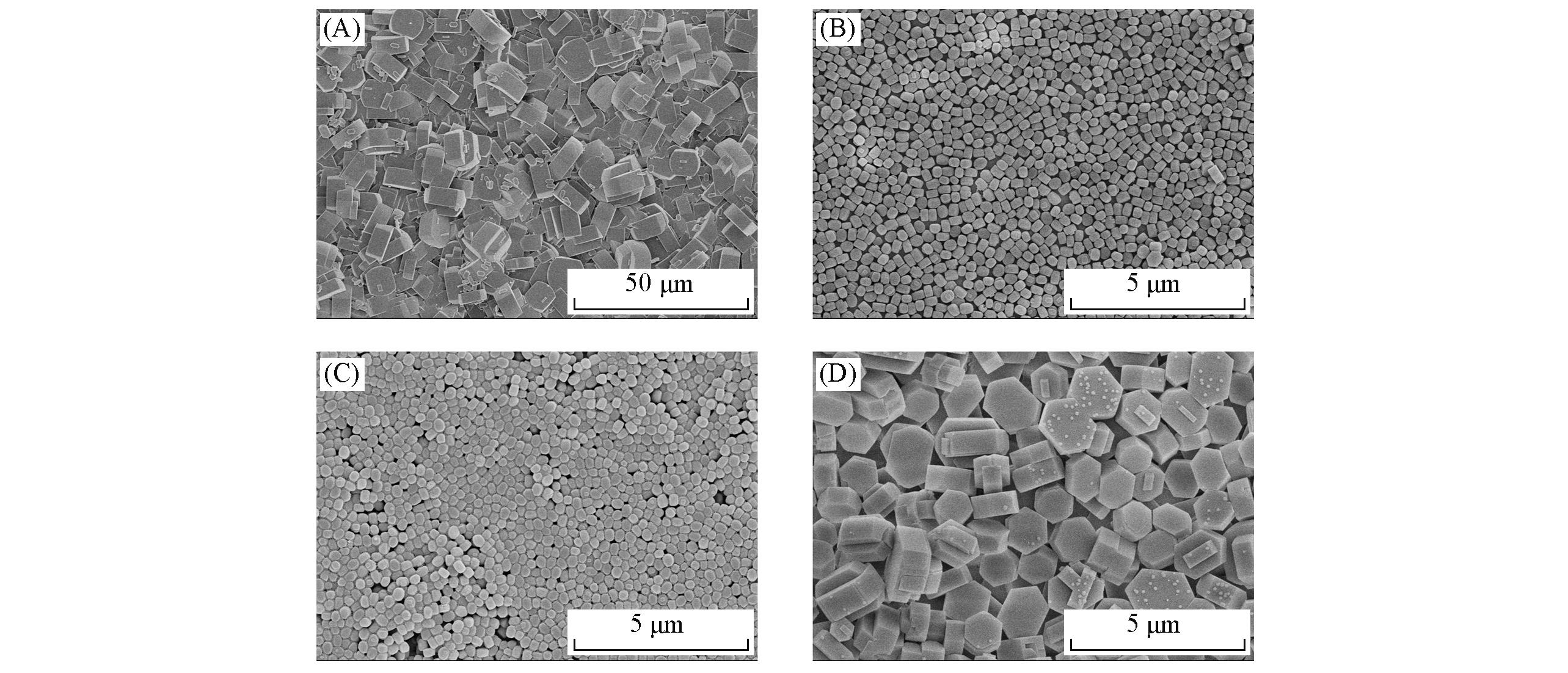
Fig.8 SEM images of TS?1 zeolite membranes grown in precursor solutions with different proportions(A) n(TEOS)∶n(TPAOH)=1∶0.10; (B) n(TEOS)∶n(TPAOH)=1∶0.20; (C) n(TEOS)∶n(H2O)=1∶20;(D) n(TEOS)∶n(H2O)=1∶100.
