

高等学校化学学报 ›› 2017, Vol. 38 ›› Issue (6): 1059.doi: 10.7503/cjcu20160797
陈东星1, 施锦渝2, 陈秋芳2, 张芮2, 龚国清2, 徐云根1,2( ), 朱启华1,2(
), 朱启华1,2( )
)
收稿日期:2016-11-16
出版日期:2017-06-10
发布日期:2017-05-23
作者简介:联系人简介: 徐云根, 男, 博士, 教授, 博士生导师, 主要从事抗肿瘤、 镇痛抗炎和心血管疾病方面的研究. E-mail: 基金资助:
CHEN Dongxing1, SHI Jinyu2, CHEN Qiufang2, ZHANG Rui2, GONG Guoqing2, XU Yungen1,2,*( ), ZHU Qihua1,2,*(
), ZHU Qihua1,2,*( )
)
Received:2016-11-16
Online:2017-06-10
Published:2017-05-23
Contact:
XU Yungen,ZHU Qihua
E-mail:xyg@cpu.edu.cn;zhuqihua@cpu.edu.cn
Supported by:摘要:
以具有四氢苯并[4,5]咪唑并[1,2-a]吡嗪新骨架的凝血酶抑制剂1为先导化合物, 设计合成了14个氨基甲酸酯衍生物(2a~6a, 2b~6b和7~10). 同时, 在化合物1结构的基础上, 引入具有抗血栓活性的川芎醇(HTMP), 设计合成了新型结构的化合物11. 目标化合物的结构均经1H NMR, 13C NMR和HRMS确证. 生物活性测试结果显示, 所有目标化合物对凝血酶诱导的血小板聚集均有一定的抑制活性, 其中化合物4b 的抑制活性[IC50=(0.11±0.08) μmol/L]强于对照药达比加群酯[IC50=(0.60±0.05) μmol/L]. 在体内抗血栓活性测试中, 化合物4b能以剂量依赖的方式减少大鼠静脉血栓的形成. 化合物11对凝血酶诱导的血小板聚集的抑制活性较弱, 但其抑制大鼠静脉血栓形成的作用与达比加群酯相当, 这可能是由于化合物11在体内水解为川芎醇和化合物1, 两者协同产生抗血栓作用所致.
中图分类号:
TrendMD:
陈东星, 施锦渝, 陈秋芳, 张芮, 龚国清, 徐云根, 朱启华. 具有四氢苯并[4,5]咪唑并[1,2-a]吡嗪骨架凝血酶抑制剂的设计合成及生物活性研究. 高等学校化学学报, 2017, 38(6): 1059.
CHEN Dongxing, SHI Jinyu, CHEN Qiufang, ZHANG Rui, GONG Guoqing, XU Yungen, ZHU Qihua. Design, Synthesis and Biological Evaluation of Thrombin Inhibitors with 1,2,3,4-Tetrahydrobenzo[4,5]imidazo[1,2-a]pyrazine Nucleus†. Chem. J. Chinese Universities, 2017, 38(6): 1059.
Scheme 3 Synthetic routes of target compounds 2a—6a, 2b—6b, 7—10Reagents and conditions: a. i: HCl, EtOH, -5—0 ℃, 6 h; ii: (NH4)2CO3, EtOH, 25 ℃, 12 h, 38.7%; b. CH3I, K2CO3, DMF, r. t., 88.6%; c. K2CO3, THF/H2O, 0—25 ℃, 1 h, 40.3%—66.0%; d. i: LiOH, EtOH, H2O, 2 h; ii: CH3CO2H, pH=6.0, 29.9%.
Scheme 4 Synthetic routes of target compound 11Reagents and conditions: a. Pyridine, CH2Cl2, 0 ℃ to r. t., 12 h, 82.1%; b. K2CO3, THF/H2O, 0 ℃ to r. t., 12 h, 35.7%.
| Compd. | Appearance | Yield(%) | m. p./℃ | HRMS(calcd. ), m/z[M+H]+ |
|---|---|---|---|---|
| 2a | White solid | 44.2 | 114—116 | 597.2823(597.2820) |
| 2b | White solid | 48.3 | 106—108 | 669.3044(669.3031) |
| 3a | White solid | 40.3 | 108—110 | 625.3148(625.3133) |
| 3b | White solid | 44.6 | 94—96 | 725.3673(725.3657) |
| 4a | White solid | 48.0 | 160—162 | 625.3141(625.3133) |
| 4b | White solid | 66.0 | 148—150 | 725.3662(725.3657) |
| 5a | White solid | 42.6 | 108—110 | 639.3287(639.3289) |
| 5b | White solid | 47.4 | 88—90 | 753.3978(753.3970) |
| 6a | White solid | 43.7 | 130—132 | 659.2979(659.2976) |
| 6b | White solid | 54.3 | 120—122 | 793.3354(793.3344) |
| 7 | White solid | 29.9 | > 250 | 511.2453(511.2452) |
| 8 | White solid | 57.4 | 112—114 | 639.3295(639.3289) |
| 9 | White solid | 59.6 | 82—84 | 667.3603(667.3602) |
| 10 | White solid | 54.5 | 94—96 | 673.3145(673.3133) |
| 11 | Yellow solid | 35.7 | 104—106 | 703.3356(703.3351) |
Table 1 Appearance, yields, melting points and HRMS data of target compounds 2a—6a, 2b—6b and 7—11
| Compd. | Appearance | Yield(%) | m. p./℃ | HRMS(calcd. ), m/z[M+H]+ |
|---|---|---|---|---|
| 2a | White solid | 44.2 | 114—116 | 597.2823(597.2820) |
| 2b | White solid | 48.3 | 106—108 | 669.3044(669.3031) |
| 3a | White solid | 40.3 | 108—110 | 625.3148(625.3133) |
| 3b | White solid | 44.6 | 94—96 | 725.3673(725.3657) |
| 4a | White solid | 48.0 | 160—162 | 625.3141(625.3133) |
