

高等学校化学学报 ›› 2019, Vol. 40 ›› Issue (4): 712.doi: 10.7503/cjcu20180663
代佳男, 杨子祺, 魏忠林, 曹军刚, 梁大鹏, 段海峰( ), 林英杰(
), 林英杰( )
)
收稿日期:2018-09-29
出版日期:2019-12-24
发布日期:2018-12-24
作者简介:联系人简介: 段海峰, 男, 博士, 教授, 主要从事有机合成方法学方面的研究. E-mail:
基金资助:
DAI Jianan, YANG Ziqi, WEI Zhonglin, CAO Jungang, LIANG Dapeng, DUAN Haifeng*( ), LIN Yingjie*(
), LIN Yingjie*( )
)
Received:2018-09-29
Online:2019-12-24
Published:2018-12-24
Contact:
DUAN Haifeng,LIN Yingjie
E-mail:duanhf@jlu.edu.cn;linyj@jlu.edu.cn
Supported by:摘要:
采用2-异氰基联苯与N,N-二甲基甲酰胺(DMF)作为反应原料, 在四丁基氯化铵(TBAC)、 磷酸氢二钾(K2HPO4)和叔丁基过氧化氢(TBHP)组成的催化氧化体系作用下, 通过一步构建2个C—C键, 以较高的产率合成了一系列6-氨基甲酰基取代的啡啶类化合物(产率高达86%). 同时利用该方法研究了一系列含有推电子和吸电子取代基的2-异氰基联苯衍生物的普适性, 为具有药物活性的该类分子的合成提供了一种新的方法.
中图分类号:
TrendMD:
代佳男, 杨子祺, 魏忠林, 曹军刚, 梁大鹏, 段海峰, 林英杰. TBAC/TBHP体系下6-氨基甲酰基取代的啡啶类化合物的合成. 高等学校化学学报, 2019, 40(4): 712.
DAI Jianan,YANG Ziqi,WEI Zhonglin,CAO Jungang,LIANG Dapeng,DUAN Haifeng,LIN Yingjie. Synthesis of 6-Aminoformyl-substituted Phenanthridines Promoted by TBAC/TBHP System†. Chem. J. Chinese Universities, 2019, 40(4): 712.
| Compd. | Appearance | Yield(%) | Melting point/℃ | HRMS, m/z[M+H]+(calcd.) |
|---|---|---|---|---|
| 3a | Yellow oil | 75 | 251.1176(251.1179) | |
| 3b | White solid | 61 | 150—152 | 265.1336(265.1335) |
| 3c | White solid | 69 | 123—125 | 265.1332(265.1335) |
| 3d | White solid | 74 | 146—148 | 265.1335(265.1335) |
| 3e | White solid | 81 | 129—131 | 279.1490(279.1492) |
| 3f | White solid | 59 | 227—229 | 281.1282(281.1285) |
| 3g | White solid | 56 | 153—155 | 285.0786(285.0789) |
| 3h | White solid | 65 | 163—165 | 319.1053(319.1053) |
| 3i | Green oil | 62 | 327.1490(327.1492) | |
| 3j | White solid | 65 | 204—206 | 285.0786(285.0789) |
| 3k | Yellow solid | 78 | 129—131 | 279.1492(279.1492) |
| 3l | Yellow oil | 59 | 295.1440(295.1441) | |
| 3m | White solid | 86 | 228—230 | 287.0993(287.0990) |
| 3n | White solid | 53 | 234—236 | 319.0400(319.0400) |
| 3o | White solid | 60 | 196—198 | 351.1490(351.1492) |
Table 1 Appearance, yields and HRMS data of compounds 3a—3o
| Compd. | Appearance | Yield(%) | Melting point/℃ | HRMS, m/z[M+H]+(calcd.) |
|---|---|---|---|---|
| 3a | Yellow oil | 75 | 251.1176(251.1179) | |
| 3b | White solid | 61 | 150—152 | 265.1336(265.1335) |
| 3c | White solid | 69 | 123—125 | 265.1332(265.1335) |
| 3d | White solid | 74 | 146—148 | 265.1335(265.1335) |
| 3e | White solid | 81 | 129—131 | 279.1490(279.1492) |
