

高等学校化学学报 ›› 2017, Vol. 38 ›› Issue (4): 591.doi: 10.7503/cjcu20160798
严畅1, 邹瑜1, 傅俊杰2, 黄张建1, 张大永1( ), 张奕华1(
), 张奕华1( )
)
收稿日期:2016-11-16
出版日期:2017-04-10
发布日期:2017-03-13
作者简介:联系人简介: 张大永, 男, 博士, 教授, 博士生导师, 主要从事新药分子的设计、 合成及其生物活性研究. E-mail:基金资助:
YAN Chang1, ZOU Yu1, FU Junjie2, HUANG Zhangjian1, ZHANG Dayong1,*( ), ZHANG Yihua1,*(
), ZHANG Yihua1,*( )
)
Received:2016-11-16
Online:2017-04-10
Published:2017-03-13
Contact:
ZHANG Dayong,ZHANG Yihua
E-mail:cpuzdy@163.com;zyhtgd@163.com
Supported by:摘要:
以O2-2,4-二硝基苯基偶氮二醇盐(PABA/NO)为先导化合物, 选择适当的仲胺作为偶氮
二醇盐中相应的胺片段, 并用碳氮键取代苯环5位的碳氧酯键, 设计合成了化合物2a, 2b和4a~4j, 以期获得活性更强且稳定性好的抗肿瘤药物. 目标化合物经1H NMR, 13C NMR及HRMS进行了结构确证. 生物活性测试结果表明, 目标化合物可不同程度地抑制结肠癌HCT-116细胞的增殖, 其中化合物4h的活性最强(IC50=7.945±0.421 μmol/L), 优于PABA/NO(IC50=12.134±0.675 μmol/L). NO释放实验结果表明, 此类化合物的NO释放量与细胞毒性呈正相关. 化合物4h在HCT-116细胞中释放NO的量最多, 约是正常细胞的2倍. 此外, 化合物4h在大鼠血浆中的体外稳定性显著优于PABA/NO, 值得进一步研究.
中图分类号:
TrendMD:
严畅, 邹瑜, 傅俊杰, 黄张建, 张大永, 张奕华. O2-2,4-二硝基苯基偶氮二醇盐类化合物的设计、 合成及抗肿瘤活性. 高等学校化学学报, 2017, 38(4): 591.
YAN Chang, ZOU Yu, FU Junjie, HUANG Zhangjian, ZHANG Dayong, ZHANG Yihua. Design, Synthesis and Biological Evaluation of Novel O2-(2,4-Dinitrophenyl)diazeniumdiolates as Anti-tumor Agents†. Chem. J. Chinese Universities, 2017, 38(4): 591.
Scheme 3 Synthetic route of compounds 4a—4ja. NO, NaOMe/MeOH/Et2O, nanometer-sized TiO2, 25 ℃, 48 h; b. 1-fluoro-2,4-dinitrobenzene, Me2CO, NaHCO3(mass fraction 5%), N2, 0—25 ℃; c. 1,5-difluoro-2,4-dinitrobenzene, Me2CO, NaHCO3(mass fraction 5%), N2, 0—25 ℃; d. R2, K2CO3, Me2CO, 0 ℃—r. t.