| 4b | White solid | 66.0 | 148—150 | 725.3662(725.3657) |
| 5a | White solid | 42.6 | 108—110 | 639.3287(639.3289) |
| 5b | White solid | 47.4 | 88—90 | 753.3978(753.3970) |
| 6a | White solid | 43.7 | 130—132 | 659.2979(659.2976) |
| 6b | White solid | 54.3 | 120—122 | 793.3354(793.3344) |
| 7 | White solid | 29.9 | > 250 | 511.2453(511.2452) |
| 8 | White solid | 57.4 | 112—114 | 639.3295(639.3289) |
| 9 | White solid | 59.6 | 82—84 | 667.3603(667.3602) |
| 10 | White solid | 54.5 | 94—96 | 673.3145(673.3133) |
| 11 | Yellow solid | 35.7 | 104—106 | 703.3356(703.3351) |
| Compd. | 1H NMR(300 MHz), δa | 13C NMR(75 MHz), δb |
|---|---|---|
| 2a | 9.14(brs, 2H), 7.87(d, J=8.3 Hz, 2H), 7.43(s, 1H), 7.38(d, J=8.3 Hz, 2H), 7.32—7.21(m, 2H), 7.21—7.06(m, 5H), 4.34—4.24(m, 1H), 4.15—3.99(m, 5H), 3.96(q, J=7.2 Hz, 2H), 3.91—3.81(m, 1H), 3.57—3.45(m, 1H), 3.29—3.20(m, 1H), 3.07—2.84(m, 2H), 2.58(t, J=7.1 Hz, 2H), 1.18(t, J=6.9 Hz, 3H), 1.11(t, J=7.1 Hz, 3H) | 171.08, 170.63, 167.19, 163.87, 152.30, 142.83, 141.49, 140.86, 134.73, 132.51, 129.67, 129.05(2C), 128.78(2C), 127.33(2C), 127.28(2C), 126.19, 122.90, 119.95, 107.88, 60.78, 60.07, 55.29, 46.34, 42.50, 40.98, 38.95, 32.10, 13.93, 13.61 |
| 2b | 9.59(brs, 2H), 7.85—7.59(m, 3H), 7.26—7.02(m, 9H), 5.89—5.63(m, 1H), 4.65—4.44(m, 1H), 4.41—3.80(m, 11H), 3.62—3.27(m, 2H), 2.76(t, J=7.2 Hz, 2H), 1.36(t, J=6.8 Hz, 3H), 1.32—1.18(m, 6H) | 171.12, 170.38, 166.89, 164.02, 154.40, 150.11, 142.91, 141.21, 141.00, 134.20, 132.49, 130.10, 129.33(2C), 128.79(2C), 127.36(2C), 126.94(2C), 126.21, 123.18, 120.30, 107.95, 61.68, 60.89, 60.08, 53.49, 46.34, 41.36, 38.73, 36.87, 32.12, 13.91(2C), 13.62 |
| 3a | 9.07(brs, 2H), 7.89(d, J=8.0 Hz, 2H), 7.46(s, 1H), 7.40(d, J=8.2 Hz, 2H), 7.34—7.21(m, 3H), 7.21—7.18(m, 2H), 7.18—7.15(m, 1H), 7.15—7.10(m, 1H), 4.38—4.19(m, 1H), 4.11(t, J=7.0 Hz, 2H), 4.07—4.03(m, 1H), 3.99(q, J=7.1 Hz, 2H), 3.94—3.85(m, 1H), 3.81(d, J=6.6 Hz, 2H), 3.60—3.44(m, 1H), 3.31—3.20(m, 1H), 3.08—2.87(m, 2H), 2.61(t, J=7.1 Hz, 2H), 1.99—1.81(m, 1H), 1.14(t, J=7.1 Hz, 3H), 0.91(d, J=6.7 Hz, 6H) | 171.12, 170.64, 167.26, 164.34, 152.41, 142.93, 141.48, 140.98, 134.80, 132.90, 129.69, 129.11(2C), 128.78(2C), 127.35(2C), 127.23(2C), 126.16, 122.92, 120.06, 107.83, 71.25, 60.07, 55.42, 46.36, 42.65, 41.11, 39.04, 32.13, 27.33, 18.77(2C), 13.62 |
| 3b | 9.07(brs, 2H), 7.88(s, 2H), 7.51(s, 1H), 7.35(d, J=8.6 Hz, 1H), 7.31—7.27(m, 1H), 7.27—7.21(m, 3H), 7.21—7.18(m, 2H), 7.18—7.04(m, 2H), 5.69—5.38(m, 1H), 4.45—4.27(m, 1H), 4.24—4.15(m, 1H), 4.11(t, J=7.2 Hz, 2H), 3.99(q, J=7.1 Hz, 3H), 3.81(d, J=6.6 Hz, 2H), 3.76—3.66(m, 1H), 3.60—3.51(m, 1H), 3.49—3.39(m, 1H), 3.33—3.24(m, 2H), 2.61(t, J=7.1 Hz, 2H), 1.97—1.81(m, 1H), 1.63—1.45(m, 1H), 1.14(t, J=7.1 Hz, 3H), 0.91(d, J=6.6 Hz, 6H), 0.83(s, 3H), 0.68(s, 3H) | 171.10, 170.36, 166.91, 164.15, 154.56, 150.06, 142.91, 141.22, 140.77, 134.20, 132.77, 130.10, 129.32(2C), 128.79(2C), 127.36(2C), 126.98(2C), 126.21, 123.16, 120.30, 107.96, 71.85, 71.30, 60.06, 53.58, 46.33, 41.31, 39.21, 37.24, 32.11, 27.30(2C), 18.75(2C), 18.50(2C), 13.62 |