| 3f | White solid | 59 | 227—229 | 281.1282(281.1285) |
| 3g | White solid | 56 | 153—155 | 285.0786(285.0789) |
| 3h | White solid | 65 | 163—165 | 319.1053(319.1053) |
| 3i | Green oil | 62 | 327.1490(327.1492) | |
| 3j | White solid | 65 | 204—206 | 285.0786(285.0789) |
| 3k | Yellow solid | 78 | 129—131 | 279.1492(279.1492) |
| 3l | Yellow oil | 59 | 295.1440(295.1441) | |
| 3m | White solid | 86 | 228—230 | 287.0993(287.0990) |
| 3n | White solid | 53 | 234—236 | 319.0400(319.0400) |
| 3o | White solid | 60 | 196—198 | 351.1490(351.1492) |
| Compd. | 1H NMR(400 MHz, CDCl3), δ | 13C NMR(101 MHz, CDCl3), δ | |
|---|---|---|---|
| 3a | 8.68(d, J=8.2 Hz, 1H), 8.62(d, J=8.4 Hz, 1H), 8.21(d, J=8.1 Hz, 1H), 8.09(d, J=8.1 Hz, 1H), 7.91(t, J=7.7 Hz, 1H), 7.82—7.69(m, 3H), 3.33(s, 3H), 2.95(s, 3H) | 168.3, 156.4, 143.2, 133.4, 131.5, 130.4, 129.2, 128.1, 127.9, 127.2, 124.2, 123.3, 122.5, 122.3, 38.5, 35.0 | |
| 3b | 8.64(d, J=8.3 Hz, 1H), 8.37(s, 1H), 8.07(t, J=8.8 Hz, 2H), 7.86(t, J=7.6 Hz, 1H), 7.68(t, J=7.6 Hz, 1H), 7.59(d, J=8.3 Hz, 1H), 3.31(s, 3H), 2.93(s, 3H), 2.64(s, 3H). | 168.4, 155.4, 141.5, 137.9, 133.0, 131.2, 130.9, 130.0, 127.8, 127.1, 124.0, 123.3, 122.4, 121.8, 38.4, 34.9, 22.1 | |
| 3c | 8.85(d, J=8.4 Hz, 1H), 8.24(d, J=9.3 Hz, 1H), 7.96(d, J=7.9 Hz, 1H), 7.80—7.67(m, 3H), 7.61(t, J=7.7 Hz, 1H), 3.31(s, 3H), 3.15(s, 3H), 2.90(s, 3H) | 168.7, 157.2, 144.4, 135.8, 135.7, 132.7, 130.6, 128.3, 127.5, 127.0, 126.7, 125.8, 125.7, 124.6, 38.3, 34.9, 26.9 | |
| 3d | 8.58—8.51(m, 2H), 8.20—8.14(m, 1H), 7.83(s, 1H), 7.76—7.66(m, 3H), 3.32(s, 3H), 2.93(s, 3H), 2.58(s, 3H) | 168.4, 156.1, 142.8, 138.2, 133.3, 131.2, 130.2, 128.6, 127.7, 126.4, 124.3, 123.4, 122.3, 122.0, 38.4, 34.9, 21.8 | |
| 3e | 8.63(d, J=8.4 Hz, 1H), 8.22(s, 1H), 8.08(d, J=8.1 Hz, 1H), 7.82(t, J=7.7 Hz, 1H), 7.66(t, J=7.6 Hz, 1H), 7.45(s, 1H), 3.32(s, 3H), 2.93(s, 3H), 2.81(s, 3H), 2.59(s, 3H) | 168.8, 153.6, 140.2, 138.1, 137.3, 133.3, 131.6, 130.8, 127.5, 127.0, 123.9, 123.2, 122.6, 119.6, 38.6, 35.0, 22.1, 18.5 | |
| 3f | 8.57(d, J=9.1 Hz, 1H), 8.51(dd, J=6.2, 3.2 Hz, 1H), 8.17(dd, J=6.2, 3.1 Hz, 1H), 7.75—7.65(m, 2H), 7.51(dd, J=9.1, 2.6 Hz, 1H), 7.41(d, J=2.5 Hz, 1H), 3.97(s, 3H), 3.32(s, 3H), 2.95(s, 3H) | 168.3, 159.2, 155.3, 142.3, 130.2, 128.1, 127.9, 127.9, 124.6, 124.4, 124.1, 122.4, 121.7, 106.8, 55.8, 38.5, 35.1 | |
