

高等学校化学学报 ›› 2020, Vol. 41 ›› Issue (1): 162.doi: 10.7503/cjcu20190340
收稿日期:2019-06-17
出版日期:2020-01-10
发布日期:2019-11-21
通讯作者:
周琦
E-mail:zhouxq301@sina.com
基金资助:Received:2019-06-17
Online:2020-01-10
Published:2019-11-21
Contact:
Qi ZHOU
E-mail:zhouxq301@sina.com
Supported by:摘要:
采用快速凝固与脱合金相结合的方法制备了纳米多孔Ni, 经热处理氧化获得纳米多孔NiO, 利用X射线衍射仪(XRD)、 扫描电子显微镜(SEM)、 透射电子显微镜(TEM)和氮气吸附-脱附仪(BET)对纳米多孔Ni和NiO的物相、 形貌结构和孔径分布进行了表征, 并通过循环伏安、 稳态极化和电化学阻抗分析研究了电极的电催化析氧性能. 结果表明, 由Ni30Al70所得纳米多孔Ni具有多层次纳米多孔结构, 在10 mA/cm 2电流密度下析氧过电位仅为224 mV, 交换电流密度为0.63297 mA/cm 2, 表观活化自由能为40.297 kJ/mol, 经1000次循环后, 过电位降低了5 mV(j=10 mA/cm 2), 表现出良好的催化稳定性和耐久性; 热处理氧化降低了NiO的比表面积与电化学活性面积, 平衡电位下扩散传质速率明显减小, 析氧活性较Ni电极有所下降.
中图分类号:
TrendMD:
任向荣,周琦. 纳米多孔Ni和NiO的制备及电催化析氧性能. 高等学校化学学报, 2020, 41(1): 162.
REN Xiangrong,ZHOU Qi. Preparation of Nanoporous Ni and NiO and Their Electrocatalytic Activities for Oxygen Evolution Reaction †. Chem. J. Chinese Universities, 2020, 41(1): 162.
| Alloy | w(%) | ||
|---|---|---|---|
| O | Ni | Al | |
| Ni25Al75 | 7.35 | 88.55 | 4.10 |
| Ni30Al70 | 5.27 | 91.54 | 3.18 |
Table 1 Composition of dealloyed Ni25Al75 and Ni30Al70
| Alloy | w(%) | ||
|---|---|---|---|
| O | Ni | Al | |
| Ni25Al75 | 7.35 | 88.55 | 4.10 |
| Ni30Al70 | 5.27 | 91.54 | 3.18 |
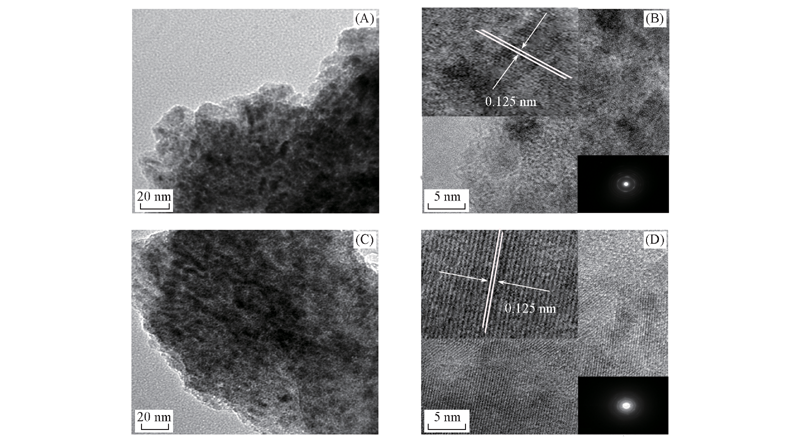
Fig.4 TEM(A, C) and HRTEM(B, D) images of dealloyed Ni25Al75(A, B) and Ni30Al70(C, D) The insets in upper left corner and the lower right corner of (B) and (D) show a local enlarged image and a selected area electron diffraction pattern of the corresponding samples, respectively.