| 4a | 9.03(brs, 2H), 7.83(d, J=8.2 Hz, 2H), 7.43(s, 1H), 7.36(d, J=8.2 Hz, 2H), 7.31—7.19(m, 3H), 7.18—7.15(m, 2H), 7.15—7.08(m, 2H), 4.32—4.20(m, 1H), 4.08(t, J=7.1 Hz, 2H), 4.04—4.00(m, 1H), 3.96(q, J=7.1 Hz, 2H), 3.91—3.81(m, 1H), 3.56—3.45(m, 1H), 3.28—3.18(m, 1H), 3.04—2.86(m, 2H), 2.58(t, J=7.1 Hz, 2H), 1.42(s, 9H), 1.11(t, J=7.1 Hz, 3H) | 171.20, 170.63, 166.66, 162.89, 152.33, 142.95, 141.35, 140.93, 134.78, 132.98, 129.68, 129.16(2C), 128.80(2C), 127.36(2C), 127.19(2C), 126.16, 123.00, 120.11, 107.85, 79.37, 60.10, 55.42, 46.37, 42.63, 41.12, 39.01, 32.09, 27.69(3C), 13.64 |
| 4b | 9.05(brs, 2H), 7.94(d,J=5.6 Hz, 2H), 7.50(s, 1H), 7.39—7.10(m, 9H), 5.60—5.43(m, 1H), 4.44—4.28(m, 1H), 4.26—4.15(m, 1H), 4.11(t, J=6.7 Hz, 2H), 3.99(q, J=7.1 Hz, 2H), 3.94—3.83(m, 1H), 3.57—3.39(m, 1H), 3.30—3.20(m, 2H), 2.61(t, J=7.2 Hz, 2H), 1.45(s, 9H), 1.34—1.19(m, 3H), 1.14(t, J=7.1 Hz, 3H), 1.12—0.99(m, 6H) | 171.13, 170.40, 166.19, 162.93, 153.23, 150.49, 142.94, 141.26, 140.97, 134.26, 132.62, 130.03, 129.38(2C), 128.81(2C), 127.37(2C), 126.96(2C), 126.21, 123.15, 120.26, 107.98, 80.74, 79.50, 60.07, 53.66, 46.35, 41.43, 39.11, 36.29, 32.12, 27.69(6C), 13.63 |
| 5a | 9.18(brs, 2H), 7.89(d, J=8.3 Hz, 2H), 7.46(s, 1H), 7.40(d, J=8.3 Hz, 2H), 7.35—7.21(m, 3H), 7.21—7.15(m, 3H), 7.15—7.10(m, 1H), 4.32—4.21(m, 1H), 4.10(t, J=6.9 Hz, 2H), 4.07—3.94(m, 5H), 3.94—3.82(m, 1H), 3.59—3.47(m, 1H), 3.31—3.20(m, 1H), 3.06—2.88(m, 2H), 2.61(t, J=6.9 Hz, 2H), 1.67—1.54(m, 2H), 1.37—1.27(m, 4H), 1.14(t, J=7.1 Hz, 3H), 0.88(t, J=7.0 Hz, 3H) | 171.18, 170.65, 167.21, 164.06, 152.19, 142.85, 141.48, 140.88, 134.74, 132.63, 129.69, 129.18(2C), 128.81(2C), 127.35(2C), 127.28(2C), 126.21, 122.99, 120.05, 107.90, 65.19, 60.13, 55.38, 46.36, 42.49, 41.09, 38.96, 32.07, 28.03, 27.57, 21.91, 13.64, 13.50 |
| 5b | 9.13(brs, 2H), 7.90(s, 2H), 7.50(s, 1H), 7.39—7.28(m, 2H), 7.28—7.22(m, 3H), 7.22—7.07(m, 4H), 5.65—5.41(m, 1H), 4.46—4.29(m, 1H), 4.28—4.16(m, 1H), 4.11(t, J=6.7 Hz, 2H), 4.05—3.86(m, 5H), 3.76—3.61(m, 1H), 3.61—3.41(m, 2H), | 171.16, 170.39, 166.71, 164.36, 154.57, 150.08, 142.97, 141.28, 140.90, 134.24, 132.77, 130.15, 129.37(2C), 128.82(2C), 127.39(2C), 126.93(2C), 126.22, 123.22, 120.37, 107.95, 65.94, |
| Compd. | 1H NMR(300 MHz), δa | 13C NMR(75 MHz), δb |
| 5b | 3.32—3.22(m, 2H), 2.61(t, J=7.0 Hz, 2H), 1.68—1.52(m, 2H), 1.39—1.17(m, 8H), 1.14(t, J=7.1 Hz, 3H), 1.11—0.95(m, 2H), 0.95—0.72(m, 6H) | 65.18, 60.10, 53.61, 46.37, 41.37, 39.24, 37.12, 32.16, 28.05, 27.97, 27.60, 27.49, 21.91, 21.79, 13.66, 13.48, 13.45 |
| 6a | 9.06(brs, 2H), 7.90(d,J=7.9 Hz, 2H), 7.46(s, 1H), 7.44—7.36(m, 5H), 7.36—7.32(m, 1H), 7.33—7.27(m, 2H), 7.27—7.21(m, 2H), 7.21—7.10(m, 4H), 5.10(s, 2H), 4.33—4.22(m, 1H), 4.10(t, J=7.0 Hz, 2H), 4.06—4.03(m, 1H), 3.99(q, J=7.0 Hz, 2H), 3.94—3.80(m, 1H), 3.59—3.47(m, 1H), 3.31—3.20(m, 1H), 3.09—2.82(m, 2H), 2.61(t, J=7.0 Hz, 2H), 1.14(t, J=7.1 Hz, 3H) | 171.13, 170.64, 167.43, 163.97, 152.34, 142.95, 141.67, 140.96, 136.18, 134.78, 132.67, 129.74, 129.17(2C), 128.79(2C), 127.88(2C), 127.67(2C), 127.42, 127.36(2C), 127.23(2C), 126.16, 122.96, 120.08, 107.84, 66.66, 60.08, 55.38, 46.38, 42.61, 41.08, 39.05, 32.13, 13.64 |
