

Chem. J. Chinese Universities ›› 2025, Vol. 46 ›› Issue (10): 20250123.doi: 10.7503/cjcu20250123
• Organic Chemistry • Previous Articles Next Articles
ZHANG Lingzhi1( ), JU Qiurong2, GUAN Zhe3, ZHU Qihua2, ZHANG Tingting1, YANG Shiqin1, XU Yungen2
), JU Qiurong2, GUAN Zhe3, ZHU Qihua2, ZHANG Tingting1, YANG Shiqin1, XU Yungen2
Received:2025-04-25
Online:2025-10-10
Published:2025-06-25
Contact:
ZHANG Lingzhi
E-mail:zhanglingzhicpu@163.com
Supported by:CLC Number:
TrendMD:
ZHANG Lingzhi, JU Qiurong, GUAN Zhe, ZHU Qihua, ZHANG Tingting, YANG Shiqin, XU Yungen. Design, Synthesis and Biological Evaluation of ERK Inhibitors with Isoindolin-1-one Structure[J]. Chem. J. Chinese Universities, 2025, 46(10): 20250123.
| Compd. | Appearance | Yield(%) | m. p./℃ | Compd. | Appearance | Yield(%) | m. p./℃ |
|---|---|---|---|---|---|---|---|
| 3 | Yellow solid | 39.4 | 128—130 | 17b | White solid | 54.3 | 222—224 |
| 6 | Yellow solid | 86.2 | 150—152 | 17c | White solid | 80.2 | 192—194 |
| 7* | Yellow solid | — | — | 17d | White solid | 50.5 | 181—183 |
| 8 | White solid | 37.4 | 130—132 | 17e | White solid | 89.2 | 177—179 |
| 11a | White solid | 69.4 | 140—142 | 17f | White solid | 25.5 | 243—245 |
| 11b | White solid | 95.6 | 129—130 | 17g | Yellow solid | 59.3 | 134—136 |
| 14a | Yellow solid | 46.3 | 182—184 | 17h | Yellow solid | 50.8 | 126—128 |
| 14b | Yellow solid | 78.0 | 113—115 | 21 | White solid | 64.7 | 222—224 |
| 15a | Yellow solid | 81.7 | >250 | 22 | White solid | 78.7 | 176—178 |
| 15b | White solid | 40.5 | 209—211 | 24a | Yellow solid | 62.8 | 190—192 |
| 17a | White solid | 63.4 | 246—248 | 24b | Yellow solid | 94.2 | 240—242 |
Table 1 Appearances and yields of compounds11a, 11b, 14a, 14b, 15a, 15b, 17a—17h, 21, 24a, 24b
| Compd. | Appearance | Yield(%) | m. p./℃ | Compd. | Appearance | Yield(%) | m. p./℃ |
|---|---|---|---|---|---|---|---|
| 3 | Yellow solid | 39.4 | 128—130 | 17b | White solid | 54.3 | 222—224 |
| 6 | Yellow solid | 86.2 | 150—152 | 17c | White solid | 80.2 | 192—194 |
| 7* | Yellow solid | — | — | 17d | White solid | 50.5 | 181—183 |
| 8 | White solid | 37.4 | 130—132 | 17e | White solid | 89.2 | 177—179 |
| 11a | White solid | 69.4 | 140—142 | 17f | White solid | 25.5 | 243—245 |
| 11b | White solid | 95.6 | 129—130 | 17g | Yellow solid | 59.3 | 134—136 |
| 14a | Yellow solid | 46.3 | 182—184 | 17h | Yellow solid | 50.8 | 126—128 |
| 14b | Yellow solid | 78.0 | 113—115 | 21 | White solid | 64.7 | 222—224 |
| 15a | Yellow solid | 81.7 | >250 | 22 | White solid | 78.7 | 176—178 |
| 15b | White solid | 40.5 | 209—211 | 24a | Yellow solid | 62.8 | 190—192 |
| 17a | White solid | 63.4 | 246—248 | 24b | Yellow solid | 94.2 | 240—242 |
| Compd. | 1H NMR |
|---|---|
| 3 | (300 MHz, CDCl3), δ: 8.00(s, 1H), 7.92(d, J=8.8 Hz, 2H), 6.67(d, J=8.7 Hz, 2H), 4.07(s, 2H), 3.93(s, 3H), 3.60(q, J=5.9 Hz, 2H), 3.39(t, J=5.8 Hz, 2H) |
| 6 | (300 MHz, CDCl3), δ: 8.08—7.95(m, 3H), 7.25(d, J=8.0 Hz, 2H), 4.78(s, 1H), 3.92(s, 3H), 3.44—3.35(m, 2H), 2.82(t, J=7.0 Hz, 2H), 1.43(s, 9H) |
| 8 | (300 MHz, CDCl3), δ: 8.10(s, 1H), 8.08(d, J=8.2 Hz, 2H), 7.34—7.31(d, J=2.0 Hz, 2H), 6.68(s, 1H), 4.07(s, 2H), 4.01(s, 3H), 3.64(q, J=6.7 Hz, 2H), 2.93(t, J=7.0 Hz, 2H). |
| 11a | (300 MHz, CDCl3), δ: 8.65(d, J=5.3 Hz, 1H), 8.61—8.60(m, 1H), 8.16(dd, J=9.8, 1.8 Hz, 1H), 7.68(d, J=5.3 Hz, 1H), 7.41(d, J=8.1 Hz, 1H), 3.95(s, 3H), 2.68(s, 3H) |
| 11b | (400 MHz, CDCl3), δ: 8.65(s, 1H), 8.61-8.60(d, J=2.0 Hz, 1H), 7.96(dd, J=10.0, 2.0 Hz, 1H), 7.41(d, J=8.0 Hz, 1H), 3.93(s, 3H), 2.69(s, 3H) |
| 14a | (300 MHz, CDCl3), δ: 8.71(d, J=5.3 Hz, 1H), 8.51—8,50(m, 1H), 8.46(dd, J=9.7, 1.7 Hz, 1H), 7.76(d, J=5.3 Hz, 1H), 7.64(d, J=8.0 Hz, 1H), 4.64(s, 2H), 4.46(s, 2H), 3.80(s, 3H) |
| 14b | (300 MHz, CDCl3), δ: 8.69(s, 1H), 8.44(s, 1H), 8.11(d, J=8.1 Hz, 1H), 7.63(d, J=8.1 Hz, 1H), 4.63(s, 2H), 4.46(s, 2H), 3.78(s, 3H) |
| 15a | (300 MHz, DMSO⁃d6), δ: 8.84(d, J=5.3 Hz, 1H), 8.48—8.43(m, 2H), 8.30(d, J=5.3 Hz, 1H), 7.82(d, J=8.0 Hz, 1H), 4.62(s, 2H), 4.32(s, 2H). |
| 15b | (300 MHz, DMSO⁃d6), δ: 12.98(s, 1H), 9.03(s, 1H), 8.14(s, 1H), 8.07(d, J=7.6 Hz, 1H), 7.83(d, J=8.3 Hz, 1H), 4.64(s, 2H), 4.33(s, 2H) |
| 17a | (300 MHz, DMSO⁃d6), δ: 8.85(d, J=5.2 Hz, 1H), 8.50—8.44(m, 3H), 8.31(d, J=5.3 Hz, 1H), 7.97(d, J=8.1 Hz, 2H), 7.82(d, J=8.3 Hz, 1H), 7.57—7.53(m, 2H), 6.30—6.28(m, 1H), 4.61—4.54(m, 3H), 4.30—4.15(m, 2H), 3.93—3.91(m, 4H), 3.76—3.70(m, 2H), 2.72—2.65(m, 2H) |
| 17b | (300 MHz, CDCl3), δ: 8.69(d, J=5.1 Hz, 1H), 8.41—8.38(m, 2H), 7.97(s, 1H), 7.78(d, J=8.5 Hz, 2H), 7.71(d, J=5.4 Hz, 1H), 7.56(d, J=8.2 Hz, 1H), 6.57(d, J=8.9 Hz, 2H), 4.54(s, 2H), 4.27(s, 2H), 3.94(s, 3H), 3.59—3.53(m, 2H), 3.40—3.35(m, 2H) |