| 3g | 8.55(dd, J=19.7, 8.4 Hz, 2H), 8.18(d, J=8.7 Hz, 1H), 8.07(d, J=2.0 Hz, 1H), 7.84—7.69(m, 3H), 3.32(s, 3H), 2.96(s, 3H) | 167.7, 155.1, 143.0, 134.0, 132.1, 131.7, 130.5, 129.5, 128.3, 126.4, 124.2, 124.2, 123.6, 122.1, 38.5, 35.1 | |
| 3h | 8.77(t, J=9.8 Hz, 1H), 8.65—8.56(m, 1H), 8.39(s, 1H), 8.22(t, J=8.0 Hz, 1H), 8.11—8.02(m, 1H), 7.88—7.73(m, 2H), 3.40—3.30(m, 3H), 3.04—2.93(m, 3H) | 167.5, 155.9, 143.7, 135.5, 130.5(d, J=20.4 Hz), 128.5, 127.3, 124.9, 123.6, 123.3, 122.8, 122.6, 38.6, 35.2 | |
| 3i | 8.75—8.70(m, 1H), 8.60(d, J=8.2 Hz, 1H), 8.26—8.14(m, 3H), 8.01(s, 1H), 7.74—7.71(m, 3H), 7.52(t, J=8.0 Hz, 3H), 3.32(s, 3H), 2.88(s, 3H) | 168.3, 162.6, 156.5, 143.1, 140.9, 140.0, 132.3, 130.8, 129.1, 128.1, 127.9, 127.6, 127.4, 125.1, 124.0, 123.7, 123.0, 122.2, 38.5, 35.0 | |
| 3j | 8.63—8.51(m, 2H), 8.18—8.05(m, 2H), 7.95—7.86(m, 1H), 7.79—7.66(m, 2H), 3.32(d, J=1.0 Hz, 3H), 2.93(d, J=1.3 Hz, 3H) | 168.0, 156.6, 141.6, 133.9, 132.3, 131.8, 131.8, 129.7, 128.7, 127.3, 125.3, 123.4, 122.5, 121.9, 38.4, 35.0 | |
| 3k | 8.52(d, J=8.5 Hz, 1H), 8.32(s, 1H), 8.05(d, J=8.3 Hz, 1H), 7.82(s, 1H), 7.67(d, J=8.3 Hz, 1H), 7.54(t, J=7.6 Hz, 1H), 3.31(s, 3H), 2.92(s, 3H), 2.63(s, 3H), 2.57(s, 3H) | 168.6, 155.1, 141.2, 137.9, 137.7, 133.0, 130.9, 130.4, 129.9, 126.3, 124.1, 123.5, 122.2, 121.6, 38.4, 34.9, 22.1, 21.7 | |
| 3l | 8.52(t, J=8.9 Hz, 1H), 8.26(s, 1H), 8.04(d, J=8.3 Hz, 1H), 7.57—7.42(m, 2H), 7.38(d, J=2.4 Hz, 1H), 3.95(s, 3H), 3.30(s, 3H), 2.93(s, 3H), 2.61(s, 3H) | 168.5, 162.5, 159.1, 154.3, 140.6, 137.9, 129.9, 129.9, 127.5, 124.7, 124.2, 124.0, 122.1, 121.3, 106.6, 55.7, 38.5, 35.0, 25.9 | |
| 3m | 8.52(dd, J=9.1, 5.0 Hz, 1H), 8.21—8.08(m, 2H), 7.75(dd, J=9.0, 2.4 Hz, 1H), 7.68—7.58(m, 1H), 7.53—7.44(m, 1H), 3.31(s, 3H), 2.96(s, 3H) | 167.6, 139.6, 132.7, 125.3, 124.7, 124.7, 121.0, 120.8, 118.2, 118.0, 112.1, 111.8, 107.2, 107.0, 38.5, 35.1. | |
| 3n | 8.49(d, J=8.9 Hz, 2H), 8.08(dd, J=12.8, 5.3 Hz, 2H), 7.83(dd, J=8.8, 1.9 Hz, 1H), 7.70(dd, J=8.7, 2.0 Hz, 1H), 3.32(s, 3H), 2.95(s, 3H) | 167.3, 155.3, 141.4, 134.8, 134.4, 132.4, 131.9, 130.7, 130.1, 126.6, 124.7, 124.4, 124.2, 121.8, 38.5, 35.1 | |
| 3o | 8.84(d, J=8.2 Hz, 1H), 8.76(d, J=8.4 Hz, 1H), 8.70(t, 2H), 8.52(d, J=8.1 Hz, 1H), 8.30(d, J=8.2 Hz, 1H), 7.79(t, J=7.3 Hz, 2H), 7.76—7.60(m, 4H), 3.31(s, 3H), 2.82(s, 3H) | 170.8, 152.7, 145.6, 135.1, 132.7, 130.6, 130.1, 129.4, 129.2, 129.1, 128.2, 128.1, 127.9, 127.8, 127.7, 127.3, 127.0, 125.8, 123.9, 123.7, 123.5, 120.4, 38.4, 35.4 | |