| Compd. | Appearance | Yield(%) | Chemical formula | m. p./℃ | HRMS(calcd. )[M+Na]+ |
|---|---|---|---|---|---|
| 2a | Yellow solid | 88 | C9H11N5O7 | 149—153 | 324.0564(324.0556) |
| 2b | Yellow solid | 83 | C11H13N5O7 | 148—150 | 350.0721(350.0713) |
| 4a | Yellow solid | 68 | C14H21N7O7 | 166—171 | 422.1411(422.1400) |
| 4b | Yellow solid | 62 | C16H23N7O9 | 155—158 | 480.1466(480.1455) |
| 4c | Yellow solid | 65 | C13H18N6O8 | 153—156 | 409.1093(409.1084) |
| 4d | Yellow solid | 68 | C14H20N6O8 | 143—147 | 423.1249(423.1240) |
| 4e | Yellow solid | 62 | C12H13N7O7 | 145—147 | 390.0784(390.0774) |
| 4f | Yellow solid | 70 | C16H23N7O7 | 170—173 | 448.1566(448.1557) |
| 4g | Yellow solid | 69 | C18H25N7O9 | 117—119 | 506.1622(506.1611) |
| 4h | Yellow solid | 72 | C15H20N6O8 | 146—150 | 435.1246(435.1240) |
| 4i | Yellow solid | 68 | C16H22N6O8 | 158—162 | 449.1407(449.1397) |
| 4j | Yellow solid | 71 | C14H15N7O7 | 173—176 | 416.0942(416.0931) |
Table 1 Appearance, yields, melting points and HRMS data of target compounds 2 and 4
| Compd. | Appearance | Yield(%) | Chemical formula | m. p./℃ | HRMS(calcd. )[M+Na]+ |
|---|---|---|---|---|---|
| 2a | Yellow solid | 88 | C9H11N5O7 | 149—153 | 324.0564(324.0556) |
| 2b | Yellow solid | 83 | C11H13N5O7 | 148—150 | 350.0721(350.0713) |
| 4a | Yellow solid | 68 | C14H21N7O7 | 166—171 | 422.1411(422.1400) |
| 4b | Yellow solid | 62 | C16H23N7O9 | 155—158 | 480.1466(480.1455) |
| 4c | Yellow solid | 65 | C13H18N6O8 | 153—156 | 409.1093(409.1084) |
| 4d | Yellow solid | 68 | C14H20N6O8 | 143—147 | 423.1249(423.1240) |
| 4e | Yellow solid | 62 | C12H13N7O7 | 145—147 | 390.0784(390.0774) |
| 4f | Yellow solid | 70 | C16H23N7O7 | 170—173 | 448.1566(448.1557) |
| 4g | Yellow solid | 69 | C18H25N7O9 | 117—119 | 506.1622(506.1611) |
| 4h | Yellow solid | 72 | C15H20N6O8 | 146—150 | 435.1246(435.1240) |
| 4i | Yellow solid | 68 | C16H22N6O8 | 158—162 | 449.1407(449.1397) |
| 4j | Yellow solid | 71 | C14H15N7O7 | 173—176 | 416.0942(416.0931) |
| Compd. | 1H NMR(300 MHz), δa | 13C NMR(75 MHz), δb |
|---|---|---|