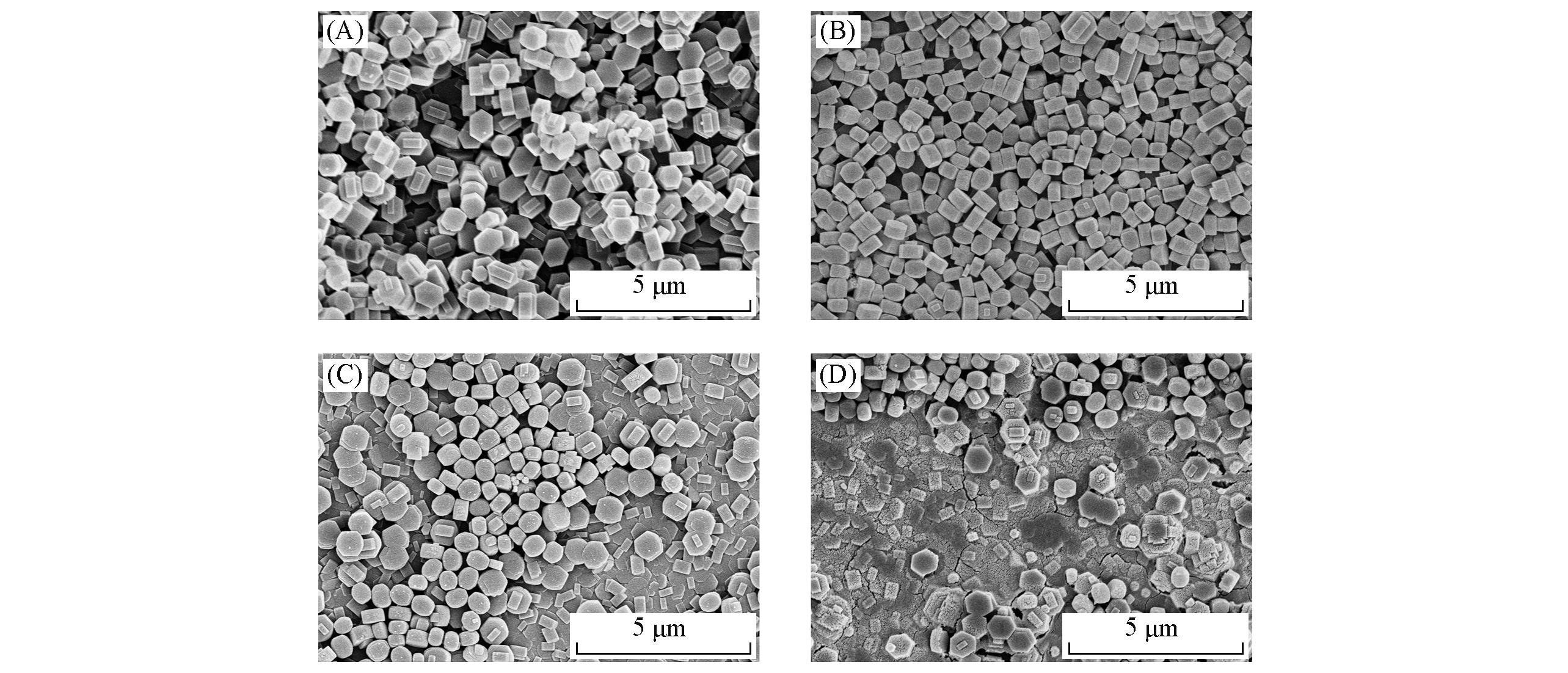
Fig.11 SEM images of TS?1 zeolite membranes prepared under different crystallization conditions(A) 170 ℃, 72 h; (B) 190 ℃, 72 h; (C) 180 ℃, 12 h; (D) 180 ℃, 96 h.
| 1 | Lai Z. P., Bonilla G., Diaz I., Nery J. G., Sujaoti K., Amat M. A., Kokkoli E., Terasaki O., Thompson R. W., Tsapatsis M., Vlachos D. G., Science, 2003, 300(5618), 456—460 |
| 2 | Lai Z. P., Tsapatsis M., Nicolich J. R., Adv. Funct. Mater., 2004, 14(7), 716—729 |
| 3 | Muller G., Narbeshuber T., Mirth G., Lercher J. A., J. Phys. Chem., 1994, 98(31), 7436—7439 |
| 4 | Caro J., Noack M., Richter-Mendau J., Marlow F., Petersohn D., Griepentrog M., Kornatowski J., J. Phys. Chem., 1993, 97(51), 13685—13690 |
| 5 | Kaerger J., J. Phys. Chem., 1991, 95(14), 5558—5560 |
| 6 | Pham T. C., Kim H. S., Yoon K. B., Science, 2011, 334(6062), 1533—1538 |
| 7 | Min B., Yang S., Korde A., Kwon Y. H., Jones C. W., Nair S., Angew. Chem. Int. Ed.,2019, 58(24), 8201—8205 |
| 8 | Lu X. F., Yang Y. W., Zhang J. J., Yan Y. S., Wang Z. B., J. Am. Chem. Soc., 2019, 141(7), 2916—2919 |
| 9 | Banihashemi F., Ibrahim A. F. M., Babaluo A. A., Lin J. Y. S., Angew. Chem. Int. Ed., 2019, 58(8), 2519—2523 |
| 10 | Xia D. Y., Peng L., Wu Z. Q., Wang L. Z., Jia Y. M., Zhang C., Gu X. H., Chem. J. Chinese Universities, 2020, 41(12), 2813—2821(夏敦焰, 彭莉, 吴政奇, 王林之, 贾逸民, 张春, 顾学红. 高等学校化学学报, 2020, 41(12), 2813—2821) |
| 11 | Su M. H., Study on Gas Separation and Shape⁃selective Catalysis Performance of Oriented Zeolite MFI Membrane, Ningxia University, Yinchuan, 2017(苏美慧. 取向MFI型分子筛膜的气体分离与择形催化性能研究, 银川: 宁夏大学, 2017) |
| 12 | Ji M. L., Liu G. Z., Chen C., Wang L., Zhang X. W., Hu S. L., Ma X. S., Appl. Catal. A: Gen., 2014, 482, 8—15 |
| 13 | Liu H., Synthesis of Orientation⁃tuned HZSM⁃5 Bi⁃layers and Its Performance in Catalytic Cracking, Tianjin University, Tianjin, 2017(刘红. 双层HZSM⁃5分子筛膜的取向调控及催化裂解性能研究, 天津: 天津大学, 2017) |
| 14 | Zhang Y. T., Zhang C., Gu X. H., Chem. J. Chinese Universities, 2021, 42(1), 289—298(张玉亭, 张春, 顾学红. 高等学校化学学报, 2021, 42(1), 289—298) |