Table 2 1H NMR and 13C NMR data of compounds 3a—3o
| Compd. | 1H NMR(400 MHz, CDCl3), δ | 13C NMR(101 MHz, CDCl3), δ | |
|---|---|---|---|
| 3a | 8.68(d, J=8.2 Hz, 1H), 8.62(d, J=8.4 Hz, 1H), 8.21(d, J=8.1 Hz, 1H), 8.09(d, J=8.1 Hz, 1H), 7.91(t, J=7.7 Hz, 1H), 7.82—7.69(m, 3H), 3.33(s, 3H), 2.95(s, 3H) | 168.3, 156.4, 143.2, 133.4, 131.5, 130.4, 129.2, 128.1, 127.9, 127.2, 124.2, 123.3, 122.5, 122.3, 38.5, 35.0 | |
| 3b | 8.64(d, J=8.3 Hz, 1H), 8.37(s, 1H), 8.07(t, J=8.8 Hz, 2H), 7.86(t, J=7.6 Hz, 1H), 7.68(t, J=7.6 Hz, 1H), 7.59(d, J=8.3 Hz, 1H), 3.31(s, 3H), 2.93(s, 3H), 2.64(s, 3H). | 168.4, 155.4, 141.5, 137.9, 133.0, 131.2, 130.9, 130.0, 127.8, 127.1, 124.0, 123.3, 122.4, 121.8, 38.4, 34.9, 22.1 | |
| 3c | 8.85(d, J=8.4 Hz, 1H), 8.24(d, J=9.3 Hz, 1H), 7.96(d, J=7.9 Hz, 1H), 7.80—7.67(m, 3H), 7.61(t, J=7.7 Hz, 1H), 3.31(s, 3H), 3.15(s, 3H), 2.90(s, 3H) | 168.7, 157.2, 144.4, 135.8, 135.7, 132.7, 130.6, 128.3, 127.5, 127.0, 126.7, 125.8, 125.7, 124.6, 38.3, 34.9, 26.9 | |
| 3d | 8.58—8.51(m, 2H), 8.20—8.14(m, 1H), 7.83(s, 1H), 7.76—7.66(m, 3H), 3.32(s, 3H), 2.93(s, 3H), 2.58(s, 3H) | 168.4, 156.1, 142.8, 138.2, 133.3, 131.2, 130.2, 128.6, 127.7, 126.4, 124.3, 123.4, 122.3, 122.0, 38.4, 34.9, 21.8 | |
| 3e | 8.63(d, J=8.4 Hz, 1H), 8.22(s, 1H), 8.08(d, J=8.1 Hz, 1H), 7.82(t, J=7.7 Hz, 1H), 7.66(t, J=7.6 Hz, 1H), 7.45(s, 1H), 3.32(s, 3H), 2.93(s, 3H), 2.81(s, 3H), 2.59(s, 3H) | 168.8, 153.6, 140.2, 138.1, 137.3, 133.3, 131.6, 130.8, 127.5, 127.0, 123.9, 123.2, 122.6, 119.6, 38.6, 35.0, 22.1, 18.5 | |
| 3f | 8.57(d, J=9.1 Hz, 1H), 8.51(dd, J=6.2, 3.2 Hz, 1H), 8.17(dd, J=6.2, 3.1 Hz, 1H), 7.75—7.65(m, 2H), 7.51(dd, J=9.1, 2.6 Hz, 1H), 7.41(d, J=2.5 Hz, 1H), 3.97(s, 3H), 3.32(s, 3H), 2.95(s, 3H) | 168.3, 159.2, 155.3, 142.3, 130.2, 128.1, 127.9, 127.9, 124.6, 124.4, 124.1, 122.4, 121.7, 106.8, 55.8, 38.5, 35.1 | |
| 3g | 8.55(dd, J=19.7, 8.4 Hz, 2H), 8.18(d, J=8.7 Hz, 1H), 8.07(d, J=2.0 Hz, 1H), 7.84—7.69(m, 3H), 3.32(s, 3H), 2.96(s, 3H) | 167.7, 155.1, 143.0, 134.0, 132.1, 131.7, 130.5, 129.5, 128.3, 126.4, 124.2, 124.2, 123.6, 122.1, 38.5, 35.1 | |
| 3h | 8.77(t, J=9.8 Hz, 1H), 8.65—8.56(m, 1H), 8.39(s, 1H), 8.22(t, J=8.0 Hz, 1H), 8.11—8.02(m, 1H), 7.88—7.73(m, 2H), 3.40—3.30(m, 3H), 3.04—2.93(m, 3H) | 167.5, 155.9, 143.7, 135.5, 130.5(d, J=20.4 Hz), 128.5, 127.3, 124.9, 123.6, 123.3, 122.8, 122.6, 38.6, 35.2 | |