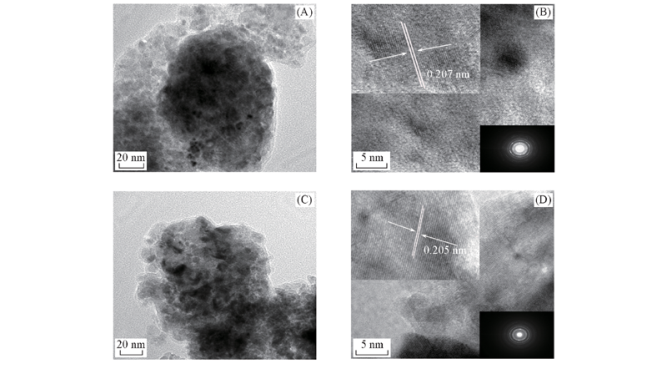
Fig.8 TEM(A, C) and HRTEM(B, D) images of NiO formed from Ni25Al75(A, B) and Ni30Al70(C, D) The insets in the upper left corner and the lower right corner of (B) and (D) show a local enlarged image and a selected area electron diffraction of the corresponding samples, respectively.
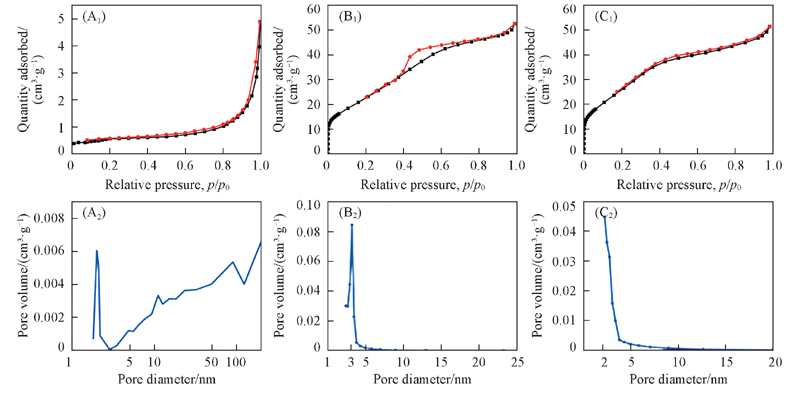
Fig.9 N2 adsorption-desorption isotherms(A1—C1) and the pore size distributions(A2—C2) of Ni and NiO (A) Nanoporous Ni formed from Ni25Al75; (B, C) nanoporous Ni and NiO formed from Ni30Al70, respectively.
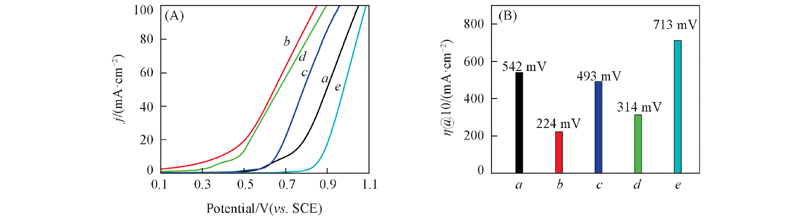
Fig.10 Anodic polarization plots of the Ni, NiO electrode(A) and overpotential histogram of the Ni, NiO electrodes obtained from graph(A) at 10 mA/cm2(B) a. Ni from Ni25Al75; b. Ni from Ni30A70; c. NiO from Ni25Al75; d. NiO from Ni30Al70; e. foam Ni.
| Electrode | b/(mV·dec-1) | a/mV | j0/(mA·cm-2) |
|---|---|---|---|
| Ni from Ni25Al75 | 261.09 | 1106.49 | 0.05781 |
| Ni from Ni30Al70 | 194.06 | 620.72 | 0.63297 |
| NiO from Ni25Al75 | 201.90 | 846.87 | 0.06390 |
| NiO from Ni30Al70 | 232.23 | 769.06 | 0.48794 |
Table 2 Kinetic parameters for oxygen evolution reaction on different electrodes*
| Electrode | b/(mV·dec-1) | a/mV | j0/(mA·cm-2) |
|---|---|---|---|
| Ni from Ni25Al75 | 261.09 | 1106.49 | 0.05781 |
| Ni from Ni30Al70 | 194.06 | 620.72 | 0.63297 |
| NiO from Ni25Al75 | 201.90 | 846.87 | 0.06390 |
| NiO from Ni30Al70 | 232.23 | 769.06 | 0.48794 |
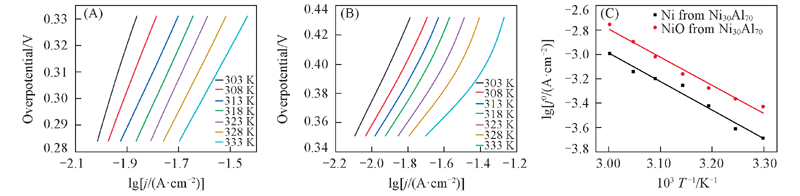
Fig.11 Tafel curves of nanoporous Ni(A) and NiO(B) obtained from Ni30Al70 at different temperatures in 1 mol/L NaOH solution and OER Arrhenius plots on the Ni, NiO electrode formed from Ni30Al70 alloy(C)
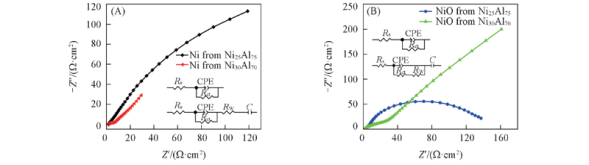
Fig.12 Nyquist plots for the Ni(A), NiO(B) electrodes at equilibrium potential Insets of (A) are equivalent circuit models of nanoporous Ni formed from Ni25Al75(up) and Ni30Al70(down) alloys, respectively; Insets of (B) are equivalent circuit models of nanoporous NiO formed from Ni25Al75(up) and Ni30Al70(down) alloys, respectively.