| 2a | 8.88(s, 1H, ArH), 8.46(d,J=9.24 Hz, 1H, ArH), 7.69(d, J=8.85 Hz, 1H, ArH), 3.82—3.92(m, 4H, NCH2CH2OH), 3.41(s, 3H, CH3) | 129.8, 129.2, 122.3, 121.8, 120.5, 117.5, 57.5, 54.8, 39.6 |
| 2b | 8.89(d,J=2.55 Hz, 1H, ArH), 8.46(dd, J1=9.3 Hz, J2=2.64 Hz, 1H, ArH), 7.68(d, J=9.27 Hz, 1H, ArH), 4.03(s, 1H, NCH2CH2CHOH), 3.63—3.88(m, 4H, 2×NCH2CH2CHOH), 1.84—2.05(m, 4H, 2×NCH2CH2CHOH) | 152.9, 142.0, 136.8, 129.8, 121.8, 118.0, 63.6, 47.4, 31.8 |
| 4a | 8.72(s, 1H, ArH), 6.98(s, 1H, ArH), 3.81—3.88(m, 4H, NCH2CH2OH), 3.33(s, 3H, CH3), 3.24—3.27(m, 4H, 2×ArNCH2CH2N), 2.58—2.63(m, 4H, 2×ArNCH2CH2N), 2.37(s, 3H, CH3) | 153.6, 149.6, 132.2, 127.3, 127.1, 105.4, 57.6, 54.8, 53.9, 50.1, 45.5, 39.6 |
| 4b | 8.72(s, 1H, ArH), 6.98(s, 1H, ArH), 3.91(t, J=5.1 Hz, 2H, NCH2CH2OH), 3.76—3.82(m, 6H, 2×ArNCH2CH2N, NCH2CH2OH), 3.34(s, 3H, CH3), 3.31—3.35(m, 7H, 2×ArNCH2CH2N, NCH3), 2.78(s, 2H, CH2) | 170.3, 153.6, 149.6, 132.3, 127.7, 127.2, 105.5, 57.7, 57.6, 54.8, 51.2, 51.1, 50.2, 39.6 |
| 4c | 8.75(s, 1H, ArH), 6.98(s, 1H, ArH), 3.85—3.89(m, 6H, 2×NCH2CH2O, NCH2CH2OH), 3.76(t, J=4.71 Hz, 2H, NCH2CH2OH), 3.34(s, 3H, CH3), 3.24(t, J=4.17 Hz, 4H, 2×NCH2CH2O) | 153.6, 149.6, 132.3, 127.4, 127.2, 105.4, 85.5, 57.6, 54.8, 50.5, 39.6 |
| 4d | 8.71(s, 1H, ArH), 6.32(s, 1H, ArH), 4.00(s, 2H, NCH2CH2OH), 3.87—3.92(m, 3H, NCH2CH2OH, NCH2CH2CHOH), 3.49(s, 3H, CH3), 3.34—3.42(m, 4H, 2×NCH2CH2CHOH), 1.23—1.28(m, 4H, 2×NCH2CH2CHOH) | 153.6, 149.8, 131.9, 129.5, 127.4, 107.1, 105.0, 64.9, 64.4, 57.6, 54.8, 48.0, 39.6, 33.5 |
| 4e | 8.95(s, 1H, ArH), 8.03(s, 1H, Imidazole), 7.86(s, 1H, ArH), 7.52(s, 1H, Imidazole), 7.14(s, 1H, Imidazole), 4.90(s, 1H, OH), 3.75(t, J=5.2 Hz, 2H, NCH2CH2OH), 3.63(t, J=4.8 Hz, 2H, NCH2CH2OH), 3.14(s, 3H, CH3) | 152.4, 137.6, 136.5, 135.3, 128.5, 124.4, 123.1, 121.0, 116.7, 57.6, 55.5, 54.7 |
| 4f | 8.73(s, 1H, ArH), 6.96(s, 1H, ArH), 3.51—3.55(m, 4H, 2×ArNCH2CH2N), 3.23—3.29(m, 5H, 2×ArNCH2CH2N, NCH2CH2CHOH), 2.58—2.63(m, 4H, 2×NCH2CH2CHOH), 2.37(s, 3H, CH3), 2.03—2.08(m, 4H, 2×NCH2CH2CHOH) | 153.0, 149.5, 132.6, 127.4, 127.2, 106.4, 63.7, 53.9, 50.1, 47.4, 45.5, 31.8 |