| 3i | 8.75—8.70(m, 1H), 8.60(d, J=8.2 Hz, 1H), 8.26—8.14(m, 3H), 8.01(s, 1H), 7.74—7.71(m, 3H), 7.52(t, J=8.0 Hz, 3H), 3.32(s, 3H), 2.88(s, 3H) | 168.3, 162.6, 156.5, 143.1, 140.9, 140.0, 132.3, 130.8, 129.1, 128.1, 127.9, 127.6, 127.4, 125.1, 124.0, 123.7, 123.0, 122.2, 38.5, 35.0 | |
| 3j | 8.63—8.51(m, 2H), 8.18—8.05(m, 2H), 7.95—7.86(m, 1H), 7.79—7.66(m, 2H), 3.32(d, J=1.0 Hz, 3H), 2.93(d, J=1.3 Hz, 3H) | 168.0, 156.6, 141.6, 133.9, 132.3, 131.8, 131.8, 129.7, 128.7, 127.3, 125.3, 123.4, 122.5, 121.9, 38.4, 35.0 | |
| 3k | 8.52(d, J=8.5 Hz, 1H), 8.32(s, 1H), 8.05(d, J=8.3 Hz, 1H), 7.82(s, 1H), 7.67(d, J=8.3 Hz, 1H), 7.54(t, J=7.6 Hz, 1H), 3.31(s, 3H), 2.92(s, 3H), 2.63(s, 3H), 2.57(s, 3H) | 168.6, 155.1, 141.2, 137.9, 137.7, 133.0, 130.9, 130.4, 129.9, 126.3, 124.1, 123.5, 122.2, 121.6, 38.4, 34.9, 22.1, 21.7 | |
| 3l | 8.52(t, J=8.9 Hz, 1H), 8.26(s, 1H), 8.04(d, J=8.3 Hz, 1H), 7.57—7.42(m, 2H), 7.38(d, J=2.4 Hz, 1H), 3.95(s, 3H), 3.30(s, 3H), 2.93(s, 3H), 2.61(s, 3H) | 168.5, 162.5, 159.1, 154.3, 140.6, 137.9, 129.9, 129.9, 127.5, 124.7, 124.2, 124.0, 122.1, 121.3, 106.6, 55.7, 38.5, 35.0, 25.9 | |
| 3m | 8.52(dd, J=9.1, 5.0 Hz, 1H), 8.21—8.08(m, 2H), 7.75(dd, J=9.0, 2.4 Hz, 1H), 7.68—7.58(m, 1H), 7.53—7.44(m, 1H), 3.31(s, 3H), 2.96(s, 3H) | 167.6, 139.6, 132.7, 125.3, 124.7, 124.7, 121.0, 120.8, 118.2, 118.0, 112.1, 111.8, 107.2, 107.0, 38.5, 35.1. | |
| 3n | 8.49(d, J=8.9 Hz, 2H), 8.08(dd, J=12.8, 5.3 Hz, 2H), 7.83(dd, J=8.8, 1.9 Hz, 1H), 7.70(dd, J=8.7, 2.0 Hz, 1H), 3.32(s, 3H), 2.95(s, 3H) | 167.3, 155.3, 141.4, 134.8, 134.4, 132.4, 131.9, 130.7, 130.1, 126.6, 124.7, 124.4, 124.2, 121.8, 38.5, 35.1 | |
| 3o | 8.84(d, J=8.2 Hz, 1H), 8.76(d, J=8.4 Hz, 1H), 8.70(t, 2H), 8.52(d, J=8.1 Hz, 1H), 8.30(d, J=8.2 Hz, 1H), 7.79(t, J=7.3 Hz, 2H), 7.76—7.60(m, 4H), 3.31(s, 3H), 2.82(s, 3H) | 170.8, 152.7, 145.6, 135.1, 132.7, 130.6, 130.1, 129.4, 129.2, 129.1, 128.2, 128.1, 127.9, 127.8, 127.7, 127.3, 127.0, 125.8, 123.9, 123.7, 123.5, 120.4, 38.4, 35.4 | |
| Entry | n(1a)/ mmol | n(2)/ mmol | n(DBU)/ mmol | n(TBAI)/ mmol | n(TBHP)/ mmol | Solvent (5 mL) | Time/h | Yieldb(%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.5 | 1 | 0.5 | 0.5 | 3 | 1,1,2-TCA | 6 | —— |
| 2 | 0.5 | 0.25 | 0.25 | 0.25 | 0.75 | 1,1,2-TCA | 6 | —— |
| 3 | 0.5 | 0.5 | 0.5 | 3 | DMF | 6 | 28 | |
| 4 | 0.5 | 0.5 | 0.5 | 3 | DMF | 12 | 28 | |