| 6b | 9.15(brs, 2H), 7.87(d, J=8.3 Hz, 2H), 7.50(s, 1H), 7.44—6.97(m, 19H), 5.69—5.47(m, 1H), 5.12(s, 2H), 4.97(s, 2H), 4.70—4.56(m, 1H), 4.42—4.24(m, 1H), 4.23—4.15(m, 1H), 4.11(t, J=6.8 Hz, 2H), 3.99(q, J=7.1 Hz, 2H), 3.68—3.37(m, 2H), 3.29—3.11(m, 1H), 2.61(t, J=7.2 Hz, 2H), 1.14(t, J=7.1 Hz, 3H) | 171.14, 170.36, 166.19, 162.93, 153.23, 149.93, 142.92, 141.22, 140.83, 136.15, 135.79, 134.18, 132.52, 130.16, 129.30(2C), 128.80(2C), 128.03(2C), 127.89(2C), 127.69(2C), 127.63(2C), 127.59, 127.44, 127.37(2C), 126.96(2C), 126.22, 123.22, 120.33, 107.97, 66.70, 66.37, 60.09, 53.73, 46.35, 41.33, 39.19, 37.16, 32.14, 13.64 |
| 7 | 9.40(s, 2H), 9.20(s, 2H), 7.81(d,J=7.7 Hz, 2H), 7.62(d, J=7.9 Hz, 2H), 7.58—7.43(m, 2H), 7.32—7.24(m, 2H), 7.24—7.19(m, 2H), 7.19—7.15(m, 1H), 7.15—7.10(m, 1H), 5.11(brs, 1H), 4.42(brs, 2H), 4.10—3.99(m, 2H), 3.66(brs, 2H), 3.49(brs, 2H), 2.77(s, 3H), 2.60—2.52(m, 2H) | 172.53, 169.36, 165.24, 148.40, 143.25, 143.03, 140.55, 133.80, 131.25, 129.87(2C), 129.23(2C), 128.10(2C), 127.99(2C), 126.74, 126.22, 123.82, 118.56, 110.43, 60.70, 49.68, 46.27, 38.45, 37.94, 37.90, 32.10 |
| 8 | 9.05(brs, 2H), 7.70(d, J=8.2 Hz, 2H), 7.47(s, 1H), 7.33—7.14(m, 9H), 4.16—4.12(m, 1H), 4.10(t, J=7.1 Hz, 2H), 4.06—4.03(m, 1H), 3.99(q, J=7.1 Hz, 2H), 3.92—3.77(m, 1H), 3.39—3.37(m, 1H),3.28—3.15(m, 2H), 2.92—2.77(m, 1H), 2.61(t, J=6.9 Hz, 2H), 2.40(s, 3H), 1.44(d, J=4.8 Hz, 9H), 1.14(t, J=7.1 Hz, 3H) | 171.18, 170.63, 166.73, 160.39, 152.10, 144.01, 142.89, 141.10, 134.40, 131.26, 129.62, 129.39(2C), 128.79(2C), 127.36(2C), 126.47(2C), 126.19, 122.80, 119.94, 107.84, 79.78, 62.11, 60.09, 48.88, 46.35, 41.98, 39.94, 36.84, 32.09, 27.64(3C), 13.64 |
| 9 | 8.94(brs, 2H), 7.74(d, J=8.3 Hz, 2H), 7.47(s, 1H), 7.37—7.00(m, 9H), 4.17—4.05(m, 3H), 4.06—3.92(m, 5H), 3.91—3.75(m, 1H), 3.55(d, J=27.6 Hz, 1H), 3.27—3.13(m, 2H), 2.94—2.75(m, 1H), 2.61(t, J=6.8 Hz, 2H), 2.40(s, 3H),1.68—1.49(m, 2H), 1.36—1.25(m, 6H), 1.14(t, J=7.1 Hz, 3H), 0.86(t, J=6.6 Hz, 3H) | 171.18, 170.58, 167.28, 161.05, 152.07, 142.97, 142.29, 141.20, 134.47, 131.79, 129.62, 129.38(2C), 128.78(2C), 127.35(2C), 126.43(2C), 126.13, 122.81, 120.06, 107.80, 65.14, 62.15, 60.08, 48.88, 46.35, 41.95, 39.84, 36.91, 32.09, 31.04, 28.30, 25.13, 22.07, 13.65, 13.55 |
| 10 | 9.15(brs, 2H), 7.89(d, J=8.2 Hz, 2H), 7.53—7.35(m, 4H), 7.35—7.28(m, 1H), 7.29—7.22(m, 3H), 7.22—7.17(m, 3H), 7.17—7.14(m, 2H), 7.15—7.05(m, 2H), 5.13(s, 2H), 4.34—4.23(m, 1H), 4.12(t, J=6.7 Hz, 2H), 4.08—4.04(m, 1H), 4.01(t, J=6.7 Hz, 2H), 3.94—3.85(m, 1H), 3.57—3.49(m, 2H), 3.06—2.90(m, 2H), 2.69(t, J=7.2 Hz, 2H), 2.42(s, 3H), 0.87(t, J=6.5 Hz, 3H) | 171.18, 170.81, 167.63, 164.43, 152.41, 142.94, 141.60, 141.19, 137.74, 134.89, 132.95, 129.62, 129.43(2C), 128.83(2C), 127.87(2C), 127.67(2C), 127.37(2C), 127.23, 126.46(2C), 126.19, 123.10, 120.06, 107.88, 64.69, 61.82, 60.11, 48.82, 46.30, 41.93, 40.76, 37.08, 31.05, 13.56 |
| 11 | 9.55(brs, 2H), 7.81(d,J=7.9 Hz, 2H), 7.72(s, 1H), 7.34(d, J=8.4 Hz, 2H), 7.31(d, J=1.3 Hz, 1H), 7.25—7.18(m, 2H), 7.16—7.05(m, 4H), 5.30(s, 2H), 4.47—4.35(m, 1H), 4.34—4.22(m, 2H), 4.09(q, J=7.3 Hz, 2H), 4.05—3.96(m, 2H), 3.80—3.71(m, 1H), 3.47—3.37(m, 1H), 3.22—3.05(m, 2H), 2.80—2.71(m, 2H), 2.60(s, 3H), 2.51(s, 3H), 2.49(s, 3H), 1.23(t, J=7.1 Hz, 3H) | 171.09, 170.65, 167.46, 163.62, 152.37, 150.41, 148.61, 148.19, 144.86, 142.75, 141.48, 140.82, 134.72, 132.45, 129.59, 128.96(2C), 128.79(2C), 127.36(2C), 127.32(2C), 126.22, 122.87, 119.89, 107.92, 65.43, 60.09, 55.30, 46.32, 42.49, 40.99, 38.93, 32.06, 21.09, 20.81, 20.08, 13.61 |