| 17c | (300 MHz, CDCl3), δ: 8.71(d, J=5.3 Hz, 1H), 8.52(s, 1H), 8.46(dd, J=8.0, 1.7 Hz, 1H), 7.76(d, J=5.3 Hz, 1H), 7.65(d, J=8.0 Hz, 1H), 4.70(s, 2H), 4.51(s, 2H), 3.70—3.64(m, 6H), 2.63—2.55(m, 6H) |
| 17d | (300 MHz, CDCl3), δ: 8.71(d, J=5.3 Hz, 1H), 8.54—8.51(m, 1H), 8.45(dd, J=9.7, 1.7 Hz, 1H), 7.76(d, J=5.3 Hz, 1H), 7.64(d, J=8.4 Hz, 1H), 4.68(s, 2H), 4.50(s, 2H), 3.75—3.63(m, 10H), 2.70—2.58(m, 6H) |
| 17e | (300 MHz, CDCl3), δ: 8.72(d, J=5.3 Hz, 1H), 8.51—8.47(m, 2H), 7.78(d, J=5.2 Hz, 1H), 7.67(d, J=7.8 Hz, 1H), 4.70(s, 2H), 4.29(s, 2H), 3.77—3.74(m, 2H), 3.59(t, J=4.6 Hz, 4H), 3.52—3.48(m, 2H) |
| 17f | (400 MHz, CDCl3), δ: 8.70—8.67(m, 1H), 8.45—8.44(m, 1H), 8.08—8.06(m, 4H), 7.63—7.62 m, 1H), 7.46—7.44(m, 2H), 6.18—6.13(m, 1H), 4.70(s, 2H), 4.58—4.53(m, 2H), 4.28(s, 2H), 3.98(s, 3H), 3.86—3.82(m, 2H), 2.68—2.61(m, 2H) |
| 17g | (300 MHz, DMSO⁃d6), δ: 9.05(s, 1H), 8.14(s, 1H), 8.08—8.04(m, 1H), 7.83(d, J=8.0 Hz, 1H), 7.32—7.28(m, 2H), 6.70(d, J=9.6 Hz, 2H), 6.02—6.00(m, 1H), 4.62—4.53(m, 4H), 4.24—4.10(m, 2H), 3.71—3.69(m, 2H), 2.90(s, 6H), 2.60—2.56(m, 2H) |
| 17h | (300 MHz, DMSO⁃d6), δ: 9.05(s, 1H), 8.14(s, 1H), 8.07(dd, J=8.0, 1.6 Hz, 1H), 7.83(d, J=8.4 Hz, 1H), 6.91—6.84(m, 2H), 6.74—6.68(m, 2H), 4.61(s, 2H), 4.55(s, 2H), 3.66—3.49(m,8H), 2.80(s, 6H) |
| 21 | (300 MHz, DMSO⁃d6⁃D2O), δ: 8.86(d, J=5.3 Hz, 1H, ArH), 8.50—8.43(m, 2H, ArH), 8.22(d, J=6.2 Hz, 1H, ArH), 7.87(d, J=7.7 Hz, 1H, ArH), 4.68(s, 2H, CONC |
| Compd. | 1H NMR |
| 22 | (300 MHz, CDCl3), δ: 8.72(d, J=5.3 Hz, 1H), 8.52(s, 1H), 8.46(dd, J=8.0, 1.6 Hz, 1H), 7.75(d, J=5.2 Hz, 1H), 7.66(d, J=8.2 Hz, 1H), 6.64(s, 1H), 4.68(s, 2H), 4.33(s, 2H), 3.52—3.44(m, 4H). |
| 24a | (300 MHz, DMSO⁃d6), δ: 8.85(d, J=5.3 Hz, 1H), 8.48(s, 1H), 8.45(dd, J=8.0, 1.6 Hz, 1H), 8.30(d, J=5.3 Hz, 1H), 8.28—8.26(m, 1H), 8.06(s, 1H), 7.80(d, J=8.1 Hz, 1H), 4.55(s, 2H), 4.51(s, 2H), 4.43(t, J=5.8 Hz, 2H), 4.16(s, 2H), 3.53—3.46(m, 6H) |
| 24b | (300 MHz, DMSO⁃d6), δ: 8.85(d, J=5.7 Hz, 1H), 8.48(s, 1H), 8.45(dd, J=7.9, 1.6 Hz, 1H), 8.30—8.26(m, 2H), 7.84(s, 1H), 7.79(d, J=8.0 Hz, 1H), 5.02(s, 1H), 4.55(s, 2H), 4.39(t, J=6.1 Hz, 2H), 4.17(s, 2H), 3.53—3.46(m, 2H), 1.45(s, 6H) |
Table 2 1 H NMR data of compounds 11a, 11b, 14a, 14b, 15a, 15b, 17a—17h, 21, 22, 24a, 24b
| Compd. | 1H NMR |
|---|---|
| 3 | (300 MHz, CDCl3), δ: 8.00(s, 1H), 7.92(d, J=8.8 Hz, 2H), 6.67(d, J=8.7 Hz, 2H), 4.07(s, 2H), 3.93(s, 3H), 3.60(q, J=5.9 Hz, 2H), 3.39(t, J=5.8 Hz, 2H) |
| 6 | (300 MHz, CDCl3), δ: 8.08—7.95(m, 3H), 7.25(d, J=8.0 Hz, 2H), 4.78(s, 1H), 3.92(s, 3H), 3.44—3.35(m, 2H), 2.82(t, J=7.0 Hz, 2H), 1.43(s, 9H) |
| 8 | (300 MHz, CDCl3), δ: 8.10(s, 1H), 8.08(d, J=8.2 Hz, 2H), 7.34—7.31(d, J=2.0 Hz, 2H), 6.68(s, 1H), 4.07(s, 2H), 4.01(s, 3H), 3.64(q, J=6.7 Hz, 2H), 2.93(t, J=7.0 Hz, 2H). |
| 11a | (300 MHz, CDCl3), δ: 8.65(d, J=5.3 Hz, 1H), 8.61—8.60(m, 1H), 8.16(dd, J=9.8, 1.8 Hz, 1H), 7.68(d, J=5.3 Hz, 1H), 7.41(d, J=8.1 Hz, 1H), 3.95(s, 3H), 2.68(s, 3H) |
| 11b | (400 MHz, CDCl3), δ: 8.65(s, 1H), 8.61-8.60(d, J=2.0 Hz, 1H), 7.96(dd, J=10.0, 2.0 Hz, 1H), 7.41(d, J=8.0 Hz, 1H), 3.93(s, 3H), 2.69(s, 3H) |
| 14a | (300 MHz, CDCl3), δ: 8.71(d, J=5.3 Hz, 1H), 8.51—8,50(m, 1H), 8.46(dd, J=9.7, 1.7 Hz, 1H), 7.76(d, J=5.3 Hz, 1H), 7.64(d, J=8.0 Hz, 1H), 4.64(s, 2H), 4.46(s, 2H), 3.80(s, 3H) |
| 14b | (300 MHz, CDCl3), δ: 8.69(s, 1H), 8.44(s, 1H), 8.11(d, J=8.1 Hz, 1H), 7.63(d, J=8.1 Hz, 1H), 4.63(s, 2H), 4.46(s, 2H), 3.78(s, 3H) |
| 15a | (300 MHz, DMSO⁃d6), δ: 8.84(d, J=5.3 Hz, 1H), 8.48—8.43(m, 2H), 8.30(d, J=5.3 Hz, 1H), 7.82(d, J=8.0 Hz, 1H), 4.62(s, 2H), 4.32(s, 2H). |
| 15b | (300 MHz, DMSO⁃d6), δ: 12.98(s, 1H), 9.03(s, 1H), 8.14(s, 1H), 8.07(d, J=7.6 Hz, 1H), 7.83(d, J=8.3 Hz, 1H), 4.64(s, 2H), 4.33(s, 2H) |
| 17a | (300 MHz, DMSO⁃d6), δ: 8.85(d, J=5.2 Hz, 1H), 8.50—8.44(m, 3H), 8.31(d, J=5.3 Hz, 1H), 7.97(d, J=8.1 Hz, 2H), 7.82(d, J=8.3 Hz, 1H), 7.57—7.53(m, 2H), 6.30—6.28(m, 1H), 4.61—4.54(m, 3H), 4.30—4.15(m, 2H), 3.93—3.91(m, 4H), 3.76—3.70(m, 2H), 2.72—2.65(m, 2H) |
| 17b | (300 MHz, CDCl3), δ: 8.69(d, J=5.1 Hz, 1H), 8.41—8.38(m, 2H), 7.97(s, 1H), 7.78(d, J=8.5 Hz, 2H), 7.71(d, J=5.4 Hz, 1H), 7.56(d, J=8.2 Hz, 1H), 6.57(d, J=8.9 Hz, 2H), 4.54(s, 2H), 4.27(s, 2H), 3.94(s, 3H), 3.59—3.53(m, 2H), 3.40—3.35(m, 2H) |
| 17c | (300 MHz, CDCl3), δ: 8.71(d, J=5.3 Hz, 1H), 8.52(s, 1H), 8.46(dd, J=8.0, 1.7 Hz, 1H), 7.76(d, J=5.3 Hz, 1H), 7.65(d, J=8.0 Hz, 1H), 4.70(s, 2H), 4.51(s, 2H), 3.70—3.64(m, 6H), 2.63—2.55(m, 6H) |
| 17d | (300 MHz, CDCl3), δ: 8.71(d, J=5.3 Hz, 1H), 8.54—8.51(m, 1H), 8.45(dd, J=9.7, 1.7 Hz, 1H), 7.76(d, J=5.3 Hz, 1H), 7.64(d, J=8.4 Hz, 1H), 4.68(s, 2H), 4.50(s, 2H), 3.75—3.63(m, 10H), 2.70—2.58(m, 6H) |