| 5 | 0.5 | 0.5 | 0.5 | 3 | DMF | 24 | 25 | |
| 6 | 0.5 | 0.5 | 0.5 | 3 | DMF | 48 | 17 |
Table 3 Effect of the proportion of each component involved in the reaction and reaction time on yield a
| Entry | n(1a)/ mmol | n(2)/ mmol | n(DBU)/ mmol | n(TBAI)/ mmol | n(TBHP)/ mmol | Solvent (5 mL) | Time/h | Yieldb(%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.5 | 1 | 0.5 | 0.5 | 3 | 1,1,2-TCA | 6 | —— |
| 2 | 0.5 | 0.25 | 0.25 | 0.25 | 0.75 | 1,1,2-TCA | 6 | —— |
| 3 | 0.5 | 0.5 | 0.5 | 3 | DMF | 6 | 28 | |
| 4 | 0.5 | 0.5 | 0.5 | 3 | DMF | 12 | 28 | |
| 5 | 0.5 | 0.5 | 0.5 | 3 | DMF | 24 | 25 | |
| 6 | 0.5 | 0.5 | 0.5 | 3 | DMF | 48 | 17 |
| Entry | Initiator | Yieldb(%) | Entry | Initiator | Yieldb(%) |
|---|---|---|---|---|---|
| 1 | TBAB | 58 | 7 | NCS | —— |
| 2 | CTAB | 61 | 8 | KI | 17 |
| 3 | TBAC | 64 | 9 | KBr | 48 |
| 4 | TEBAC | 60 | 10 | KCl | 57 |
| 5 | NIS | 18 | 11 | KF | 59 |
| 6 | NBS | 14 |
Table 4 Effect of different initiators on yield of compound 3aa
| Entry | Initiator | Yieldb(%) | Entry | Initiator | Yieldb(%) |
|---|---|---|---|---|---|
| 1 | TBAB | 58 | 7 | NCS | —— |
| 2 | CTAB | 61 | 8 | KI | 17 |
| 3 | TBAC | 64 | 9 | KBr | 48 |
| 4 | TEBAC | 60 | 10 | KCl | 57 |
| 5 | NIS | 18 | 11 | KF | 59 |
| 6 | NBS | 14 |
| Entry | Oxidant | Yieldb(%) | Entry | Oxidant | Yieldb(%) |
|---|---|---|---|---|---|
| 1 | TBHP(70% aqueous solution) | 64 | 5 | DCP | 12 |
| 2 | DTBP | 12 | 6 | TBPB | 58 |
| 3 | KPS | 40 | 7 | TBHP(65% aqueous solution ) | 60 |
| 4 | BPO | Trace | 8 | TBHP(5.5 mol/L in decane) | 61 |
Table 5 Effect of oxidant categories and specifications on yield of compound 3aa
| Entry | Oxidant | Yieldb(%) | Entry | Oxidant | Yieldb(%) |
|---|---|---|---|---|---|
| 1 | TBHP(70% aqueous solution) | 64 | 5 | DCP | 12 |
| 2 | DTBP | 12 | 6 | TBPB | 58 |
| 3 | KPS | 40 | 7 | TBHP(65% aqueous solution ) | 60 |
| 4 | BPO | Trace | 8 | TBHP(5.5 mol/L in decane) | 61 |
| Entry | Base | Yieldb(%) | Entry | Base | Yieldb(%) |
|---|---|---|---|---|---|
| 1a | 9 | 5a | K2HPO4 | 75 | |
| 2a | DBU | 64 | 6a | NaOAc | 67 |
| 3a | TEA | 13 | 7c | K2HPO4 | 76 |
| 4a | K2CO3 | 70 |
Table 6 Effect of different catalysts and reaction environment on yield of compound 3a
| Entry | Base | Yieldb(%) | Entry | Base | Yieldb(%) |
|---|---|---|---|---|---|
| 1a | 9 | 5a | K2HPO4 | 75 | |
| 2a | DBU | 64 | 6a | NaOAc | 67 |