Table 2 1H NMR and 13C NMR data of target compounds 2a—6a, 2b—6b and 7—11
| Compd. | 1H NMR(300 MHz), δa | 13C NMR(75 MHz), δb |
|---|---|---|
| 2a | 9.14(brs, 2H), 7.87(d, J=8.3 Hz, 2H), 7.43(s, 1H), 7.38(d, J=8.3 Hz, 2H), 7.32—7.21(m, 2H), 7.21—7.06(m, 5H), 4.34—4.24(m, 1H), 4.15—3.99(m, 5H), 3.96(q, J=7.2 Hz, 2H), 3.91—3.81(m, 1H), 3.57—3.45(m, 1H), 3.29—3.20(m, 1H), 3.07—2.84(m, 2H), 2.58(t, J=7.1 Hz, 2H), 1.18(t, J=6.9 Hz, 3H), 1.11(t, J=7.1 Hz, 3H) | 171.08, 170.63, 167.19, 163.87, 152.30, 142.83, 141.49, 140.86, 134.73, 132.51, 129.67, 129.05(2C), 128.78(2C), 127.33(2C), 127.28(2C), 126.19, 122.90, 119.95, 107.88, 60.78, 60.07, 55.29, 46.34, 42.50, 40.98, 38.95, 32.10, 13.93, 13.61 |
| 2b | 9.59(brs, 2H), 7.85—7.59(m, 3H), 7.26—7.02(m, 9H), 5.89—5.63(m, 1H), 4.65—4.44(m, 1H), 4.41—3.80(m, 11H), 3.62—3.27(m, 2H), 2.76(t, J=7.2 Hz, 2H), 1.36(t, J=6.8 Hz, 3H), 1.32—1.18(m, 6H) | 171.12, 170.38, 166.89, 164.02, 154.40, 150.11, 142.91, 141.21, 141.00, 134.20, 132.49, 130.10, 129.33(2C), 128.79(2C), 127.36(2C), 126.94(2C), 126.21, 123.18, 120.30, 107.95, 61.68, 60.89, 60.08, 53.49, 46.34, 41.36, 38.73, 36.87, 32.12, 13.91(2C), 13.62 |
| 3a | 9.07(brs, 2H), 7.89(d, J=8.0 Hz, 2H), 7.46(s, 1H), 7.40(d, J=8.2 Hz, 2H), 7.34—7.21(m, 3H), 7.21—7.18(m, 2H), 7.18—7.15(m, 1H), 7.15—7.10(m, 1H), 4.38—4.19(m, 1H), 4.11(t, J=7.0 Hz, 2H), 4.07—4.03(m, 1H), 3.99(q, J=7.1 Hz, 2H), 3.94—3.85(m, 1H), 3.81(d, J=6.6 Hz, 2H), 3.60—3.44(m, 1H), 3.31—3.20(m, 1H), 3.08—2.87(m, 2H), 2.61(t, J=7.1 Hz, 2H), 1.99—1.81(m, 1H), 1.14(t, J=7.1 Hz, 3H), 0.91(d, J=6.7 Hz, 6H) | 171.12, 170.64, 167.26, 164.34, 152.41, 142.93, 141.48, 140.98, 134.80, 132.90, 129.69, 129.11(2C), 128.78(2C), 127.35(2C), 127.23(2C), 126.16, 122.92, 120.06, 107.83, 71.25, 60.07, 55.42, 46.36, 42.65, 41.11, 39.04, 32.13, 27.33, 18.77(2C), 13.62 |
| 3b | 9.07(brs, 2H), 7.88(s, 2H), 7.51(s, 1H), 7.35(d, J=8.6 Hz, 1H), 7.31—7.27(m, 1H), 7.27—7.21(m, 3H), 7.21—7.18(m, 2H), 7.18—7.04(m, 2H), 5.69—5.38(m, 1H), 4.45—4.27(m, 1H), 4.24—4.15(m, 1H), 4.11(t, J=7.2 Hz, 2H), 3.99(q, J=7.1 Hz, 3H), 3.81(d, J=6.6 Hz, 2H), 3.76—3.66(m, 1H), 3.60—3.51(m, 1H), 3.49—3.39(m, 1H), 3.33—3.24(m, 2H), 2.61(t, J=7.1 Hz, 2H), 1.97—1.81(m, 1H), 1.63—1.45(m, 1H), 1.14(t, J=7.1 Hz, 3H), 0.91(d, J=6.6 Hz, 6H), 0.83(s, 3H), 0.68(s, 3H) | 171.10, 170.36, 166.91, 164.15, 154.56, 150.06, 142.91, 141.22, 140.77, 134.20, 132.77, 130.10, 129.32(2C), 128.79(2C), 127.36(2C), 126.98(2C), 126.21, 123.16, 120.30, 107.96, 71.85, 71.30, 60.06, 53.58, 46.33, 41.31, 39.21, 37.24, 32.11, 27.30(2C), 18.75(2C), 18.50(2C), 13.62 |
| 4a | 9.03(brs, 2H), 7.83(d, J=8.2 Hz, 2H), 7.43(s, 1H), 7.36(d, J=8.2 Hz, 2H), 7.31—7.19(m, 3H), 7.18—7.15(m, 2H), 7.15—7.08(m, 2H), 4.32—4.20(m, 1H), 4.08(t, J=7.1 Hz, 2H), 4.04—4.00(m, 1H), 3.96(q, J=7.1 Hz, 2H), 3.91—3.81(m, 1H), 3.56—3.45(m, 1H), 3.28—3.18(m, 1H), 3.04—2.86(m, 2H), 2.58(t, J=7.1 Hz, 2H), 1.42(s, 9H), 1.11(t, J=7.1 Hz, 3H) | 171.20, 170.63, 166.66, 162.89, 152.33, 142.95, 141.35, 140.93, 134.78, 132.98, 129.68, 129.16(2C), 128.80(2C), 127.36(2C), 127.19(2C), 126.16, 123.00, 120.11, 107.85, 79.37, 60.10, 55.42, 46.37, 42.63, 41.12, 39.01, 32.09, 27.69(3C), 13.64 |