| 17e | (300 MHz, CDCl3), δ: 8.72(d, J=5.3 Hz, 1H), 8.51—8.47(m, 2H), 7.78(d, J=5.2 Hz, 1H), 7.67(d, J=7.8 Hz, 1H), 4.70(s, 2H), 4.29(s, 2H), 3.77—3.74(m, 2H), 3.59(t, J=4.6 Hz, 4H), 3.52—3.48(m, 2H) |
| 17f | (400 MHz, CDCl3), δ: 8.70—8.67(m, 1H), 8.45—8.44(m, 1H), 8.08—8.06(m, 4H), 7.63—7.62 m, 1H), 7.46—7.44(m, 2H), 6.18—6.13(m, 1H), 4.70(s, 2H), 4.58—4.53(m, 2H), 4.28(s, 2H), 3.98(s, 3H), 3.86—3.82(m, 2H), 2.68—2.61(m, 2H) |
| 17g | (300 MHz, DMSO⁃d6), δ: 9.05(s, 1H), 8.14(s, 1H), 8.08—8.04(m, 1H), 7.83(d, J=8.0 Hz, 1H), 7.32—7.28(m, 2H), 6.70(d, J=9.6 Hz, 2H), 6.02—6.00(m, 1H), 4.62—4.53(m, 4H), 4.24—4.10(m, 2H), 3.71—3.69(m, 2H), 2.90(s, 6H), 2.60—2.56(m, 2H) |
| 17h | (300 MHz, DMSO⁃d6), δ: 9.05(s, 1H), 8.14(s, 1H), 8.07(dd, J=8.0, 1.6 Hz, 1H), 7.83(d, J=8.4 Hz, 1H), 6.91—6.84(m, 2H), 6.74—6.68(m, 2H), 4.61(s, 2H), 4.55(s, 2H), 3.66—3.49(m,8H), 2.80(s, 6H) |
| 21 | (300 MHz, DMSO⁃d6⁃D2O), δ: 8.86(d, J=5.3 Hz, 1H, ArH), 8.50—8.43(m, 2H, ArH), 8.22(d, J=6.2 Hz, 1H, ArH), 7.87(d, J=7.7 Hz, 1H, ArH), 4.68(s, 2H, CONC |
| Compd. | 1H NMR |
| 22 | (300 MHz, CDCl3), δ: 8.72(d, J=5.3 Hz, 1H), 8.52(s, 1H), 8.46(dd, J=8.0, 1.6 Hz, 1H), 7.75(d, J=5.2 Hz, 1H), 7.66(d, J=8.2 Hz, 1H), 6.64(s, 1H), 4.68(s, 2H), 4.33(s, 2H), 3.52—3.44(m, 4H). |
| 24a | (300 MHz, DMSO⁃d6), δ: 8.85(d, J=5.3 Hz, 1H), 8.48(s, 1H), 8.45(dd, J=8.0, 1.6 Hz, 1H), 8.30(d, J=5.3 Hz, 1H), 8.28—8.26(m, 1H), 8.06(s, 1H), 7.80(d, J=8.1 Hz, 1H), 4.55(s, 2H), 4.51(s, 2H), 4.43(t, J=5.8 Hz, 2H), 4.16(s, 2H), 3.53—3.46(m, 6H) |
| 24b | (300 MHz, DMSO⁃d6), δ: 8.85(d, J=5.7 Hz, 1H), 8.48(s, 1H), 8.45(dd, J=7.9, 1.6 Hz, 1H), 8.30—8.26(m, 2H), 7.84(s, 1H), 7.79(d, J=8.0 Hz, 1H), 5.02(s, 1H), 4.55(s, 2H), 4.39(t, J=6.1 Hz, 2H), 4.17(s, 2H), 3.53—3.46(m, 2H), 1.45(s, 6H) |
| Compd. | Appearance | m. p./℃ | Yield(%) | HRMS(calcd.), m/z[M+H]+ |
|---|---|---|---|---|
| 19a | White solid | >250 | 17.8 | 591.2832(591.2833) |
| 19b | White solid | 254—256 | 58.6 | 625.2437(625.2431) |
| 19c | Yellow solid | >250 | 46.7 | 587.2626(587.2622) |
| 19d | Yellow solid | 279—281 | 68.4 | 621.2236(621.2247) |
| 19e | White solid | 219—221 | 61.7 | 568.2784(568.2784) |
| 19f | Yellow solid | 170—172 | 48.6 | 481.2563(481.2555) |
| 19g | White solid | 139—141 | 44.5 | 525.2825(525.2828) |
| 19h | White solid | 188—190 | 59.1 | 456.2247(456.2234) |
| 19i | White solid | 215—217 | 65.3 | 587.2532(587.2530) |
| 19j | White solid | 222—224 | 77.2 | 612.2460(612.2441) |
| 25a | Yellow solid | 100—102 | 85.1 | 537.2574(537.2571) |
| 25b | White solid | 118—120 | 90.2 | 521.2625(521.2617) |
| 27a | White solid | 139—141 | 52.9 | 669.3078(669.3085) |
| 27b | White solid | 140—142 | 41.2 | 654.2969(654.2977) |
Table 3 Appearances, melting points, yields and HRMS data of compounds 19a—19j, 25a, 25b, 27a and 27b
| Compd. | Appearance | m. p./℃ | Yield(%) | HRMS(calcd.), m/z[M+H]+ |
|---|---|---|---|---|
| 19a | White solid | >250 | 17.8 | 591.2832(591.2833) |
| 19b | White solid | 254—256 | 58.6 | 625.2437(625.2431) |
| 19c | Yellow solid | >250 | 46.7 | 587.2626(587.2622) |
| 19d | Yellow solid | 279—281 | 68.4 | 621.2236(621.2247) |
| 19e | White solid | 219—221 | 61.7 | 568.2784(568.2784) |
| 19f | Yellow solid | 170—172 | 48.6 | 481.2563(481.2555) |
| 19g | White solid | 139—141 | 44.5 | 525.2825(525.2828) |
| 19h | White solid | 188—190 | 59.1 | 456.2247(456.2234) |
| 19i | White solid | 215—217 | 65.3 | 587.2532(587.2530) |
| 19j | White solid | 222—224 | 77.2 | 612.2460(612.2441) |
| 25a | Yellow solid | 100—102 | 85.1 | 537.2574(537.2571) |
| 25b | White solid | 118—120 | 90.2 | 521.2625(521.2617) |
| 27a | White solid | 139—141 | 52.9 | 669.3078(669.3085) |
| 27b | White solid | 140—142 | 41.2 | 654.2969(654.2977) |
| Compd. | 1H NMR | 13C NMR |
|---|---|---|
| 19a | (300 MHz, CDCl3), δ: 8.50(s, 1H), 8.38(d, J=5.3 Hz, 1H), 8.30(dd, J=8.1, 1.7 Hz, 1H), 8.11—8.08(m, 3H), 7.59(d, J=8.0 Hz, 1H), 7.49—7.44(m, 2H), 7.08(d, J=5.3 Hz, 1H), 6.20—6.15(m, 1H), 5.26(d, J=8.4 Hz, 1H), 4.69(s, 2H), 4.60(s, 1H), 4.55(s, 1H), 4.31—4.30(m, 2H), 4.23—4.19(m, 1H), 4.07—4.0(m, 5H), 3.90—3.82(m, 2H), 3.66—3.58(m, 2H), 2.69—2.64(m, 2H), 2.15—2.11(m, 2H), 1.63— 1.58(m, 2H) | (75 MHz, DMSO⁃d6), δ: 167.99, 166.75, 166.58, 162.27, 161.27, 159.65, 146.15, 144.91, 140.60, 134.82, 134.38, 133.14, 130.31, 126.21, 125.47, 124.34, 121.72, 121.43, 121.14,106.14, 72.75, 66.59, 60.72, 51.03, 47.21, 44.05, 36.44, 32.98, 27.33 |