| Electrode | Rs/(Ω·cm-2) | CPE/F | Rct/(Ω·cm-2) | C/F | Warburg Y0/ (Ω-1·cm-2· |
|---|---|---|---|---|---|
| Ni from Ni25Al75 | 1.724 | 0.005269 | 456.6 | | |
| Ni from Ni30Al70 | 1.587 | 0.004831 | 4.125 | 0.521 | 0.1069 |
| NiO from Ni25Al75 | 1.773 | 0.01208 | 173.1 | | |
| NiO from Ni30Al70 | 1.864 | 0.00539 | 6.754 | 0.371 | 0.01938 |
Table 3 Fitted parameters of the electrode equivalent circuit on porous Ni, NiO electrodes at equilibrium potential*
| Electrode | Rs/(Ω·cm-2) | CPE/F | Rct/(Ω·cm-2) | C/F | Warburg Y0/ (Ω-1·cm-2· |
|---|---|---|---|---|---|
| Ni from Ni25Al75 | 1.724 | 0.005269 | 456.6 | | |
| Ni from Ni30Al70 | 1.587 | 0.004831 | 4.125 | 0.521 | 0.1069 |
| NiO from Ni25Al75 | 1.773 | 0.01208 | 173.1 | | |
| NiO from Ni30Al70 | 1.864 | 0.00539 | 6.754 | 0.371 | 0.01938 |
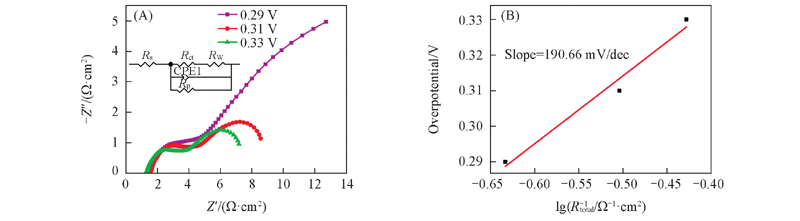
Fig.13 Nyquist plots for the Ni electrode formed from Ni30Al70 alloy at different overpotential(A) and the overpotential vs. lgRtotal-1 plot(B) The inset is equivalent circuit model of nanoporous Ni formed from Ni30Al70 alloy at different overpotential.