| 3a | TEA | 13 | 7c | K2HPO4 | 76 |
| 4a | K2CO3 | 70 |
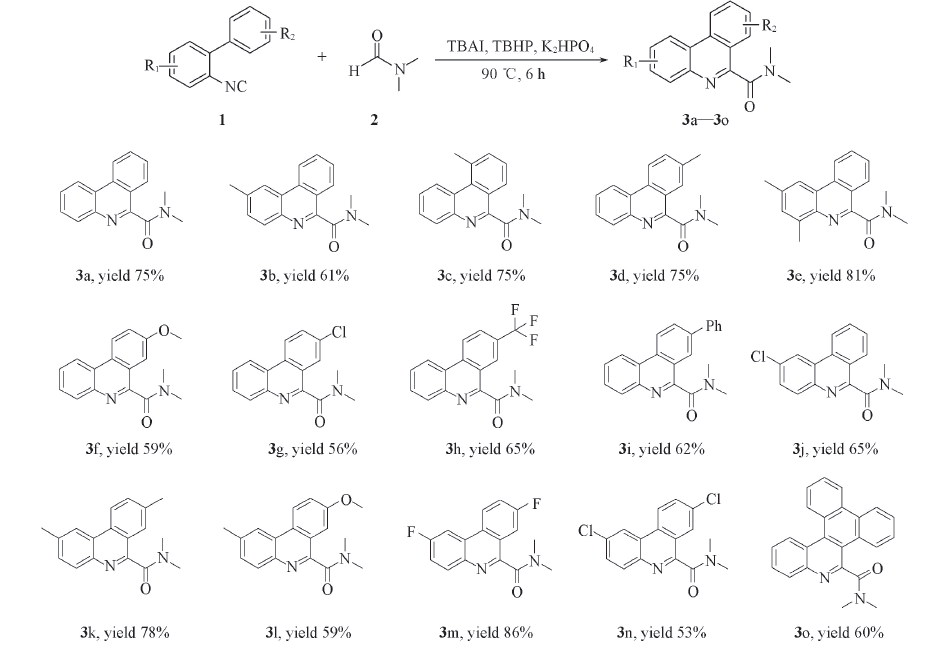
Scheme 2 Substrate scope of 2-isocyanobiphenyls Reaction conditions: 1a(0.50 mmol), K2HPO4(0.50 mmol), TBAI(0.50 mmol), TBHP(70% aqueous solution 3.00 mmol), DMF(5 mL), 90 ℃ 6 h. Isolated yield.
| [1] | Suffness M., Cordell G.A., Alkaloids Chemistry & Pharmacology, INC Press, London 1985, 25, 330—355 |
| [2] | Nakanishi T., Suzuki M., J. Nat.Prod.,1998, 61, 1263—1267 |
| [3] | Nakanishi T., Suzuki M., Atsuya S. A, Takashi K., J. Nat.Prod.,1992, 62, 864—867 |
| [4] | Nakanishi T., Suzuki M., Org.Lett., 2010, 30, 985—988 |
| [5] | Nakanishi T., Masuda A., Suwa M., Akiyama Y. J., Hoshino-Abe N., Suzuki M., Bioorg. Med. Chem.Lett.,2000, 10, 2321—2323 |
| [6] | Johnstone T. C., Alexander S. M., Lin W., Lippard S. J., J. Am. Chem.Soc.,2013, 136, 116—118 |
| [7] | Simenon S., Rios J. L., Villar A., Die Pharmazie,1989, 44, 593—599 |
| [8] | Theodosiou E., Purchartová K., Stamatis H., Kolisis F., Krěn V., Phytochem.Rev.,2014, 13, 1—18 |
| [9] | Brewster W. K., Nichols D. E., Riggs R. M., Mottola D. M., Lovenberg T. W., Lewis M. H., Mailman R. B., J. Med.Chem.,1990, 33, 1756—1764 |
| [10] | Chen H., Long H. T., Cui X. J., Zhou J., Xu M., Yuan G., J. Am. Chem.Soc.,2014, 136, 2583—2591 |
| [11] | Sripada L., Teske J. A., Deiters A., Org. Biomol.Chem.,2008, 6, 263—265 |
| [12] | Abdel-Halim O. B., Morikawa T., Ando S., Matsuda H., Yoshikawa M.,J. Nat.Prod.,2004, 67, 1119—1124 |
| [13] | Hashmi A. S. K., Braun I., Nösel P., Schädlich J., Wieteck M., Rudolph M., Rominger F., Angew. Chem. Int.Ed.,2012, 51, 4456—4460 |
| [14] | Guo A., Han J. B., Tang X. Y., Org.Lett.,2018, 20, 2351—2355 |
| [15] | Feng S. B., Li T., Du C. L., Chen P., Song D. P., Li J. L., Xie X. G., She X. G., Chem.Commun.,2017, 53, 4585—4588 |
| [16] | Li L. Y., Chen H. G., Mei M. G., Zhou L., Chem.Commun.,2017, 53, 11544—11547 |
| [17] | Wang Y. X., Wang J. H., Li G. X., He G., Chen G.,Org.Lett.,2017, 19, 1442—1445 |
| [18] | Song W. H., Yan P. P., Shen D., Chen Z. T., Zeng X. F., Zhong G. F., J. Org.Chem.,2017, 82, 4444—4448 |
| [19] | Rong J., Deng L., Tan P., Ni C. F., Gu Y. C., Hu J. B., Angew. Chem. Int.Ed.,2016, 55, 2743—2747 |
| [20] | Leifert D., Studer A., Angew. Chem. Int.Ed.,2016, 55, 11660—11663 |
| [21] | Xu Y. L., Chen Y. Y., Xie Q., Shao L. M., J. Org.Chem.,2016, 81, 8426—8435 |