| 19b | (300 MHz, DMSO⁃d6), δ: 8.51(s, 1H), 8.44(s, 1H), 8.04—7.96(m, 4H), 7.75(d, J=8.4 Hz, 1H), 7.57—7.54(m, 2H), 6.29(s, 1H), 4.59—4.54(m, 4H), 4.29—4.15(m, 2H), 3.92—3.85(m, 6H), 3.76—3.73(m, 2H), 3.41—3.37(m, 2H), 2.66—2.54(m, 2H), 1.87—1.83(m, 2H), 1.55—1.51(m, 2H) | (75 MHz, acetic⁃d4), δ: 169.74, 167.74, 160.38, 145.52, 144.50, 141.34, 135.50, 134.44, 132.86, 131.57, 128.40, 126.53, 125.14, 124.67, 122.99, 120.76, 115.97, 115.73, 104.31, 99.06, 66.06, 51.68, 47.26, 35.99, 31.92, 26.88, 19.64, 19.38, 19.12 |
| 19c | (400 MHz, DMSO⁃d6), δ: 9.56(s, 1H), 8.52—8.40(m, 4H), 8.48(s, 1H), 7.98(d, J=8.3 Hz, 2H), 7.92(s, 1H), 7.79(d, J=8.0 Hz, 1H), 7.57(t, J=7.2 Hz, 3H), 7.42(d, J=5.2 Hz, 1H), 6.30(s, 1H), 4.61—4.55(m, 4H), 4.30(s, 1H), 4.16(s, 1H), 3.92(s, 3H), 3.83(s, 3H), 3.76—3.70(m, 2H), 2.67—2.54(m, 2H) | (75 MHz, aectic⁃d4), δ: 169.65, 167.47, 160.51, 145.56, 143.16, 141.20, 140.14, 139.16, 136.38, 135.48, 134.41, 132.39, 131.43, 128.45, 126.49, 125.09, 123.97, 123.11, 121.59, 114.73, 107.02, 96.29, 90.58, 51.74, 38.06, 35.93, 35.45, 19.64, 19.38, 19.12 |
| 19d | (400 MHz, DMSO⁃d6), δ: 9.83(s, 1H), 8.58(s, 1H), 8.51(s, 1H), 8.11(s, 1H), 8.06(d, J=8.0 Hz, 1H), 7.98(d, J=8.5 Hz, 2H), 7.83—7.79(m, 2H), 7.56(dd, J=8.2, 6.2 Hz, 2H), 7.52(s, 1H), 6.30(s, 1H), 4.61—4.55(m, 4H), 4.30(s, 1H), 4.16(s, 1H), 3.92(s, 3H), 3.79—3.71(m, 5H), 2.66—2.54(m, 2H) | (75 MHz, acetic⁃d4), δ: 169.76, 167.58, 159.65, 157.54, 149.08, 145.21, 144.61, 141.60, 134.40, 132.93, 131.68, 126.63, 125.18, 124.70, 123.17, 122.56, 120.93, 119.97, 116.95, 101.59, 98.04, 90.16, 86.42, 64.75, 51.73, 37.95, 36.24, 19.65, 19.38, 19.12 |
| 19e | (300 MHz, CDCl3), δ: 8.44(s, 1H), 8.38(d, J=5.3 Hz, 1H), 8.26(dd, J=7.5, 1.7 Hz, 1H), 7.99(s, 1H), 7.86(d, J=8.5 Hz, 2H), 7.52(d, J=8.2 Hz, 1H), 7.04(d, J=5.0 Hz, 1H), 6.62(d, J=8.5 Hz, 2H), 4.48(s, 1H), 4.28(s, 2H), 4.21—4.15(m, 1H), 4.06—4.02(m, 2H), 3.94(s, 3H), 3.65—3.53(m, 4H), 3.36(t, J=6.2 Hz, 2H), 2.15—2.09(m, 2H), 1.66—1.60(m, 2H) | (75 MHz, DMSO⁃d6), δ: 168.70, 168.35, 163.20, 162.11, 162.04, 149.70, 145.42, 144.93, 137.35, 132.83, 130.49, 127.37, 124.37, 121.45, 118.97, 114.94, 112.21, 106.16, 66.58, 51.22, 47.10, 45.43, 42.29, 36.14, 32.83, 27.27 |
| 19f | (300 MHz, CDCl3), δ: 8.42(s, 1H), 8.33(d, J=5.2 Hz, 1H), 8.24(d, J=9.8 Hz, 1H), 7.53(d, J=8.9 Hz, 1H), 7.01(d, J=5.5 Hz, 1H), 5.15(d, J=6.8 Hz, 1H), 4.60(s, 2H), 4.44(s, 2H), 4.19—4.09(m, 1H), 4.01—3.97(m, 2H), 3.64—3.52(m, 8H), 2.57—2.50(m, 6H), 2.08—2.04(m, 2H), 1.84(brs, 1H), 1.63—1.52(m, 2H) | (75 MHz, DMSO⁃d6), δ: 167.92, 166.32, 163.17, 162.27, 159.61, 144.87, 137.47, 133.10, 130.30, 124.31, 121.43, 106.14, 66.59, 60.54, 58.96, 53.63, 53.23, 50.99, 47.21, 44.59, 43.87, 41.93, 32.98(Affected by steric hindrance, the four carbon atoms in piperazine show different displacements) |
| 19g | (300 MHz, CDCl3), δ: 8.48(s, 1H, ArH), 8.39(d, J=5.2 Hz, 1H), 8.30(dd, J=8.0, 1.7 Hz, 1H), 7.58(d, J=8.2 Hz, 1H), 7.07(d, J=6.0 Hz, 1H), 5.18(d, J=7.8 Hz, 1H), 4.65(s, 2H), 4.49(s, 2H), 4.24—4.17(m, 1H), 4.06—4.02(m, 2H), 3.75—3.58(m, 12H), 2.72—2.64(m, 6H), 2.16—2.09(m, 2H), 1.65—1.61(m, 3H) | (75 MHz, DMSO⁃d6), δ:167.92, 166.33, 162.27, 159.72, 144.87, 137.48, 133.11, 130.31, 124.33, 121.44, 116.58, 106.15, 72.69, 68.67, 66.59, 60.71, 57.57, 53.59, 53.18, 50.99, 47.21, 44.57, 43.88, 32.98. |
| 19h | (300 MHz, CDCl3), δ: 8.50(s, 1H), 8.39(d, J=5.5 Hz, 1H), 8.32(dd, J=8.0, 1.7 Hz, 1H), 7.61(d, J=7.7 Hz, 1H), 7.18—7.15(m, 1H), 7.08(d, J=5.3 Hz, 1H), 5.46—5.42(m, 1H), 4.67(s, 2H), 4.29(s, 2H), 4.22—4.18(m, 1H), 4.07—4.03(m, 2H), 3.75(t, J=4.5 Hz, 2H), 3.67—3.58(m, 6H), 3.51—3.48(m, 2H), 2.16—2.10(m, 2H), 1.69—1.63(m, 3H) | (75 MHz, DMSO⁃d6), δ: 168.34, 167.88, 163.17, 162.27, 159.64, 144.88, 137.44, 133.14, 130.29, 124.28, 121.44, 106.12, 72.58, 69.35, 66.60, 60.65, 51.08, 47.22, 45.25, 36.44, 32.97 |
| 19i | (300 MHz, DMSO⁃d6), δ: 8.45(s, 1H), 8.03(s, 1H), 7.99(d, J=6.5 Hz, 1H), 7.76(d, J=9.0 Hz, 1H), 7.63(s, 1H), 7.33—7.28(m, 2H), 6.71(d, J=9.3 Hz, 2H), 6.02—6.00(m, 1H), 4.58—4.45(m, 4H), 4.23—4.10(m, 2H), 3.90—3.85(m, 3H), 3.74—3.67(m, 4H), 2.90(s, 6H), 2.59—2.55(m, 2H), 1.88—1.82(m, 2H), 1.58—1.49(m, 2H) | (75 MHz, acetic⁃d4), δ: 169.76, 163.66, 157.73, 146.51, 145.71, 144.43, 132.93, 131.53, 126.45, 124.63, 122.99, 120.45, 120.09, 115.69, 110.54, 109.86, 108.66, 98.84, 66.06, 51.64, 47.23, 45.42, 31.94, 25.79, 19.64, 19.38, 19.11 |
| 19j | (300 MHz, DMSO⁃d6), δ: 8.45(s, 1H), 8.04(s, 1H), 7.99(d, J=8.4 Hz, 1H), 7.75(d, J=7.9 Hz, 1H), 7.63(s, 1H), 6.90—6.86(m, 2H), 6.73—6.68(s, 2H), 4.56(d, J=11.9 Hz, 4H), 3.95—3.84(m, 3H), 3.63(d, J=15.9 Hz, 4H), 3.41—3.37(m, 2H), 3.05—2.96(m, 4H), 2.78(s, 6H), 1.86—1.81(m, 2H), 1.59—1.46(m, 2H) | (75 MHz, acetic⁃d4), δ: 169.74, 167.59, 163.30, 144.42, 143.85, 136.64, 135.56, 135.16, 132.94, 131.48, 126.61, 124.62, 123.01, 120.62, 117.38, 110.20, 66.06, 47.24, 45.56, 31.93, 25.89, 19.65, 19.38, 19.12 |