| 4g | 8.72(s, 1H, ArH), 6.96(s, 1H, ArH), 3.72(s, 3H, CH3), 3.29—3.33(m, 5H, 2×ArNCH2CH2N, NCH2CH2CHOH), 3.22(s, 2H, NCH2COOMe), 2.63—2.66(m, 8H, 2×NCH2CH2CHOH, 2×ArNCH2CH2N), 2.02—2.05(m, 4H, 2×NCH2CH2CHOH) | 170.3, 153.0, 149.5, 132.6, 127.5, 127.2, 106.4, 63.7, 58.3, 57.7, 51.9, 51.2, 50.3, 47.4, 31.8 |
| 4h | 8.74(s, 1H, ArH), 6.96(s, 1H, ArH), 3.88(t, J=4.5 Hz, 4H, 2×NCH2CH2O), 3.81—3.86(m, 1H, NCH2CH2CHOH), 3.55—3.58(m, 4H, 2×NCH2CH2CHOH), 3.24(t, J=4.38 Hz, 4H, 2×NCH2CH2O), 1.84—2.06(m, 4H, 2×NCH2CH2CHOH) | 150.8, 150.7, 129.9, 127.5, 107.4, 106.3, 66.5, 66.4, 65.1, 51.5, 51.3, 48.4, 47.7, 33.7, 32.1 |
| 4i | 8.61(s, 1H, ArH), 7.12(s, 1H, ArH), 4.85(s, 2H, 2×OH), 3.75—3.79(m, 2H, 2×NCH2CH2CHOH), 3.42—3.45(m, 4H, 2×ArNCH2CH2CHOH), 3.12—3.15(m, 4H, 2×NCH2CH2CHOH), 1.85—1.88(m, 8H, 4×NCH2CH2CHOH) | 153.0, 149.6, 132.3, 127.3, 126.9, 106.0, 64.4, 63.7, 48.0, 47.4, 33.4, 31.8 |
| 4j | 8.95(s, 1H, ArH), 8.05(s, 1H, Imidazole), 7.96(s, 1H, ArH), 7.53(s, 1H, Imidazole), 7.14(s, 1H, Imidazole), 4.85(s, 1H, OH), 3.71(s, 1H, NCH2CH2CHOH), 3.44—3.51(m, 4H, 2×NCH2CH2CHOH), 1.58—1.85(m, 4H, NCH2CH2CHOH) | 141.7, 137.6, 134.1, 130.2, 129.3, 128.0, 126.9, 123.9, 120.3, 64.3, 47.8, 33.6 |
Table 2 1H NMR and 13C NMR data of target compounds 2 and 4
| Compd. | 1H NMR(300 MHz), δa | 13C NMR(75 MHz), δb |
|---|---|---|
| 2a | 8.88(s, 1H, ArH), 8.46(d,J=9.24 Hz, 1H, ArH), 7.69(d, J=8.85 Hz, 1H, ArH), 3.82—3.92(m, 4H, NCH2CH2OH), 3.41(s, 3H, CH3) | 129.8, 129.2, 122.3, 121.8, 120.5, 117.5, 57.5, 54.8, 39.6 |
| 2b | 8.89(d,J=2.55 Hz, 1H, ArH), 8.46(dd, J1=9.3 Hz, J2=2.64 Hz, 1H, ArH), 7.68(d, J=9.27 Hz, 1H, ArH), 4.03(s, 1H, NCH2CH2CHOH), 3.63—3.88(m, 4H, 2×NCH2CH2CHOH), 1.84—2.05(m, 4H, 2×NCH2CH2CHOH) | 152.9, 142.0, 136.8, 129.8, 121.8, 118.0, 63.6, 47.4, 31.8 |
| 4a | 8.72(s, 1H, ArH), 6.98(s, 1H, ArH), 3.81—3.88(m, 4H, NCH2CH2OH), 3.33(s, 3H, CH3), 3.24—3.27(m, 4H, 2×ArNCH2CH2N), 2.58—2.63(m, 4H, 2×ArNCH2CH2N), 2.37(s, 3H, CH3) | 153.6, 149.6, 132.2, 127.3, 127.1, 105.4, 57.6, 54.8, 53.9, 50.1, 45.5, 39.6 |
| 4b | 8.72(s, 1H, ArH), 6.98(s, 1H, ArH), 3.91(t, J=5.1 Hz, 2H, NCH2CH2OH), 3.76—3.82(m, 6H, 2×ArNCH2CH2N, NCH2CH2OH), 3.34(s, 3H, CH3), 3.31—3.35(m, 7H, 2×ArNCH2CH2N, NCH3), 2.78(s, 2H, CH2) | 170.3, 153.6, 149.6, 132.3, 127.7, 127.2, 105.5, 57.7, 57.6, 54.8, 51.2, 51.1, 50.2, 39.6 |