| 4b | 9.05(brs, 2H), 7.94(d,J=5.6 Hz, 2H), 7.50(s, 1H), 7.39—7.10(m, 9H), 5.60—5.43(m, 1H), 4.44—4.28(m, 1H), 4.26—4.15(m, 1H), 4.11(t, J=6.7 Hz, 2H), 3.99(q, J=7.1 Hz, 2H), 3.94—3.83(m, 1H), 3.57—3.39(m, 1H), 3.30—3.20(m, 2H), 2.61(t, J=7.2 Hz, 2H), 1.45(s, 9H), 1.34—1.19(m, 3H), 1.14(t, J=7.1 Hz, 3H), 1.12—0.99(m, 6H) | 171.13, 170.40, 166.19, 162.93, 153.23, 150.49, 142.94, 141.26, 140.97, 134.26, 132.62, 130.03, 129.38(2C), 128.81(2C), 127.37(2C), 126.96(2C), 126.21, 123.15, 120.26, 107.98, 80.74, 79.50, 60.07, 53.66, 46.35, 41.43, 39.11, 36.29, 32.12, 27.69(6C), 13.63 |
| 5a | 9.18(brs, 2H), 7.89(d, J=8.3 Hz, 2H), 7.46(s, 1H), 7.40(d, J=8.3 Hz, 2H), 7.35—7.21(m, 3H), 7.21—7.15(m, 3H), 7.15—7.10(m, 1H), 4.32—4.21(m, 1H), 4.10(t, J=6.9 Hz, 2H), 4.07—3.94(m, 5H), 3.94—3.82(m, 1H), 3.59—3.47(m, 1H), 3.31—3.20(m, 1H), 3.06—2.88(m, 2H), 2.61(t, J=6.9 Hz, 2H), 1.67—1.54(m, 2H), 1.37—1.27(m, 4H), 1.14(t, J=7.1 Hz, 3H), 0.88(t, J=7.0 Hz, 3H) | 171.18, 170.65, 167.21, 164.06, 152.19, 142.85, 141.48, 140.88, 134.74, 132.63, 129.69, 129.18(2C), 128.81(2C), 127.35(2C), 127.28(2C), 126.21, 122.99, 120.05, 107.90, 65.19, 60.13, 55.38, 46.36, 42.49, 41.09, 38.96, 32.07, 28.03, 27.57, 21.91, 13.64, 13.50 |
| 5b | 9.13(brs, 2H), 7.90(s, 2H), 7.50(s, 1H), 7.39—7.28(m, 2H), 7.28—7.22(m, 3H), 7.22—7.07(m, 4H), 5.65—5.41(m, 1H), 4.46—4.29(m, 1H), 4.28—4.16(m, 1H), 4.11(t, J=6.7 Hz, 2H), 4.05—3.86(m, 5H), 3.76—3.61(m, 1H), 3.61—3.41(m, 2H), | 171.16, 170.39, 166.71, 164.36, 154.57, 150.08, 142.97, 141.28, 140.90, 134.24, 132.77, 130.15, 129.37(2C), 128.82(2C), 127.39(2C), 126.93(2C), 126.22, 123.22, 120.37, 107.95, 65.94, |
| Compd. | 1H NMR(300 MHz), δa | 13C NMR(75 MHz), δb |
| 5b | 3.32—3.22(m, 2H), 2.61(t, J=7.0 Hz, 2H), 1.68—1.52(m, 2H), 1.39—1.17(m, 8H), 1.14(t, J=7.1 Hz, 3H), 1.11—0.95(m, 2H), 0.95—0.72(m, 6H) | 65.18, 60.10, 53.61, 46.37, 41.37, 39.24, 37.12, 32.16, 28.05, 27.97, 27.60, 27.49, 21.91, 21.79, 13.66, 13.48, 13.45 |
| 6a | 9.06(brs, 2H), 7.90(d,J=7.9 Hz, 2H), 7.46(s, 1H), 7.44—7.36(m, 5H), 7.36—7.32(m, 1H), 7.33—7.27(m, 2H), 7.27—7.21(m, 2H), 7.21—7.10(m, 4H), 5.10(s, 2H), 4.33—4.22(m, 1H), 4.10(t, J=7.0 Hz, 2H), 4.06—4.03(m, 1H), 3.99(q, J=7.0 Hz, 2H), 3.94—3.80(m, 1H), 3.59—3.47(m, 1H), 3.31—3.20(m, 1H), 3.09—2.82(m, 2H), 2.61(t, J=7.0 Hz, 2H), 1.14(t, J=7.1 Hz, 3H) | 171.13, 170.64, 167.43, 163.97, 152.34, 142.95, 141.67, 140.96, 136.18, 134.78, 132.67, 129.74, 129.17(2C), 128.79(2C), 127.88(2C), 127.67(2C), 127.42, 127.36(2C), 127.23(2C), 126.16, 122.96, 120.08, 107.84, 66.66, 60.08, 55.38, 46.38, 42.61, 41.08, 39.05, 32.13, 13.64 |
| 6b | 9.15(brs, 2H), 7.87(d, J=8.3 Hz, 2H), 7.50(s, 1H), 7.44—6.97(m, 19H), 5.69—5.47(m, 1H), 5.12(s, 2H), 4.97(s, 2H), 4.70—4.56(m, 1H), 4.42—4.24(m, 1H), 4.23—4.15(m, 1H), 4.11(t, J=6.8 Hz, 2H), 3.99(q, J=7.1 Hz, 2H), 3.68—3.37(m, 2H), 3.29—3.11(m, 1H), 2.61(t, J=7.2 Hz, 2H), 1.14(t, J=7.1 Hz, 3H) | 171.14, 170.36, 166.19, 162.93, 153.23, 149.93, 142.92, 141.22, 140.83, 136.15, 135.79, 134.18, 132.52, 130.16, 129.30(2C), 128.80(2C), 128.03(2C), 127.89(2C), 127.69(2C), 127.63(2C), 127.59, 127.44, 127.37(2C), 126.96(2C), 126.22, 123.22, 120.33, 107.97, 66.70, 66.37, 60.09, 53.73, 46.35, 41.33, 39.19, 37.16, 32.14, 13.64 |