| 25a | (300 MHz, DMSO⁃d6), δ: 8.40(s, 1H), 8.37(d, J=5.2 Hz, 1H | (75 MHz, DMSO⁃d6), δ: 168.24, 167.44, 161.73, 159.12, 151.81, 148.31, 144.41, 143.97, 132.59, 129.84, 124.17, 123.78, 120.96, 105.63, 77.24, 71.49, 66.09, 63.51, 60.12, 50.51, 48.54, 46.72, 44.89, 32.47 |
| 25b | (300 MHz, DMSO⁃d6), δ: 8.40—8.24(m, 4H), 7.83(s, 1H), 7.70(d, J=8.1 Hz, 1H), 7.26(s, 1H), 7.26(s, 1H), 7.23(d, J=5.2 Hz, 1H), 5.01(s, 1H), 4.51(s, 2H), 4.39(t, J=5.9 Hz, 2H), 4.15(s, 2H), 4.05—3.98(m, 1H), 3.90—3.87(m, 2H), 3.54—3.37(m, 4H), 1.90—1.85(m, 2H), 1.59—1.51(m, 2H), 1.45(s, 6H) | (75 MHz, DMSO⁃d6), δ: 167.56, 167.14, 160.75, 158.35, 155.10, 154.71, 143.63, 136.03, 131.47, 129.23, 123.04, 120.10, 104.90, 99.87, 66.26, 65.29, 49.86, 47.58, 45.82, 44.07, 31.52, 29.54, 21.84 |
| 27a | (300 MHz, CDCl3), δ: 9.03(s, 1H), 8.13(t, J=1.3 Hz, 1H), 7.98—7.94(m, 3H), 7.89(dd, J=9.3, 2.5 Hz, 1H), 7.75(d, J=8.7 Hz, 2H), 7.44(d, J=1.3 Hz, 2H), 6.69(d, J=9.3 Hz, 1H), 6.51(d, J=8.7 Hz, 1H), 5.34(hept, J=6.8 Hz, 1H), 4.81(s, 1H), 3.89(s, 3H), 3.74—3.67(m, 2H), 3.49—3.32(m, 4H), 3.13—2.99(m, 2H), 2.843—2.53(m, 3H), 2.06—1.99(m, 4H), 1.42—1.40(m, 6H) | (75 MHz, CDCl3), δ: 172.20, 170.86, 163.17, 161.73, 149.06, 143.99, 142.20, 139.37, 138.19, 131.61, 127.51, 125.25, 121.85, 120.73, 120.59, 119.75, 113.31, 112.30, 111.49, 110.99, 63.37, 57.65, 56.97, 53.15, 46.86, 43.70, 37.28, 36.16, 22.04, 13.93 |
| 27b | (300 MHz, CDCl3), δ: 9.04(s, 1H), 8.31(s, 1H), 8.06—7.94(m, 6H), 7.55—7.45(m, 3H), 7.34(s, 1H), 6.77(d, J=9.3 Hz, 1H), 5.41(hept, J=7.4 Hz, 1H), 3.97(s, 3H), 3.69—3.62(m, 2H), 3.50—3.47(m, 1H), 3.38—3.32(m, 1H), 2.99 — 2.71(m, 6H), 2.68—2.64(m, 1H), 2.11(s, 3H), 2.05—2.03(m, 1H), 1.51—1.49(m, 6H) | (75 MHz, CDCl3), δ: 171.13, 170.33, 162.54, 161.78, 144.33, 141.88, 140.13, 139.21, 138.28, 131.90, 131.49,129.18, 129.09, 126.46, 121.28, 120.71, 120.46, 113.64, 110.89, 110.81, 63.35, 58.36, 57.68, 53.56, 46.90, 40.02, 36.65, 36.28, 35.33, 22.07, 13.90 |
Table 4 1H NMR and 13C NMR data of compounds 19a—19j, 25a, 25b, 27a and 27b
| Compd. | 1H NMR | 13C NMR |
|---|---|---|
| 19a | (300 MHz, CDCl3), δ: 8.50(s, 1H), 8.38(d, J=5.3 Hz, 1H), 8.30(dd, J=8.1, 1.7 Hz, 1H), 8.11—8.08(m, 3H), 7.59(d, J=8.0 Hz, 1H), 7.49—7.44(m, 2H), 7.08(d, J=5.3 Hz, 1H), 6.20—6.15(m, 1H), 5.26(d, J=8.4 Hz, 1H), 4.69(s, 2H), 4.60(s, 1H), 4.55(s, 1H), 4.31—4.30(m, 2H), 4.23—4.19(m, 1H), 4.07—4.0(m, 5H), 3.90—3.82(m, 2H), 3.66—3.58(m, 2H), 2.69—2.64(m, 2H), 2.15—2.11(m, 2H), 1.63— 1.58(m, 2H) | (75 MHz, DMSO⁃d6), δ: 167.99, 166.75, 166.58, 162.27, 161.27, 159.65, 146.15, 144.91, 140.60, 134.82, 134.38, 133.14, 130.31, 126.21, 125.47, 124.34, 121.72, 121.43, 121.14,106.14, 72.75, 66.59, 60.72, 51.03, 47.21, 44.05, 36.44, 32.98, 27.33 |
| 19b | (300 MHz, DMSO⁃d6), δ: 8.51(s, 1H), 8.44(s, 1H), 8.04—7.96(m, 4H), 7.75(d, J=8.4 Hz, 1H), 7.57—7.54(m, 2H), 6.29(s, 1H), 4.59—4.54(m, 4H), 4.29—4.15(m, 2H), 3.92—3.85(m, 6H), 3.76—3.73(m, 2H), 3.41—3.37(m, 2H), 2.66—2.54(m, 2H), 1.87—1.83(m, 2H), 1.55—1.51(m, 2H) | (75 MHz, acetic⁃d4), δ: 169.74, 167.74, 160.38, 145.52, 144.50, 141.34, 135.50, 134.44, 132.86, 131.57, 128.40, 126.53, 125.14, 124.67, 122.99, 120.76, 115.97, 115.73, 104.31, 99.06, 66.06, 51.68, 47.26, 35.99, 31.92, 26.88, 19.64, 19.38, 19.12 |
| 19c | (400 MHz, DMSO⁃d6), δ: 9.56(s, 1H), 8.52—8.40(m, 4H), 8.48(s, 1H), 7.98(d, J=8.3 Hz, 2H), 7.92(s, 1H), 7.79(d, J=8.0 Hz, 1H), 7.57(t, J=7.2 Hz, 3H), 7.42(d, J=5.2 Hz, 1H), 6.30(s, 1H), 4.61—4.55(m, 4H), 4.30(s, 1H), 4.16(s, 1H), 3.92(s, 3H), 3.83(s, 3H), 3.76—3.70(m, 2H), 2.67—2.54(m, 2H) | (75 MHz, aectic⁃d4), δ: 169.65, 167.47, 160.51, 145.56, 143.16, 141.20, 140.14, 139.16, 136.38, 135.48, 134.41, 132.39, 131.43, 128.45, 126.49, 125.09, 123.97, 123.11, 121.59, 114.73, 107.02, 96.29, 90.58, 51.74, 38.06, 35.93, 35.45, 19.64, 19.38, 19.12 |
| 19d | (400 MHz, DMSO⁃d6), δ: 9.83(s, 1H), 8.58(s, 1H), 8.51(s, 1H), 8.11(s, 1H), 8.06(d, J=8.0 Hz, 1H), 7.98(d, J=8.5 Hz, 2H), 7.83—7.79(m, 2H), 7.56(dd, J=8.2, 6.2 Hz, 2H), 7.52(s, 1H), 6.30(s, 1H), 4.61—4.55(m, 4H), 4.30(s, 1H), 4.16(s, 1H), 3.92(s, 3H), 3.79—3.71(m, 5H), 2.66—2.54(m, 2H) | (75 MHz, acetic⁃d4), δ: 169.76, 167.58, 159.65, 157.54, 149.08, 145.21, 144.61, 141.60, 134.40, 132.93, 131.68, 126.63, 125.18, 124.70, 123.17, 122.56, 120.93, 119.97, 116.95, 101.59, 98.04, 90.16, 86.42, 64.75, 51.73, 37.95, 36.24, 19.65, 19.38, 19.12 |