| 4c | 8.75(s, 1H, ArH), 6.98(s, 1H, ArH), 3.85—3.89(m, 6H, 2×NCH2CH2O, NCH2CH2OH), 3.76(t, J=4.71 Hz, 2H, NCH2CH2OH), 3.34(s, 3H, CH3), 3.24(t, J=4.17 Hz, 4H, 2×NCH2CH2O) | 153.6, 149.6, 132.3, 127.4, 127.2, 105.4, 85.5, 57.6, 54.8, 50.5, 39.6 |
| 4d | 8.71(s, 1H, ArH), 6.32(s, 1H, ArH), 4.00(s, 2H, NCH2CH2OH), 3.87—3.92(m, 3H, NCH2CH2OH, NCH2CH2CHOH), 3.49(s, 3H, CH3), 3.34—3.42(m, 4H, 2×NCH2CH2CHOH), 1.23—1.28(m, 4H, 2×NCH2CH2CHOH) | 153.6, 149.8, 131.9, 129.5, 127.4, 107.1, 105.0, 64.9, 64.4, 57.6, 54.8, 48.0, 39.6, 33.5 |
| 4e | 8.95(s, 1H, ArH), 8.03(s, 1H, Imidazole), 7.86(s, 1H, ArH), 7.52(s, 1H, Imidazole), 7.14(s, 1H, Imidazole), 4.90(s, 1H, OH), 3.75(t, J=5.2 Hz, 2H, NCH2CH2OH), 3.63(t, J=4.8 Hz, 2H, NCH2CH2OH), 3.14(s, 3H, CH3) | 152.4, 137.6, 136.5, 135.3, 128.5, 124.4, 123.1, 121.0, 116.7, 57.6, 55.5, 54.7 |
| 4f | 8.73(s, 1H, ArH), 6.96(s, 1H, ArH), 3.51—3.55(m, 4H, 2×ArNCH2CH2N), 3.23—3.29(m, 5H, 2×ArNCH2CH2N, NCH2CH2CHOH), 2.58—2.63(m, 4H, 2×NCH2CH2CHOH), 2.37(s, 3H, CH3), 2.03—2.08(m, 4H, 2×NCH2CH2CHOH) | 153.0, 149.5, 132.6, 127.4, 127.2, 106.4, 63.7, 53.9, 50.1, 47.4, 45.5, 31.8 |
| 4g | 8.72(s, 1H, ArH), 6.96(s, 1H, ArH), 3.72(s, 3H, CH3), 3.29—3.33(m, 5H, 2×ArNCH2CH2N, NCH2CH2CHOH), 3.22(s, 2H, NCH2COOMe), 2.63—2.66(m, 8H, 2×NCH2CH2CHOH, 2×ArNCH2CH2N), 2.02—2.05(m, 4H, 2×NCH2CH2CHOH) | 170.3, 153.0, 149.5, 132.6, 127.5, 127.2, 106.4, 63.7, 58.3, 57.7, 51.9, 51.2, 50.3, 47.4, 31.8 |
| 4h | 8.74(s, 1H, ArH), 6.96(s, 1H, ArH), 3.88(t, J=4.5 Hz, 4H, 2×NCH2CH2O), 3.81—3.86(m, 1H, NCH2CH2CHOH), 3.55—3.58(m, 4H, 2×NCH2CH2CHOH), 3.24(t, J=4.38 Hz, 4H, 2×NCH2CH2O), 1.84—2.06(m, 4H, 2×NCH2CH2CHOH) | 150.8, 150.7, 129.9, 127.5, 107.4, 106.3, 66.5, 66.4, 65.1, 51.5, 51.3, 48.4, 47.7, 33.7, 32.1 |
| 4i | 8.61(s, 1H, ArH), 7.12(s, 1H, ArH), 4.85(s, 2H, 2×OH), 3.75—3.79(m, 2H, 2×NCH2CH2CHOH), 3.42—3.45(m, 4H, 2×ArNCH2CH2CHOH), 3.12—3.15(m, 4H, 2×NCH2CH2CHOH), 1.85—1.88(m, 8H, 4×NCH2CH2CHOH) | 153.0, 149.6, 132.3, 127.3, 126.9, 106.0, 64.4, 63.7, 48.0, 47.4, 33.4, 31.8 |