| η/V | Rs/(Ω·cm-2) | CPE/F | Rct/(Ω·cm-2) | Rp/(Ω·cm-2) | Warburg Y0/ (Ω-1·cm-2·S0.5) |
|---|---|---|---|---|---|
| 0.29 | 1.551 | 0.01469 | 3.021 | 30.36 | 0.2573 |
| 0.31 | 1.6 | 0.0027 | 1.95 | 8.745 | 0.1455 |
| 0.33 | 1.344 | 0.003215 | 1.639 | 7.347 | 0.1733 |
Table 4 Fitted parameters of the electrode equivalent circuit on porous Ni electrode formed from Ni30Al70 alloy at different overpotentials
| η/V | Rs/(Ω·cm-2) | CPE/F | Rct/(Ω·cm-2) | Rp/(Ω·cm-2) | Warburg Y0/ (Ω-1·cm-2·S0.5) |
|---|---|---|---|---|---|
| 0.29 | 1.551 | 0.01469 | 3.021 | 30.36 | 0.2573 |
| 0.31 | 1.6 | 0.0027 | 1.95 | 8.745 | 0.1455 |
| 0.33 | 1.344 | 0.003215 | 1.639 | 7.347 | 0.1733 |
| E/V(vs. SCE) | 1015/(cm2·s-1) | |
|---|---|---|
| Ni | NiO | |
| 0.16 | 3.653 | 0.3442 |
| 0.21 | 4.628 | 0.2108 |
| 0.26 | 6.507 | 0.2685 |
| 0.31 | 15.520 | 0.3689 |
| 0.36 | 69.970 | 0.6541 |
Table 5 Diffusion coefficient values of Ni and NiO electrodes formed from Ni30Al70 alloy at different potentials
| E/V(vs. SCE) | 1015/(cm2·s-1) | |
|---|---|---|
| Ni | NiO | |
| 0.16 | 3.653 | 0.3442 |
| 0.21 | 4.628 | 0.2108 |
| 0.26 | 6.507 | 0.2685 |
| 0.31 | 15.520 | 0.3689 |
| 0.36 | 69.970 | 0.6541 |
| Electrode | Cdl/μF | S/cm2 | r | (j0/r)/(mA·cm-2) |
|---|---|---|---|---|
| Ni | 642333 | 32117 | 32117 | 1.9708×10-5 |
| NiO | 562440 | 28122 | 28122 | 1.7351×10-5 |
Table 6 Surface parameters of Ni and NiO electrode formed from Ni30Al70 alloy*
| Electrode | Cdl/μF | S/cm2 | r | (j0/r)/(mA·cm-2) |
|---|---|---|---|---|
| Ni | 642333 | 32117 | 32117 | 1.9708×10-5 |
| NiO | 562440 | 28122 | 28122 | 1.7351×10-5 |
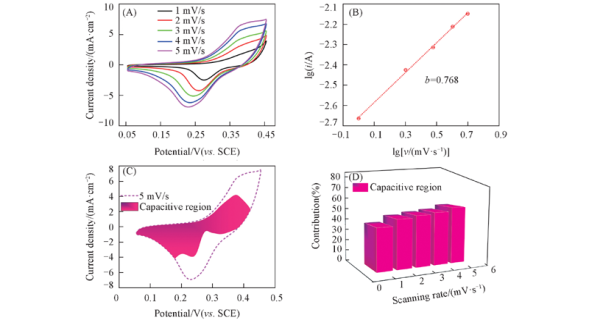
Fig.15 Electrochemical properties and kinetics of nanoporous Ni formed from Ni30Al70 alloy (A) CV curves at 1—5 mV/s; (B) relationship between lgip and lgv; (C) area contribution of capacitance curve at 5 mV/s; (D) contribution diagram of capacitance at different scanning rates.
| [1] |
Rausch B., Symes M. D ., Chisholm G., Cronin L.,. Science, 2014,345(6202), 1326— 1330
doi: 10.1126/science.1257443 URL pmid: 25214625 |
| [2] | Feng X. L., Qu Z. K., Chen J., Wang D. D., Chen X., Yang W. S., Chem. J. Chinese Universities, 2017,38(11), 1999— 2005 |
| ( 冯晓磊, 曲宗凯, 陈俊, 王登登, 陈旭, 杨文胜. 高等学校化学学报, 2017,38(11), 1999— 2005) | |
| [3] | Zhao D. D., Zhang N., Pu L. Z., Shao Q., Huang X. Q., J. Electrochem., 2018,24(5), 52— 62 |
| ( 赵丹丹, 张楠, 卜令正, 邵琪, 黄小青. 电化学, 2018,24(5), 52— 62) | |
| [4] | Liu W., Fabrication of Transition Metal Based Nanosheet Array Electrodes and Their Catalytic Performance Toward Photo-electrochemical Oxygen Evolution Reaction, Zhejiang University, Hangzhou, 2018 |
| (刘伟. 过渡金属基纳米片阵列电极材料的制备及其光/电催化产氧性能研究,.杭州: 浙江大学, 2018) | |