| 15 | Li Y. C., Zhu G. F., Wang Y., Chai Y. M., Liu C. G., Micropor. Mesopor. Mater., 2021, 312, 110790 |
| 16 | Lu X. F., Peng Y., Wang Z. B., Yan Y. S., Micropor. Mesopor. Mater., 2016, 230, 49—57 |
| 17 | Ji M. L., Liu G. Z., Wang L., Zhang X. W., AIChE. J., 2014, 60(6), 1964—1968 |
| 18 | Liu H., Liu G. Z., Zhang X. W., Zhao D. D., Wang L., Micropor. Mesopor. Mater., 2017, 244, 164—170 |
| 19 | Wang Z. X., Yan W. F., Tian D. Y., Cao X. J., Yu J. H., Xu R. R., Acta Phys. Chim. Sin., 2010, 26(7), 2044—2048(王周翔, 闫文付, 田大勇, 曹学静, 于吉红, 徐如人. 物理化学学报, 2010, 26(7), 2044—2048) |
| 20 | Jiang X., Zhuang Z., Xin F., Micropor. Mesopor. Mater., 2013, 172, 141—145 |
| 21 | Wang X. D., Zhang B. Q., Liu X. F., Lin J. Y. S., Adv. Mater., 2006, 18(24), 3261—3265 |
| 22 | Lang L., Liu X. F., Zhang B. Q., Appl. Surf. Sci., 2009, 255(9), 4886—4890 |
| 23 | Wang Z. B., Yan Y. S., Chem. Mater., 2001, 13(3), 1101—1107 |
| 24 | Aguado S., Mcleary E. E., Nijmeijer A., Luiten M., Jansen J. C., Kapteijn F., Micropor. Mesopor. Mater., 2009, 120(1/2), 165— 169 |
| 25 | Hrabanek P., Zikanova A., Drahokoupil J., Prokopova O., Brabec L., Jirka I., Matejkova M., Fila V., de La Iglesia O., Kocirik M., Micropor. Mesopor. Mater., 2013, 174, 154—162 |
| 26 | Ji M. L., Liu G. Z., Wang L., Zhang X. W., Fuel, 2014, 134, 180—188 |
| 27 | Fu D. L., Schmidt J. E., Pletcher P., Karakilic P., Ye X., Vis C. M., Bruijnincx P. C. A., Filez M., Mandemaker L. D. B., Winnubst L., Weckhuysen B. M., Angew. Chem. Int. Ed., 2018, 57(38), 12458—12462 |
| 28 | Ji M. L., Controllable Fabrication and Catalytic Activity of b⁃Oriented HZSM⁃5 Coatings, Tianjin University, Tianjin, 2014(纪镁铃. b轴取向HZSM⁃5催化薄膜的可控制备及性能, 天津: 天津大学, 2014) |
| 29 | Wang Z. B., Yan Y. S., Micropor. Mesopor. Mater., 2001, 48(1—3), 229—238 |
| 30 | Li S., Li Z. J., Bozhilov K. N., Chen Z. W., Yan Y. S., J. Am. Chem. Soc., 2004, 126(34), 10732—10737 |
| 31 | Ji M. L., Liu G. Z., Chen C., Wang L., Zhang X. W., Micropor. Mesopor. Mater., 2012, 155, 117—123 |