| 4j | 8.95(s, 1H, ArH), 8.05(s, 1H, Imidazole), 7.96(s, 1H, ArH), 7.53(s, 1H, Imidazole), 7.14(s, 1H, Imidazole), 4.85(s, 1H, OH), 3.71(s, 1H, NCH2CH2CHOH), 3.44—3.51(m, 4H, 2×NCH2CH2CHOH), 1.58—1.85(m, 4H, NCH2CH2CHOH) | 141.7, 137.6, 134.1, 130.2, 129.3, 128.0, 126.9, 123.9, 120.3, 64.3, 47.8, 33.6 |
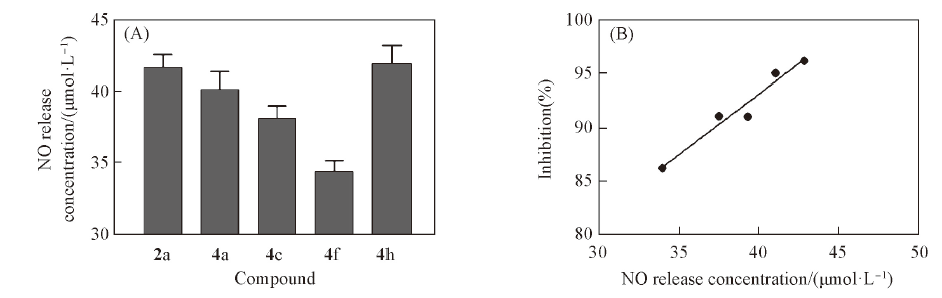
Fig.2 Quantitative measurement of intracellular NO production(A), the positive correlation between NO release in HCT-116 cells and anti-proliferative activity of target compounds against HCT-116 cells(B)
| [1] | Strange R. C., Jones P. W., Fryer A. A., Toxicol. Lett., 2000, 112/113, 357—363 |
| [2] | Hayes J. D., Flanagan J. U., Jowsey I. R., Annu. Rev. Pharmacol. Toxicol., 2005, 45, 51—88 |
| [3] | Obrien M. L., Tew K. D., Eur. J. Cancer, 1996, 32(6), 967—978 |
| [4] | Habig W. H., Pabst M. J., Jakoby W. B., J. Biol. Chem., 1974, 249(22), 7130—7139 |
| [5] | Ji X., Pal A., Kalathur R., Hu X., Gu Y., Saavedra J. E., Buzard G. S., Srinivasan A., Keefer L. K., Singh S. V., Drug Des. Devel. Ther., 2008, 2, 123—130 |
| [6] | Kim Y., Maciag A. E., Cao Z., Deschamps J. R., Saavedra J. E., Keefer L. K., Holland R. J., Bioorg. Med. Chem., 2015, 23, 4980—4988 |
| [7] | Huerta S., Chilka S., Bonavida B., Int. J. Oncol., 2008, 33(5), 909—927 |
| [8] | Burke A. J., Sullivan F. J., Giles F. J., Glynn S. A., Carcinogenesis., 2013, 34(3), 503—512 |
| [9] | Mocellin S., Bronte V., Nitti D., Med. Res. Rev., 2007, 27(3), 317—352 |
| [10] | He L. Q., Gu H. X., Yin D. K., Zhang Y. H., Wang X. S., Chem. J. Chinese Universities, 2010, 31(8), 1541—1547 |
| (何黎琴, 顾宏霞, 尹登科, 张奕华, 王效山. 高等学校化学学报, 2010,31(8), 1541—1547) | |
| [11] | Saavedra J. E., Dunams T. M., Flippen J. L., Keefer L. K., J. Org. Chem., 1992, 57(23), 6134—6138 |
| [12] | Saavedra J. E., Srinivasan A., Buzard G. S., Davies K. M., Waterhouse D. J., J. Med. Chem., 2006, 49(3), 1157—1164 |
| [13] | Findlay V. J., Townsend D. M., Saavedra J. E., Buzard G. S., Citro M. L., Keefer L. K., Ji X., Tew K. D., Mol. Pharmacol., 2004, 65(5), 1070—1079 |
| [14] | Chakrapani H., Wilde T. C., Citro M. L., Bioorg. Med. Chem., 2008, 16, 2657—2664 |
| [15] | Sies H., Free. Radic. Biol. Med., 1999, 27(9/10), 916—921 |