| 19e | (300 MHz, CDCl3), δ: 8.44(s, 1H), 8.38(d, J=5.3 Hz, 1H), 8.26(dd, J=7.5, 1.7 Hz, 1H), 7.99(s, 1H), 7.86(d, J=8.5 Hz, 2H), 7.52(d, J=8.2 Hz, 1H), 7.04(d, J=5.0 Hz, 1H), 6.62(d, J=8.5 Hz, 2H), 4.48(s, 1H), 4.28(s, 2H), 4.21—4.15(m, 1H), 4.06—4.02(m, 2H), 3.94(s, 3H), 3.65—3.53(m, 4H), 3.36(t, J=6.2 Hz, 2H), 2.15—2.09(m, 2H), 1.66—1.60(m, 2H) | (75 MHz, DMSO⁃d6), δ: 168.70, 168.35, 163.20, 162.11, 162.04, 149.70, 145.42, 144.93, 137.35, 132.83, 130.49, 127.37, 124.37, 121.45, 118.97, 114.94, 112.21, 106.16, 66.58, 51.22, 47.10, 45.43, 42.29, 36.14, 32.83, 27.27 |
| 19f | (300 MHz, CDCl3), δ: 8.42(s, 1H), 8.33(d, J=5.2 Hz, 1H), 8.24(d, J=9.8 Hz, 1H), 7.53(d, J=8.9 Hz, 1H), 7.01(d, J=5.5 Hz, 1H), 5.15(d, J=6.8 Hz, 1H), 4.60(s, 2H), 4.44(s, 2H), 4.19—4.09(m, 1H), 4.01—3.97(m, 2H), 3.64—3.52(m, 8H), 2.57—2.50(m, 6H), 2.08—2.04(m, 2H), 1.84(brs, 1H), 1.63—1.52(m, 2H) | (75 MHz, DMSO⁃d6), δ: 167.92, 166.32, 163.17, 162.27, 159.61, 144.87, 137.47, 133.10, 130.30, 124.31, 121.43, 106.14, 66.59, 60.54, 58.96, 53.63, 53.23, 50.99, 47.21, 44.59, 43.87, 41.93, 32.98(Affected by steric hindrance, the four carbon atoms in piperazine show different displacements) |
| 19g | (300 MHz, CDCl3), δ: 8.48(s, 1H, ArH), 8.39(d, J=5.2 Hz, 1H), 8.30(dd, J=8.0, 1.7 Hz, 1H), 7.58(d, J=8.2 Hz, 1H), 7.07(d, J=6.0 Hz, 1H), 5.18(d, J=7.8 Hz, 1H), 4.65(s, 2H), 4.49(s, 2H), 4.24—4.17(m, 1H), 4.06—4.02(m, 2H), 3.75—3.58(m, 12H), 2.72—2.64(m, 6H), 2.16—2.09(m, 2H), 1.65—1.61(m, 3H) | (75 MHz, DMSO⁃d6), δ:167.92, 166.33, 162.27, 159.72, 144.87, 137.48, 133.11, 130.31, 124.33, 121.44, 116.58, 106.15, 72.69, 68.67, 66.59, 60.71, 57.57, 53.59, 53.18, 50.99, 47.21, 44.57, 43.88, 32.98. |
| 19h | (300 MHz, CDCl3), δ: 8.50(s, 1H), 8.39(d, J=5.5 Hz, 1H), 8.32(dd, J=8.0, 1.7 Hz, 1H), 7.61(d, J=7.7 Hz, 1H), 7.18—7.15(m, 1H), 7.08(d, J=5.3 Hz, 1H), 5.46—5.42(m, 1H), 4.67(s, 2H), 4.29(s, 2H), 4.22—4.18(m, 1H), 4.07—4.03(m, 2H), 3.75(t, J=4.5 Hz, 2H), 3.67—3.58(m, 6H), 3.51—3.48(m, 2H), 2.16—2.10(m, 2H), 1.69—1.63(m, 3H) | (75 MHz, DMSO⁃d6), δ: 168.34, 167.88, 163.17, 162.27, 159.64, 144.88, 137.44, 133.14, 130.29, 124.28, 121.44, 106.12, 72.58, 69.35, 66.60, 60.65, 51.08, 47.22, 45.25, 36.44, 32.97 |
| 19i | (300 MHz, DMSO⁃d6), δ: 8.45(s, 1H), 8.03(s, 1H), 7.99(d, J=6.5 Hz, 1H), 7.76(d, J=9.0 Hz, 1H), 7.63(s, 1H), 7.33—7.28(m, 2H), 6.71(d, J=9.3 Hz, 2H), 6.02—6.00(m, 1H), 4.58—4.45(m, 4H), 4.23—4.10(m, 2H), 3.90—3.85(m, 3H), 3.74—3.67(m, 4H), 2.90(s, 6H), 2.59—2.55(m, 2H), 1.88—1.82(m, 2H), 1.58—1.49(m, 2H) | (75 MHz, acetic⁃d4), δ: 169.76, 163.66, 157.73, 146.51, 145.71, 144.43, 132.93, 131.53, 126.45, 124.63, 122.99, 120.45, 120.09, 115.69, 110.54, 109.86, 108.66, 98.84, 66.06, 51.64, 47.23, 45.42, 31.94, 25.79, 19.64, 19.38, 19.11 |
| 19j | (300 MHz, DMSO⁃d6), δ: 8.45(s, 1H), 8.04(s, 1H), 7.99(d, J=8.4 Hz, 1H), 7.75(d, J=7.9 Hz, 1H), 7.63(s, 1H), 6.90—6.86(m, 2H), 6.73—6.68(s, 2H), 4.56(d, J=11.9 Hz, 4H), 3.95—3.84(m, 3H), 3.63(d, J=15.9 Hz, 4H), 3.41—3.37(m, 2H), 3.05—2.96(m, 4H), 2.78(s, 6H), 1.86—1.81(m, 2H), 1.59—1.46(m, 2H) | (75 MHz, acetic⁃d4), δ: 169.74, 167.59, 163.30, 144.42, 143.85, 136.64, 135.56, 135.16, 132.94, 131.48, 126.61, 124.62, 123.01, 120.62, 117.38, 110.20, 66.06, 47.24, 45.56, 31.93, 25.89, 19.65, 19.38, 19.12 |
| 25a | (300 MHz, DMSO⁃d6), δ: 8.40(s, 1H), 8.37(d, J=5.2 Hz, 1H | (75 MHz, DMSO⁃d6), δ: 168.24, 167.44, 161.73, 159.12, 151.81, 148.31, 144.41, 143.97, 132.59, 129.84, 124.17, 123.78, 120.96, 105.63, 77.24, 71.49, 66.09, 63.51, 60.12, 50.51, 48.54, 46.72, 44.89, 32.47 |
| 25b | (300 MHz, DMSO⁃d6), δ: 8.40—8.24(m, 4H), 7.83(s, 1H), 7.70(d, J=8.1 Hz, 1H), 7.26(s, 1H), 7.26(s, 1H), 7.23(d, J=5.2 Hz, 1H), 5.01(s, 1H), 4.51(s, 2H), 4.39(t, J=5.9 Hz, 2H), 4.15(s, 2H), 4.05—3.98(m, 1H), 3.90—3.87(m, 2H), 3.54—3.37(m, 4H), 1.90—1.85(m, 2H), 1.59—1.51(m, 2H), 1.45(s, 6H) | (75 MHz, DMSO⁃d6), δ: 167.56, 167.14, 160.75, 158.35, 155.10, 154.71, 143.63, 136.03, 131.47, 129.23, 123.04, 120.10, 104.90, 99.87, 66.26, 65.29, 49.86, 47.58, 45.82, 44.07, 31.52, 29.54, 21.84 |
| 27a | (300 MHz, CDCl3), δ: 9.03(s, 1H), 8.13(t, J=1.3 Hz, 1H), 7.98—7.94(m, 3H), 7.89(dd, J=9.3, 2.5 Hz, 1H), 7.75(d, J=8.7 Hz, 2H), 7.44(d, J=1.3 Hz, 2H), 6.69(d, J=9.3 Hz, 1H), 6.51(d, J=8.7 Hz, 1H), 5.34(hept, J=6.8 Hz, 1H), 4.81(s, 1H), 3.89(s, 3H), 3.74—3.67(m, 2H), 3.49—3.32(m, 4H), 3.13—2.99(m, 2H), 2.843—2.53(m, 3H), 2.06—1.99(m, 4H), 1.42—1.40(m, 6H) | (75 MHz, CDCl3), δ: 172.20, 170.86, 163.17, 161.73, 149.06, 143.99, 142.20, 139.37, 138.19, 131.61, 127.51, 125.25, 121.85, 120.73, 120.59, 119.75, 113.31, 112.30, 111.49, 110.99, 63.37, 57.65, 56.97, 53.15, 46.86, 43.70, 37.28, 36.16, 22.04, 13.93 |