| 32 | Lai R., Yan Y. S., Gavalas G. R., Micropor. Mesopor. Mater., 2000, 37(1/2), 9—19 |
| 33 | Wu S. C., Luo X., Long Y. F., Zhang L., Xu B. J., Huang R., J. Mater. Eng., 2020, 48(11), 99—107(吴胜财, 罗弦, 龙永富, 张露, 徐本军, 黄润. 材料工程, 2020, 48(11), 99—107) |
| 34 | Xu K., Lv D. Y., Xun C. Y., Zheng Y. F., Ge Z. H., J. Mater. Sci. Eng., 2005, (4), 629—632(许可, 吕德义, 郇昌永, 郑遗凡, 葛忠华. 材料科学与工程学报, 2005, (4), 629—632) |
| 35 | Wu Q. M., Wang Y. Q., Meng X. J., Xiao F. S., Chem. J. Chinese Universities, 2021, 42(1), 21—28(吴勤明, 王叶青, 孟祥举, 肖丰收. 高等学校化学学报, 2021, 42(1), 21—28) |
| 36 | Li Y. C., Li Y. X., Zhu G. F., Fan J., Feng X., Chai Y. M., Liu C. G., Ind. Eng. Chem. Res., 2020, 59(20), 9364—9371 |
| 37 | Peng Y., Lu X. F., Wang Z. B., Yan Y. S., Angew. Chem. Int. Ed., 2015, 54(19), 5709—5712 |
| [1] | 王隆杰, 范鸿川, 秦渝, 曹秋娥, 郑立炎. 金属有机框架材料在分离分析领域的研究进展[J]. 高等学校化学学报, 2021, 42(4): 1167. |
| [2] | 姜悦, 覃远航, 钮东方, 张新胜, 周兴贵, 孙世刚, 袁渭康. 纳米碳纤维的表面性质和微结构对氧还原催化活性的影响[J]. 高等学校化学学报, 2012, 33(05): 1001. |
| [3] | 王新平, 陈志方, 倪华钢, 沈之荃. 端羟基化聚苯乙烯的表面性质[J]. 高等学校化学学报, 2005, 26(9): 1747. |
| [4] | 敦惠娟, 魏雨, 宋秀芹, 陈立仁. 层层纳米自组装锆基色谱填料的表面性质研究[J]. 高等学校化学学报, 2005, 26(11): 2040. |
| [5] | 计剑, 沈家骢. 十八烷基聚氧乙烯表面空间结构和蛋白质吸附行为研究[J]. 高等学校化学学报, 2004, 25(3): 580. |
| [6] | 计剑, 封麟先, 沈家骢. 白蛋白原位复合生物医用功能材料的研究(Ⅰ)──材料的合成和表面结构研究[J]. 高等学校化学学报, 2002, 23(11): 2196. |
| [7] | 周钰明, 徐群华, 黄静艳. 全氟烷基乙基丙烯酸酯共聚物的制备和表面性质[J]. 高等学校化学学报, 2001, 22(12): 2126. |
| [8] | 徐文萍, 何静, 孙鹏, 段雪. 过渡金属改性MCM-41的结构及对苯催化氧化的研究[J]. 高等学校化学学报, 1999, 20(9): 1429. |
| [9] | 牛振江, 姚士冰, 周绍民. 电沉积条件对非晶态Fe-Mo合金结构及表面性质的影响[J]. 高等学校化学学报, 1998, 19(8): 1312. |
| [10] | 冯丽娟, 陈诵英, 彭少逸, 魏昭彬, 辛勤. 超细Mo/Al2O3催化剂(Ⅱ)──加氢脱硫表面结构性质研究[J]. 高等学校化学学报, 1995, 16(1): 103. |
| [11] | 纪庆绪, 程时远, 李建宗. MMA-BA无皂乳液共聚合(Ⅰ)[J]. 高等学校化学学报, 1992, 13(6): 853. |
| [12] | 顾东民, 杨昌正, 余学海. 多嵌段聚硅氧烷聚脲共聚物及双离子型聚硅氨酯的表面性质的研究[J]. 高等学校化学学报, 1989, 10(3): 280. |
| [13] | 徐僖, 宫晓颐, 刘学恕. 乙烯等离子体处理的云母表面结构及表面性质[J]. 高等学校化学学报, 1989, 10(1): 97. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||