| [22] | Pan C. D., Zhang H. L., Han J., Cheng Y. X., Zhu C. J., Chem.Commun.,2015, 51, 3786—3788 |
| [23] | Tobisu M., Koh K., Furukawa T., Chatani N., Angew. Chem. Int.Ed.,2012, 51, 11363—11366 |
| [24] | Jiang H., Cheng Y. Z., Wang R. Z., Zheng M. M., Zhang Y., Yu S. Y., Angew. Chem. Int.Ed.,2013, 52, 13289—13292 |
| [25] | Zhang B., Mück-Lichtenfeld C., Daniliuc C. G., Angew. Chem. Int.Ed.,2013, 125, 10992—10995 |
| [26] | Zhang Z., Tang X., Dolbier W. R. Jr., Org.Lett.,2015, 17, 4401—4403 |
| [27] | Wang L., Sha W.X., Dai Q., Feng X. M., Wu W. T., Peng H. B., Chen B., Cheng B., Org. Lett., 2014, 16, 20882091— |
| [28] | Li Z. J., Fan F. H., Yang J., Liu Z. Q., Org.Lett.,2014, 16, 3396—3399 |
| [29] | Wang L., Zhu H., Cuo S. G., Cheng J., Yu J. T., Chem.Commun.,2014, 50, 10864—10867 |
| [30] | Li Y. W., Qiu G. Y. S., Ding Q. P., Wu J.,Tetrahedron,2014, 70, 4652—4656 |
| [31] | Zhang B., Daniliuc C. G., Studer A., Org.Lett.,2013, 16, 250—253 |
| [32] | Yang X. L., Chen F., Zhou N. N., Yu W., Han B., Org.Lett.,2014, 16, 6476—6479 |
| [33] | Larock R.C., Comprehensive Organic Transformations, Willey-VCH Press, New York, 2010, 966 |
| [34] | Sewald N., Jakubke H.D., Peptides: Chemistry and Biology, Willey-VCH Press, New York, 2002, 192 |
| [35] | Cupido T., Tulla-Puche J., Spengler J., Curr. Opin. Drug.Disc.,2007, 10, 768—783 |
| [36] | Bode J. W., Curr. Opin. Drug.Disc., 2006, 9, 765—775 |
| [37] | Humphrey J. M., Chamberlin A. R.,Chem.Rev.,1997, 97, 2243—2266 |
| [38] | Hudson D., J. Org.Chem., 1988, 53, 617—624 |
| [39] | Bray B. L., Nat. Rev. Drug Discov., 2003, 2, 587—593 |
| [40] | Allen C. L., Williams J. M. J., Chem. Soc.Rev.,2011, 40, 3405—3415 |
| [41] | Pattabiraman V. R., Bode J. W., Nature,2011, 480, 471—479 |
| [42] | Montallbetti C. A. G. N., Falque V., Tetrahedron,2005, 61, 10827—10852 |
| [43] | Ghosh S., Bhaumik A., Mondal J., Mallik J., Sengupta S., Mukhopadhyay C., Green Chem.,2012, 14, 3220—3229 |
| [44] | Gooben L. J., Ohlmann D. M., Lange P. P., Synthesis,2009, 2009, 160—164 |
| [45] | Liu Z. J., Zhang J., Chen S. L., Shi E. B., Xu Y., Wan X. B., Angew. Chem. Int.Ed.,2012, 51, 3231—3235 |
| [46] | Pearlman B. A., Wells A., Zaks A., Zhang T. Y., Green Chem.,2007, 9, 411—420 |
| [1] | 林俊洁, 王爽, 李伟强, 崔鑫, 黄超. 微波辅助无催化剂高效串联环化合成吡啶[2,3⁃d]嘧啶衍生物[J]. 高等学校化学学报, 2020, 41(12): 2749. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||