| [5] |
Lee Y., Jin S., May K. J ., Perry E. E., Yang S. H.,. Journal of Physical Chemistry Letters, 2015,3(3), 399— 404
doi: 10.1021/jz2016507 URL pmid: 26285858 |
| [6] |
Prathap M. U. A ., Satpati B., Srivastava R.,. Electrochemical Acta, 2014,130, 368— 380
doi: 10.1016/j.electacta.2014.03.043 URL |
| [7] |
Fominykh K., Feckl J. M ., Sicklinger J., Doblinger M., Bocklein S., Ziegler J., Peter L., Rathousky J., Scheidt E. W., Bein T., Dina F. R.,. Advanced Functional Materials, 2014,24(21), 3123— 3129
doi: 10.1002/adfm.201303600 URL |
| [8] |
Wei G., Xia Z. M ., Cao F. X., Ho J. C., Zheng J., Qu Y. Q.,. Advanced Functional Materials, 2018,28(11), 1706056
doi: 10.1002/adfm.v28.11 URL |
| [9] |
Zou X., Zhang Y ., Chemical Society Reviews, 2015,44(15), 5148— 5180
doi: 10.1039/C4CS00448E URL |
| [10] |
Bain W., Yang Z., Strasser P., Yang R ., Journal of Power Sources, 2014,250(3), 196— 203
doi: 10.1016/j.jpowsour.2013.11.024 URL |
| [11] |
Chen R., Wang H. Y ., Miao J., Yang H., Liu B.,. Nano Energy, 2015,11, 333— 340
doi: 10.1016/j.nanoen.2014.11.021 URL |
| [12] | Zhou Q., Zheng B., Li Z. Y., Wang Y. F., Feng J. W., Chinese Journal of Inorganic Chemistry, 2017,33(8), 1416— 1422 |
| ( 周琦, 郑斌, 李志洋, 王亚飞, 冯基伟. 无机化学学报, 2017,33(8), 1416— 1422) | |
| [13] |
Wang X., Qi Z., Zhao C., Wang W., Zhang Z ., Journal of Physical Chemistry C, 2009,113(30), 13139— 13150
doi: 10.1021/jp902490u URL |
| [14] |
Babar P. T ., Lokhande A. C., Gang M. G., Pawar B. S., Pawar S. M., Kim J. H.,. Journal of Industrial & Engineering Chemistry, 2017,60, 493— 497
doi: 10.1002/bab.1888 URL pmid: 31954377 |
| [15] |
Li J., Luo F., Zhao Q., Li Z., Yuan H., Xiao D ., Journal of Materials Chemistry A, 2014,2(13), 4690— 4697
doi: 10.1039/c3ta14694d URL |
| [16] | Gao X. S., Preparation and Performance of Mesoporous Binary Metal Oxide Nanorods as Oxygen Evolution Catalyst, Taiyuan University of Technology, Taiyuan, 2017 |
| ( 高旭升. 介孔二元金属氧化物纳米棒析氧催化剂的制备及性能研究, 太原: 太原理工大学, 2017) | |
| [17] | Chen G., Study on the Synthesis and Properties of NiO-based Electrode Materials for Supercapacitors, Yunnan University, Kunming, 2016 |
| (陈刚. 基于NiO超级电容器电极材料的制备及其性能研究, 昆明: 云南大学, 2016) | |
| [18] |
Thi T. V ., Rai A. K., Gim J., Kim J.,. Journal of Power Sources, 2015,292, 23— 30
doi: 10.1016/j.jpowsour.2015.05.029 URL |
| [19] |
Zhang J., Cai G., Zhou D., Tang H., Wang X., Gu C., Tu J ., Journal of Materials Chemistry C, 2014,2(34), 7013— 7021
doi: 10.1039/c4tc01033g URL |
| [20] |
Zhu L., Cai Q., Liao F., Sheng M., Wu B., Shao M ., Electrochemistry Communications, 2015,52(15), 29— 33
doi: 10.1016/j.elecom.2015.01.012 URL |
| [21] |