| 27b | (300 MHz, CDCl3), δ: 9.04(s, 1H), 8.31(s, 1H), 8.06—7.94(m, 6H), 7.55—7.45(m, 3H), 7.34(s, 1H), 6.77(d, J=9.3 Hz, 1H), 5.41(hept, J=7.4 Hz, 1H), 3.97(s, 3H), 3.69—3.62(m, 2H), 3.50—3.47(m, 1H), 3.38—3.32(m, 1H), 2.99 — 2.71(m, 6H), 2.68—2.64(m, 1H), 2.11(s, 3H), 2.05—2.03(m, 1H), 1.51—1.49(m, 6H) | (75 MHz, CDCl3), δ: 171.13, 170.33, 162.54, 161.78, 144.33, 141.88, 140.13, 139.21, 138.28, 131.90, 131.49,129.18, 129.09, 126.46, 121.28, 120.71, 120.46, 113.64, 110.89, 110.81, 63.35, 58.36, 57.68, 53.56, 46.90, 40.02, 36.65, 36.28, 35.33, 22.07, 13.90 |
| Compd. | R1a | R2 | R3a | ERK2 IC50/(nmol∙L-1) |
|---|---|---|---|---|
| 19a | 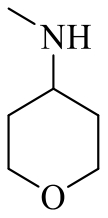 | H | 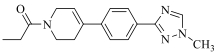 | 16 |
| 19b | Cl | 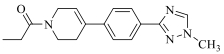 | 15 | |
| 19c | 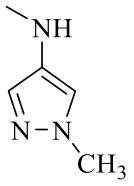 | H | 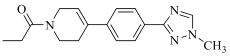 | 97 |
| 19d | Cl | 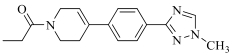 | 121 | |
| 19e | 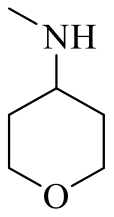 | H | 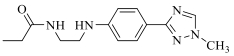 | 20 |
| 19f | 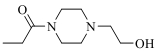 | 9085 | ||
| 19g | 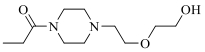 | >10000 | ||
| 19h | 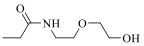 | 3475 | ||
| 19i | 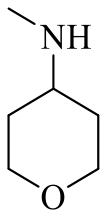 | Cl | 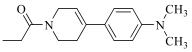 | 96 |
| 19j | 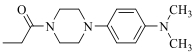 | 150 | ||
| 25a | 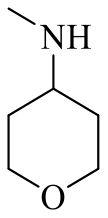 | H | 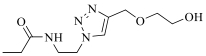 | 2152 |
| 25b | 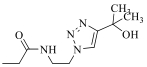 | 2254 | ||
| MK⁃8353 b | — | — | — | 1.7 |
Table 5 Inhibitory activities of compounds 19a—19j, 25a and 25b on EKR2
| Compd. | R1a | R2 | R3a | ERK2 IC50/(nmol∙L-1) |
|---|---|---|---|---|
| 19a |  | H |  | 16 |
| 19b | Cl |  | 15 | |
| 19c |  | H |  | 97 |
| 19d | Cl |  | 121 | |
| 19e |  | H |  | 20 |
| 19f |  | 9085 | ||
| 19g |  | >10000 | ||
| 19h |  | 3475 | ||
| 19i |  | Cl |  | 96 |
| 19j |  | 150 | ||
| 25a |  | H |  | 2152 |
| 25b |  | 2254 | ||
| MK⁃8353 b | — | — | — | 1.7 |
| Compd. | IC50 a /(μmol∙L-1) | |||
|---|---|---|---|---|
| A375 | A2058 | COLO⁃205 | HT⁃29 | |
| 19a | 1.13±0.05 | 7.82±0.06 | 9.30±0.09 | >50 |
| 19b | 2.89±0.04 | 2.69±0.04 | 2.29±0.05 | 5.62±0.06 |
| 19e | 3.29±0.01 | 7.22±0.03 | 2.82±0.04 | >50 |
| 30a | >50 | 9.72±0.05 | 21.96±0.03 | 26.14±0.01 |
| 30b | 3.04±0.03 | 1.73±0.04 | 1.08±0.05 | 3.16±0.04 |
| MK-8353 b | 0.04±0.04 | 0.04±0.03 | 0.01±0.05 | 0.57±0.06 |
Table 6 Anti-proliferation activities of five selected compounds
| Compd. | IC50 a /(μmol∙L-1) | |||
|---|---|---|---|---|
| A375 | A2058 | COLO⁃205 | HT⁃29 | |
| 19a | 1.13±0.05 | 7.82±0.06 | 9.30±0.09 | >50 |
| 19b | 2.89±0.04 | 2.69±0.04 | 2.29±0.05 | 5.62±0.06 |
| 19e | 3.29±0.01 | 7.22±0.03 | 2.82±0.04 | >50 |
| 30a | >50 | 9.72±0.05 | 21.96±0.03 | 26.14±0.01 |
| 30b | 3.04±0.03 | 1.73±0.04 | 1.08±0.05 | 3.16±0.04 |
| MK-8353 b | 0.04±0.04 | 0.04±0.03 | 0.01±0.05 | 0.57±0.06 |
| [1] | Zhang W., Liu H. T., Cell Res., 2002, 12(1), 9—18 |
| [2] | Raman M., Chen W., Cobb M. H., Oncogene, 2007, 26(22), 3100—3112 |
| [3] | Lavoie H., Gagnon J., Therrien M., Nat. Rev. Mol. Cell Biol., 2020, 21(10), 607—632 |
| [4] | Guo Y. J., Pan W. W., Liu S. B., Shen Z. F., Xu Y., Hu L. L., Exp. Ther. Med., 2020, 19(3), 1997—2007 |
| [5] | Degirmenci U., Wang M., Hu J. C., Cells, 2020, 9(1), 198 |
| [6] | Maik⁃Rachline G., Hacohen⁃Lev⁃Ran A., Seger R., Int. J. Mol. Sci., 2019, 20(5),1194 |
| [7] | Liang T. T., Wang W. J., Hao S. Y., He G. C., Xu Y. G., J. China Pharm. Univ., 2020, 51(3), 260—269 |
| 梁停停, 王文杰, 郝思远, 何光超, 徐云根. 中国药科大学学报, 2020, 51(3), 260—269 | |
| [8] | Song Y. L., Bi Z. F., Liu Y., Qin F. R., Wei Y. Q., Wei X. W., Genes Dis., 2023, 10(1), 76—88 |
| [9] | Smorodinsky⁃Atias K., Soudah N., Engelberg D., Cells, 2020, 9(1), 129 |
| [10] | Pan X. L., Pei J. P., Wang A. X., Shuai W., Feng L., Bu F. Q., Zhu Y. M., Zhang L., Wang G., Ouyang L., Acta Pharm. Sin. B, 2022, 12(5), 2171—2192 |
| [11] | Timofeev O., Giron P., Lawo S., Pichler M., Noeparast M., NPJ Precis. Oncol., 2024, 8(1), 70 |
| [12] | Xiao H., Wang A. X., Shuai W., Qian Y. P., Wu C. Y., Wang X., Yang P. P., Sun Q., Wang G., Ouyang L., Sun Q., Signal Transduct. Target. Ther., 2025, 10(1), 70 |
| [13] | McCubrey J. A., Steelman L. S., Chappell W. H., Abrams S. L., Franklin R. A., Montalto G., Cervello M., Libra M., Candido S., Malaponte G., Mazzarino M. C., Fagone P., Nicoletti F., Bäsecke J., Mijatovic S., Maksimovic⁃Ivanic D., Milella M., Tafuri A., Chiarini F., Evangelisti C., Cocco L., Martelli A. M., Oncotarget, 2012, 3(10), 1068—1111 |
| [20] | Heightman T. D., Berdini V., Braithwaite H., Buck I. M., Cassidy M., Castro J., Courtin A., Day J. E. H., East C., Fazal L., Graham B., Griffiths⁃Jones C. M., Lyons J. F., Martins V., Muench S., Munck J. M., Norton D., O'Reilly M., Palmer N., Pathuri P., Reader M., Rees D. C., Rich S. J., Richardson C., Saini H., Thompson N. T., Wallis N. G., Walton H., Wilsher N. E., Woolford A. J. A., Cooke M., Cousin D., Onions S., Shannon J., Watts J., Murray C. W., J. Med. Chem., 2018, 61(11), 4978—4992 |