| 7 | 9.40(s, 2H), 9.20(s, 2H), 7.81(d,J=7.7 Hz, 2H), 7.62(d, J=7.9 Hz, 2H), 7.58—7.43(m, 2H), 7.32—7.24(m, 2H), 7.24—7.19(m, 2H), 7.19—7.15(m, 1H), 7.15—7.10(m, 1H), 5.11(brs, 1H), 4.42(brs, 2H), 4.10—3.99(m, 2H), 3.66(brs, 2H), 3.49(brs, 2H), 2.77(s, 3H), 2.60—2.52(m, 2H) | 172.53, 169.36, 165.24, 148.40, 143.25, 143.03, 140.55, 133.80, 131.25, 129.87(2C), 129.23(2C), 128.10(2C), 127.99(2C), 126.74, 126.22, 123.82, 118.56, 110.43, 60.70, 49.68, 46.27, 38.45, 37.94, 37.90, 32.10 |
| 8 | 9.05(brs, 2H), 7.70(d, J=8.2 Hz, 2H), 7.47(s, 1H), 7.33—7.14(m, 9H), 4.16—4.12(m, 1H), 4.10(t, J=7.1 Hz, 2H), 4.06—4.03(m, 1H), 3.99(q, J=7.1 Hz, 2H), 3.92—3.77(m, 1H), 3.39—3.37(m, 1H),3.28—3.15(m, 2H), 2.92—2.77(m, 1H), 2.61(t, J=6.9 Hz, 2H), 2.40(s, 3H), 1.44(d, J=4.8 Hz, 9H), 1.14(t, J=7.1 Hz, 3H) | 171.18, 170.63, 166.73, 160.39, 152.10, 144.01, 142.89, 141.10, 134.40, 131.26, 129.62, 129.39(2C), 128.79(2C), 127.36(2C), 126.47(2C), 126.19, 122.80, 119.94, 107.84, 79.78, 62.11, 60.09, 48.88, 46.35, 41.98, 39.94, 36.84, 32.09, 27.64(3C), 13.64 |
| 9 | 8.94(brs, 2H), 7.74(d, J=8.3 Hz, 2H), 7.47(s, 1H), 7.37—7.00(m, 9H), 4.17—4.05(m, 3H), 4.06—3.92(m, 5H), 3.91—3.75(m, 1H), 3.55(d, J=27.6 Hz, 1H), 3.27—3.13(m, 2H), 2.94—2.75(m, 1H), 2.61(t, J=6.8 Hz, 2H), 2.40(s, 3H),1.68—1.49(m, 2H), 1.36—1.25(m, 6H), 1.14(t, J=7.1 Hz, 3H), 0.86(t, J=6.6 Hz, 3H) | 171.18, 170.58, 167.28, 161.05, 152.07, 142.97, 142.29, 141.20, 134.47, 131.79, 129.62, 129.38(2C), 128.78(2C), 127.35(2C), 126.43(2C), 126.13, 122.81, 120.06, 107.80, 65.14, 62.15, 60.08, 48.88, 46.35, 41.95, 39.84, 36.91, 32.09, 31.04, 28.30, 25.13, 22.07, 13.65, 13.55 |
| 10 | 9.15(brs, 2H), 7.89(d, J=8.2 Hz, 2H), 7.53—7.35(m, 4H), 7.35—7.28(m, 1H), 7.29—7.22(m, 3H), 7.22—7.17(m, 3H), 7.17—7.14(m, 2H), 7.15—7.05(m, 2H), 5.13(s, 2H), 4.34—4.23(m, 1H), 4.12(t, J=6.7 Hz, 2H), 4.08—4.04(m, 1H), 4.01(t, J=6.7 Hz, 2H), 3.94—3.85(m, 1H), 3.57—3.49(m, 2H), 3.06—2.90(m, 2H), 2.69(t, J=7.2 Hz, 2H), 2.42(s, 3H), 0.87(t, J=6.5 Hz, 3H) | 171.18, 170.81, 167.63, 164.43, 152.41, 142.94, 141.60, 141.19, 137.74, 134.89, 132.95, 129.62, 129.43(2C), 128.83(2C), 127.87(2C), 127.67(2C), 127.37(2C), 127.23, 126.46(2C), 126.19, 123.10, 120.06, 107.88, 64.69, 61.82, 60.11, 48.82, 46.30, 41.93, 40.76, 37.08, 31.05, 13.56 |
| 11 | 9.55(brs, 2H), 7.81(d,J=7.9 Hz, 2H), 7.72(s, 1H), 7.34(d, J=8.4 Hz, 2H), 7.31(d, J=1.3 Hz, 1H), 7.25—7.18(m, 2H), 7.16—7.05(m, 4H), 5.30(s, 2H), 4.47—4.35(m, 1H), 4.34—4.22(m, 2H), 4.09(q, J=7.3 Hz, 2H), 4.05—3.96(m, 2H), 3.80—3.71(m, 1H), 3.47—3.37(m, 1H), 3.22—3.05(m, 2H), 2.80—2.71(m, 2H), 2.60(s, 3H), 2.51(s, 3H), 2.49(s, 3H), 1.23(t, J=7.1 Hz, 3H) | 171.09, 170.65, 167.46, 163.62, 152.37, 150.41, 148.61, 148.19, 144.86, 142.75, 141.48, 140.82, 134.72, 132.45, 129.59, 128.96(2C), 128.79(2C), 127.36(2C), 127.32(2C), 126.22, 122.87, 119.89, 107.92, 65.43, 60.09, 55.30, 46.32, 42.49, 40.99, 38.93, 32.06, 21.09, 20.81, 20.08, 13.61 |
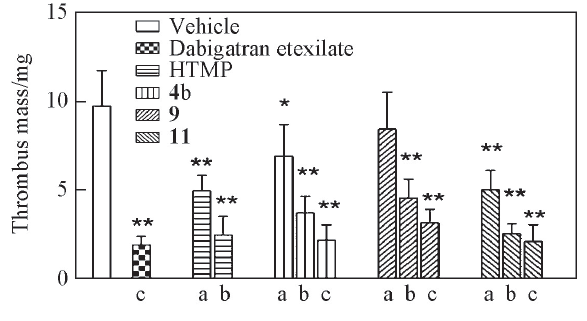
Fig.1 Antithrombotic effect of tested compounds, dabigatran etexilate and HTMP in vivoAverage thrombus mass is the mean±SD from eight independent experiments. Statistical significance compared with the vehicle group is *P<0.05, **P<0.01. Dose/(mg·kg-1): a. 1; b. 5; c. 20.