Jeyaprabha C., Sathiyanarayanan S., Venkatachari G ., Applied Surface Science, 2006,253(2), 432— 438
doi: 10.1016/j.apsusc.2005.12.081 URL |
| [22] |
Rakhi R. B ., Chen W., Hedhili M. N., Cha D., Alshareef H. N., ACS Appl. Mater. Interfaces, 2014,6(6), 4196— 4206
doi: 10.1021/am405849n URL pmid: 24580967 |
| [23] |
Min S., Zhao C., Chen G., Zhang Z., Qian X ., Electrochemical Acta, 2014,135(22), 336— 344
doi: 10.1016/j.electacta.2014.05.032 URL |
| [24] |
Zhu L., Lin H., Li Y., Liao F., Lifshitz Y., Sheng M., Shao M ., Nature Communications, 2016,7, 12272
doi: 10.1038/ncomms12272 URL pmid: 27447292 |
| [25] | Wang Y., Sheng M. Q., Weng W. P., Xu J. F., Cao M. Q., Chinese Journal of Materials Research, 2017,31(10), 55— 62 |
| (王玉, 盛敏奇, 翁文凭, 许继芳, 曹孟秋. 材料研究学报, 2017,31(10), 55— 62) | |
| [26] | Shen L., Lv H., Chen S. Q ., Kopold P., Aken P. A., Wu X. J., Maier J., Yu Y.,. Advanced Materials, 2017,29(27), 1602— 1620 |
| [27] | Wang Y. K., Fabrication of Transition Metal Sulfide as Electrode Material and Application for Lithium Ion Capacitor, Lanzhou University of Technology, Lanzhou, 2019 |
| (王雲锴. 过渡金属硫化物电极材料的设计与锂离子电容器应用, 兰州: 兰州理工大学, 2019) | |
| [28] |
Bao J. Z., Wang S. L., Acta Physico-Chimica Sinica, 2011,27(12), 2849— 2856
doi: 10.3866/PKU.WHXB20112849 URL |
|
( 鲍晋珍, 王森林. 物理化学学报, 2011,27(12), 2849— 2856)
doi: 10.3866/PKU.WHXB20112849 URL |
|
| [29] |
Wang L. P., Wang S. L., Duan Q. H., Chinese Journal of Applied Chemistry, 2013,30(6), 690— 697
doi: 10.3724/SP.J.1095.2013.20385 URL |
|
( 王丽品, 王森林, 段钱花. 应用化学, 2013,30(6), 690— 697)
doi: 10.3724/SP.J.1095.2013.20385 URL |
| [1] | 常建红, 徐国杰, 李辉, 方千荣. 基于醌基的共价有机框架用于电催化析氧反应[J]. 高等学校化学学报, 2020, 41(7): 1609. |
| [2] | 金娥,宋开绪,崔丽莉. 双金属磷化物和杂原子共修饰碳材料的制备及电催化性能[J]. 高等学校化学学报, 2020, 41(6): 1362. |
| [3] | 刘璐,伍含月,李静,佘岚. 铁镍合金催化剂的结构调控及对电化学析氧反应的催化性能[J]. 高等学校化学学报, 2020, 41(5): 1083. |
| [4] | 韩志英,李佑稷,陈飞台,汤森培,王鹏. 同轴静电纺丝法制备ZnO/Ag2O纳米纤维材料及其光电催化性能研究[J]. 高等学校化学学报, 2020, 41(2): 308. |
| [5] | 姜媛媛, 李伯语, 逯一中, 吴同舜, 韩冬雪. 无电沉积硼化镍材料对水氧化的电催化性能[J]. 高等学校化学学报, 2020, 41(12): 2774. |
| [6] | 周琦, 李志洋, 汪帆. Mo对脱合金制备的Ni-Mo电极骨架结构与析氢性能的影响[J]. 高等学校化学学报, 2019, 40(8): 1717. |
| [7] | 张敏, 陈梦伟, 高虹, 毕研峰. 基于磺酰基杯[4]芳烃的Co16笼簇的合成、 结构及电化学性质[J]. 高等学校化学学报, 2019, 40(10): 2052. |
| [8] | 蒋一兰, 袁龙, 王西阳, 黄科科, 冯守华. 缺陷调控对钙钛矿结构锰酸镧催化性质的影响[J]. 高等学校化学学报, 2018, 39(3): 416. |
| [9] | 陈晨, 李丽, 陈金华, 张小华, 许杰, 李益波, 韦杰. Pt-CeO2/聚苯乙烯磺酸盐功能化碳纳米管复合物的制备及对甲醇的电催化氧化性能[J]. 高等学校化学学报, 2018, 39(1): 157. |
| [10] | 孔庆梅, 蒋玉芝, 陈冲, 周益明, 陆天虹, 陈煜, 唐亚文. Ir/CNTs催化剂的制备及对氨的电催化氧化[J]. 高等学校化学学报, 2010, 31(11): 2260. |
| [11] | 王彦恩,唐亚文,周益明,高颖,刘长鹏,陆天虹,. Fe对Pt-Fe/C催化剂电催化氧还原反应活性的影响[J]. 高等学校化学学报, 2007, 28(4): 743. |
| [12] | 崔成强, 姚士冰, 周绍民. RuO2电极的XPS和UPS研究[J]. 高等学校化学学报, 1989, 10(4): 392. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