| [21] | Kampmann S. S., Skelton B. W., Yeoh G. C., Abraham L. J., Lengkeek N. A., Stubbs K. A., Heath C. H., Stewart S.G., Tetrahedron, 2015, 71(42), 8140—8149 |
| [22] | Zhang L., Chen Y. Q., Li W. H., Chem. Res. Chinese Universities, 2025, 41(1), 146—154 |
| [14] | Ji D. Z., Zhang L. Z., Zhu Q. H., Bai Y., Wu Y. Y., Xu Y. G., Eur. J. Med. Chem., 2019, 164, 334—341 |
| [15] | Cevik U. A., Saglik B. N., Osmaniye D., Levent S., Cavusoglu B. K., Karaduman A. B., Atlid O., Eklioglu O. A., Kaplancikli Z. A., J. Enzyme Inhib. Med. Chem., 2020, 35(1), 1657—1673 |
| [16] | Giampietro N. C., Demeter D. A., Diagne A. B., Esguerra K. V. N., Heemstra R. J., Schuldt R. A., Barton T. J., Horty L. G., Sparks T. C., Watson G. B., Molecules Having Certain Pesticidal Utilities, and Intermediates, Compositions, and Processes Rrelated Thereto, WO 2021011722 A1, 2021⁃01⁃21 |
| [17] | Boyington A. J., Seath C. P., Zearfoss A. M., Xu Z. H., Jui N. T., J. Am. Chem. Soc., 2019, 141(9), 4147—4153 |
| [18] | Jin B., Tao Y., Yang H. L., Chem. Res. Chinese Universities, 2018, 34(6), 912—917 |
| [19] | Tan S., Li F., Park S., Kim S., Org. Chem. Front., 2019, 6(23), 3854—3858 |
| [1] | LI Dong, PU Xue, DENG Li, WU Qilin, JU Anqi. Fabrication of ZIF-67-Derived Hollow Flower-like Ni0.3Co2.7S/MoS2 Composite Catalysts for Hydrogen Production via Water Electrolysis [J]. Chem. J. Chinese Universities, 2025, 46(9): 20250144. |
| [2] | CHEN Yangmi, SHE Huixian, WANG Qing, YANG Jiaqiang. Synthesis and Antibacterial Activities of Osthole Oxime Ether Derivatives [J]. Chem. J. Chinese Universities, 2025, 46(9): 20250147. |
| [3] | SHE Huixian, CHEN Yangmi, YANG Jiaqiang. Design, Synthesis and Antibacterial Activities of Thioxime Amide Derivatives Containing Thiopeptide [J]. Chem. J. Chinese Universities, 2025, 46(5): 20240548. |
| [4] | LIU Dongmei, XU Yuanqiang, XIA Chao, ZHENG Jingjing, SU Xianbin. Continuous Flow Liquid Phase Synthesis of Thymopentin Using Fluoride-labile Hydrophobic Tag [J]. Chem. J. Chinese Universities, 2025, 46(4): 20240544. |
| [5] | LIU Yixuan, HU Huimin, FAN Xiaoqiang, YU Xuehua, KONG Lian, XIAO Xia, XIE Zean, ZHAO Zhen. Preparation of Pt/Mn-silicalite-1 Catalysts and Their Catalytic Performance for Propane Dehydrogenation [J]. Chem. J. Chinese Universities, 2025, 46(3): 20240460. |
| [6] | WANG Lu. Ionic Liquid-assisted Hydrothermal Synthesis of 1T-MoS2 and Its Zinc Ion Storage Performance [J]. Chem. J. Chinese Universities, 2024, 45(9): 20240145. |
| [7] | XU Ling, YIN Panpan, LU Xianfu, LI Yiming. Development and Applications of Ligation-Desulfurization Strategy in Protein Chemical Synthesis [J]. Chem. J. Chinese Universities, 2024, 45(8): 20240196. |
| [8] | LIU Hao, LIU Dongmei, SUN Haotian, XIA Chao, SU Xianbin. Continuous Flow Liquid-phase Vialox Peptide Synthesis Using Hydrophobic Silyl Tag [J]. Chem. J. Chinese Universities, 2024, 45(7): 20240024. |
| [9] | LONG Lei, WEI Wei, LUO Yunjun, LI Xiaoyu. Research Progress in Efficient Azide Methods [J]. Chem. J. Chinese Universities, 2024, 45(5): 20230511. |
| [10] | ZHAO Xiaoguang, WANG Yunlong, YIN Haibo, QU Yakun, SU Haiwei, FANG Wei. Research Progress of Electrocatalytic Ammonia Synthesis from Different Nitrogen Sources [J]. Chem. J. Chinese Universities, 2024, 45(3): 20230527. |
| [11] | WANG Gang, LIANG Shuang, SHAN Zhonggang, YING Junwu, LYU Liang, LI Bin, YANG Huibin. Design, Synthesis and Fungicidal Activity of Pyrazinamide Analogs [J]. Chem. J. Chinese Universities, 2024, 45(10): 20240369. |
| [12] | HU Wenxin, ZHAO Ying, DU Danyang, ZHANG Hongdan, CHENG Peng. Preparation of ZSM-5 Encapsulated Pt-La Bimetallic Catalysts and Their Catalytic Performance for iso-Butane Cracking [J]. Chem. J. Chinese Universities, 2024, 45(10): 20240244. |
| [13] | ZHANG Xinghong, GENG Peng, XIANG Juanjuan, YAN Jiaying, MAO Miaofu, XIAO Shuzhang. One-pot Synthesis of Room Temperature Phosphorescent Boron-difluoride Derivative for Printing [J]. Chem. J. Chinese Universities, 2024, 45(1): 20230432. |
| [14] | YANG Yingnan, SUN Qiming. Synthesis of EAB Zeolite and Its Application in Methanol-to-olefin Reaction [J]. Chem. J. Chinese Universities, 2023, 44(8): 20230119. |
| [15] | FENG Ruiqin, FANG Yun, FAN Ye, XIA Yongmei. Facile Synthesis of Gold Nanoflowers and the Catalytic Reduction of p-Nitrophenol with Sodium Borohydride [J]. Chem. J. Chinese Universities, 2023, 44(8): 20230027. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||