| [1] | Fu Y., Wang D.W., Chinese J. Clin., 2006, 34(8), 51—53 |
| (付研, 王大为. 中国临床医生, 2006, 34(8), 51—53) | |
| [2] | Mackman N., Becker R.C., Arterioscler. Thromb. Vasc. Biol., 2010, 30(3), 369—371 |
| [3] | Rupin A., Marx I., Vallez M.O., Mennecier P., Gloanec P., de Nanteuil G., Verbeuren T. J., J. Thromb. Haemost., 2011, 9(7), 1375—1382 |
| [4] | Xiao Z., Theroux P., Circulation, 1998, 97(3), 251—256 |
| [5] | Ishibashi H., Koide M., Obara S., Kumasaka Y., Tamura K., J. Stroke. Cerebrovasc. Dis., 2013, 22(5), 656—660 |
| [6] | Di Nisio M., Middeldorp S., Buller H.R., N. Engl. J. Med., 2005, 353(10), 1028—1040 |
| [7] | Yang H.R., Ren Y. J., Gao X. D., Gao Y. H., Chem. Res. Chinese Universities, 2016, 32(6), 973—978 |
| [8] | Connolly S.J., Ezekowitz M. D., Yusuf S., Eikelboom J., Oldgren J., Parekh A., Pogue J., Reilly P. A., Themeles E., Varrone J., Wang S., Alings M., Xavier D., Zhu J., Diaz R., Lewis B. S., Darius H., Diener H. C., Joyner C. D., Wallentin L., Engl. J. Med., 2009, 361(12), 1139—1151 |
| [9] | Grave S., Aust. Nurs. J., 2011, 19(6), 30—33 |
| [10] | Martins H.S., Scalabrini-Neto A., Velasco I. T., Lancet, 2007, 370(9604), 2002—2003 |
| [11] | Hauel N.H., Nar H., Priepke H., Ries U., Stassen J. M., Wienen W., J. Med. Chem., 2002, 45(9), 1757—1766 |
| [12] | Halton J.M., Lehr T., Cronin L., Lobmeyer M. T., Haertter S., Belletrutti M., Mitchell L. G., Thromb. Haemost., 2016, 116(3), 461—471 |
| [13] | Imberti D., Pomero F., Benedetti R., Fenoglio L., Intern. Emerg. Med., 2016, 11(7), 895—900 |
| [14] | Yang X.Z., Yang W. H., Xu Y. G., Diao X. J., He G. W., Gong G. Q., Eur. J. Med. Chem., 2012, 57, 21—28 |
| [15] | Yang X.Z., Diao X. J., Yang W. H., Li F., He G. W., Gong G. Q., Xu Y. G., Bioorg. Med. Chem. Lett., 2013, 23(7), 2089—2092 |
| [16] | Wang S.C., Dai P., Xu Y. G., Chen Q. F., Zhu Q. H., Gong G. Q., Arch. Pharm., 2015, 348(8), 595—605 |
| [17] | Chen D.X., Wang S. C., Diao X. J., Zhu Q. H., Shen H. L., Han X. Q., Wang Y. W., Gong G. Q., Xu Y. G., Bioorg. Med. Chem., 2015, 23(23), 7405—7416 |
| [18] | Li M., Handa S., Ikeda Y., Goto S., Thromb. Res., 2001, 104(1), 15—28 |
| [19] | Liu J.B., Li Y. X., Chen Y. W., Wu C. C., Wan Y. Y., Wei W., Xiong L. X., Zhang X., Yu S. J., Li Z. M., Chem. Res. Chinese Universities, 2016, 32(1), 41—48 |
| [20] | Jia C.Q., Yang D. Y., Che C. L., Ma Y. Q., Rui C. H., Yan X. J., Qin Z. H., Chem. J. Chinese Universities, 2016, 37(5), 892—901 |
| (贾长青, 杨冬燕, 车传亮, 马永强, 芮昌辉, 闫晓静, 覃兆海. 高等学校化学学报, 2016, 37(5), 892—901) |
| [1] | 曹凯悦, 彭金武, 李宏斌, 石埕荧, 王鹏, 刘佰军. 基于聚苯并咪唑/超支化聚合物的交联共混体系的高温质子交换膜[J]. 高等学校化学学报, 2021, 42(6): 2049. |
| [2] | 梁敏慧, 王鹏, 李宏斌, 李天洋, 曹凯悦, 彭金武, 刘振超, 刘佰军. 基于半互穿聚合物网络的高温质子交换膜的制备[J]. 高等学校化学学报, 2020, 41(12): 2845. |
| [3] | 宋西鹏, 刘金宇, 王丽华, 韩旭彤, 黄庆林. 聚苯并咪唑/聚乙烯吡咯烷酮复合质子交换膜的制备及钒液流电池性能[J]. 高等学校化学学报, 2019, 40(7): 1543. |
| [4] | 赵彩秀, 杨溢, 刘一婷, 姜影, 袁芳, 王睿, 陈冬菊. 高通量聚苯并咪唑纳滤膜的结构调控及性能[J]. 高等学校化学学报, 2018, 39(4): 785. |
| [5] | 刘治庆, 薛飞, 雷振凯, 刘晨江. 离子液体1-烷基-3-羧甲基苯并咪唑双三氟甲磺酰亚胺盐的合成及在油品中的脱硫应用[J]. 高等学校化学学报, 2016, 37(5): 886. |
| [6] | 徐燕燕, 于书平, 韩克飞, 于景华, 朱红, 汪中明. 新型聚苯并咪唑树脂的微波合成及质子交换膜的性能[J]. 高等学校化学学报, 2013, 34(6): 1547. |
| [7] | 周宇, 王大明, 赵晓刚, 周宏伟, 陈春海, 党国栋. 含苯并咪唑基团聚酰亚胺的合成与表征[J]. 高等学校化学学报, 2013, 34(10): 2427. |
| [8] | 易平贵, 阳习春, 于贤勇, 刘峥军, 刘金, 汪朝旭, 李筱芳. 八元瓜环超分子作用下2-(2-氨基-3-吡啶基)苯并咪唑的质子转移[J]. 高等学校化学学报, 2012, 33(12): 2657. |
| [9] | 董智云, 江小枝, 张大卫, 高国华. 含脲苯并咪唑类离子液体的合成及阴离子识别性能[J]. 高等学校化学学报, 2012, 33(10): 2256. |
| [10] | 易君明, 薛赛凤, 陶朱. 六元瓜环与二氯化-1,8-二(2-苯并咪唑基)辛烷的自组装模式[J]. 高等学校化学学报, 2012, 33(09): 1973. |
| [11] | 黄雪英, 孟祥高, 张妍, 王莉, 刘长林. 多苯并咪唑锰( Ⅱ )配合物的合成及对DNA凝聚的促进作用[J]. 高等学校化学学报, 2012, 33(06): 1151. |
| [12] | 赵晶, 盛丽, 徐宏杰, 房建华, 印杰. 新型磺化聚苯并咪唑的合成及性能[J]. 高等学校化学学报, 2012, 33(03): 645. |
| [13] | 王斌, 刘晨江, 王吉德, 雷振凯, 胡东林. 功能化苯并咪唑类离子液体的合成及性质[J]. 高等学校化学学报, 2012, 33(01): 76. |
| [14] | 陈炳鹏 王卓鹏 柳菁菁 王金成 于吉红. 分子筛在医学领域的应用及作用机制[J]. 高等学校化学学报, 2011, 32(3): 485. |
| [15] | 胡松青 米思奇 贾晓林 郭爱玲 陈生辉 张军 刘新泳. 苯并咪唑类缓蚀剂的3D-QSAR研究及分子设计[J]. 高等学校化学学报, 2011, 32(10): 2402. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||