| [16] | Fu J. J., Liu L., Huang Z. J., Lai Y. S., Ji H., Peng S. X., Tian J. D., Zhang Y. H., J. Med. Chem., 2013, 56(11), 4641—4655 |
| [17] | Huang Z.J., Zhang Y. H., Fang L., Zhang Z. G., Lai Y. S., Ding Y., Cao F. Q., Zhang J., Peng S. X.,Chem. Commun., 2009, (13), 1763—1765 |
| [18] | Saavedra J. E., Srinivasan A., Bonifant C. L., Chu J., Shanklin A. P., Rice W. G., Turpin J. A., Davies K. M., Keefer L. K., J. Org. Chem., 2001, 66(9), 3090—3098 |
| [19] | Fu J. J., Zou Y., Huang Z. J., Yan C., Zhou Q. M., Zhang H. B., Lai Y. S., Peng S. X., Zhang Y. H., RSC Adv., 2015, 5(25), 19445—19454 |
| [20] | Gross G., Tardio J., Kuhlmann O., Int. J. Pharm., 2012, 437(1/2), 103—109 |
| [1] | 楚宇逸, 兰畅, 罗二桂, 刘长鹏, 葛君杰, 邢巍. 单原子铈对弱芬顿效应活性位点氧还原稳定性的提升[J]. 高等学校化学学报, 2022, 43(9): 20220294. |
| [2] | 郑安妮, 金磊, 杨家强, 王赵云, 李威青, 杨防祖, 詹东平, 田中群. 5,5-二甲基乙内酰脲在化学镀铜中的作用[J]. 高等学校化学学报, 2022, 43(8): 20220191. |
| [3] | 王红宁, 黄丽, 清江, 马腾洲, 蒋伟, 黄维秋, 陈若愚. 香蒲基生物炭的活化及对VOCs吸附的应用[J]. 高等学校化学学报, 2022, 43(4): 20210824. |
| [4] | 李伟辉, 李浩博, 曾诚, 梁昊樾, 陈佳俊, 李俊勇, 李会巧. 热压法构筑锂负极聚偏氟乙烯基双功能保护层的研究[J]. 高等学校化学学报, 2022, 43(2): 20210629. |
| [5] | 常斯惠, 陈涛, 赵黎明, 邱勇隽. 离子液体增塑生物基聚丁内酰胺的热分解机理[J]. 高等学校化学学报, 2022, 43(11): 20220353. |
| [6] | 孙金时, 陈鹏, 景丽萍, 孙福兴, 刘佳. 多级孔芳香骨架材料的合成及固载硫脲催化剂的研究[J]. 高等学校化学学报, 2022, 43(10): 20220171. |
| [7] | 岳胜利, 武光宝, 李星, 李康, 黄高胜, 唐翌, 周惠琼. 准二维钙钛矿太阳能电池的研究进展[J]. 高等学校化学学报, 2021, 42(6): 1648. |
| [8] | 王红宁, 黄丽, 宋夫交, 朱婷, 黄维秋, 钟璟, 陈若愚. 中空碳纳米球的制备及VOCs吸附性能[J]. 高等学校化学学报, 2021, 42(6): 1704. |
| [9] | 王坤华, 姚纪松, 杨俊楠, 宋永慧, 刘雨莹, 姚宏斌. 金属卤化物钙钛矿纳米晶高效发光二极管的制备与器件性能优化[J]. 高等学校化学学报, 2021, 42(5): 1464. |
| [10] | 刘瑶, 邓正涛. 反溶剂法快速合成高效发光二维锡卤钙钛矿材料[J]. 高等学校化学学报, 2021, 42(12): 3774. |
| [11] | 丁鑫, 师红东, 刘扬中. 以人血清白蛋白为载体的Ru(Ⅲ)和全反式维甲酸共运输纳米药物的构建及抗肿瘤转移作用[J]. 高等学校化学学报, 2021, 42(10): 3040. |
| [12] | 张俊, 王彬, 潘莉, 马哲, 李悦生. 含咪唑离子聚乙烯离聚体的合成与性能[J]. 高等学校化学学报, 2020, 41(9): 2070. |
| [13] | 王婷婷, 雷宇涵, 林宇娟, 黄加玲, 刘翠娥, 郑凤英, 李顺兴. 脂质体封端CsPbX3(X=Cl,Br,I)纳米晶体的制备及在发光二极管中的应用[J]. 高等学校化学学报, 2020, 41(8): 1896. |
| [14] | 苗笑梅, 毛克羽, 裴勇兵, 蒋剑雄, 颜悦, 吴连斌. 超疏水多孔硅的制备及表面稳定性[J]. 高等学校化学学报, 2020, 41(7): 1499. |
| [15] | 张开翔, 刘军杰, 宋巧丽, 王丹钰, 史进进, 张海悦, 李景虹. 基于DNA纳米花的细胞自噬基因沉默用于增敏抗肿瘤化疗[J]. 高等学校化学学报, 2020, 41(7): 1461. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||