

Chem. J. Chinese Universities ›› 2019, Vol. 40 ›› Issue (10): 2156.doi: 10.7503/cjcu20190251
• Physical Chemistry • Previous Articles Next Articles
WANG Chenfeng1,XU Qiuyu1,WANG Lincai1,ZHANG Ruiqi3,PAN Xinxin3,WANG Jingwei1,*( ),HAO Weiju2,*(
),HAO Weiju2,*( )
)
Received:2019-04-30
Online:2019-10-10
Published:2019-10-16
Contact:
WANG Jingwei,HAO Weiju
E-mail:jwwang@sspu.edu.cn;wj_hao@fudan.edu.cn
Supported by:CLC Number:
TrendMD:
WANG Chenfeng, XU Qiuyu, WANG Lincai, ZHANG Ruiqi, PAN Xinxin, WANG Jingwei, HAO Weiju. Preparation of a Sandwich-like Ni-P@Ni-B/Ni Catalytic Electrode for pH-Universal Hydrogen Evolution Reaction †[J]. Chem. J. Chinese Universities, 2019, 40(10): 2156.
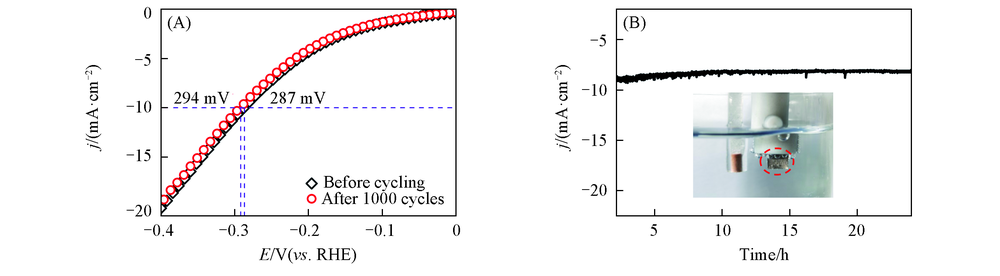
Fig.5 Durability test of the Ni-P@Ni-B/Ni electrode for HER by CV sweeping between 0 V and -0.4 V(vs. RHE) at the rate of 5 mV/s in 0.5 mol/L PBS(A) and time-dependent current density curve for Ni-P@Ni-B/Ni under static overpotential of 287 mV for 24 h in 0.5 mol/L PBS without iR correction(B) Inset: physical image of the electrode when it is working.
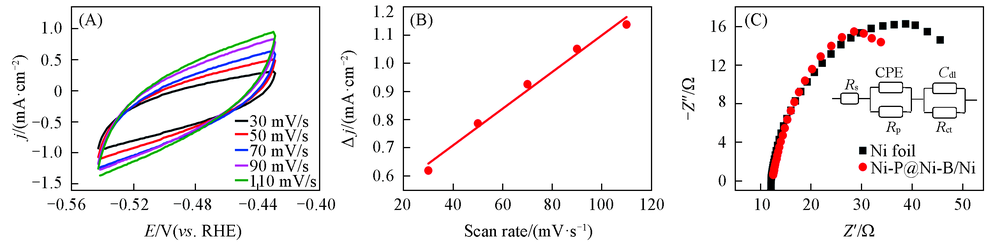
Fig.7 Cyclic voltammograms of Ni-P@Ni-B/Ni at different scan rates in 0.5 mol/L PBS(A), double layer capacitor(Cdl) diagram of Ni-P@Ni-B/Ni(B) and Nyquist plots of Ni-P@Ni-B/Ni and Ni at a current density of 10 mA/cm2 Inset shows the equivalent circuit diagram.
| Catalyst | Electrolyte | Mass loading/ (mg·cm-2) | Current density/ (mA·cm-2) | Overpotential/mV | Tafel slope/ (mV·dec-1) | Ref. |
|---|---|---|---|---|---|---|
| Ni-P@Ni-B/Ni | 0.5 mol/L PBS | 3.5 | 287 | 85 | This work | |
| 1 mol/L KOH | 3.5 | 10 | 79 | 80 | This work | |
| 0.5 mol/L H2SO4 | 3.5 | 199 | 73 | This work | ||
| CoO/CoSe2/Ti | 0.5 mol/L PBS | 2.0 | 10 | 337 | 131 | [ |
| Co-NRCNTs | 1.0 mol/L PBS | 0.28 | 10 | 540 | | [ |
| Co9S8@C | 1.0 mol/L PBS | 0.28 | 10 | 280 | | [ |
| CNF@CoS2 | 1.0 mol/L PBS | 0.6―0.8 | 10 | 360 | 113.3 | [ |
| NRs/NF | 1.0 mol/L PBS | | 10 | 204 | 81.8 | [ |
| Ni0.1Co0.9P/CC | 1.0 mol/L PBS | 0.58 | 10 | 125 | 103 | [ |
| Mo2C/MoO2 | 0.1 mol/L PBS | 0.285 | 10 | 290 | | [ |
| Co2N/TM | 1.0 mol/L PBS | 1.08 | 10 | 290 | 138 | [ |
| Syn-MoSx | 1.0 mol/L PBS | 0.523 | Onset | 94 | 111 | [ |
| Co-P-B/rGO | 0.1 mol/L PBS | 0.28 | 5 | 418 | 82 | [ |
| SiO2 PPy NTs-CFs | 1.0 mol/L PBS | 0.335 | 10 | 183 | 100.2 | [ |
| Catalyst | Electrolyte | Mass loading/ (mg·cm-2) | Current density/ (mA·cm-2) | Overpotential/mV | Tafel slope/ (mV·dec-1) | Ref. |
|---|---|---|---|---|---|---|
| Ni-P@Ni-B/Ni | 0.5 mol/L PBS | 3.5 | 287 | 85 | This work | |
| 1 mol/L KOH | 3.5 | 10 | 79 | 80 | This work | |
| 0.5 mol/L H2SO4 | 3.5 | 199 | 73 | This work | ||
| CoO/CoSe2/Ti | 0.5 mol/L PBS | 2.0 | 10 | 337 | 131 | [ |
| Co-NRCNTs | 1.0 mol/L PBS | 0.28 | 10 | 540 | | [ |
| Co9S8@C | 1.0 mol/L PBS | 0.28 | 10 | 280 | | [ |
| CNF@CoS2 | 1.0 mol/L PBS | 0.6―0.8 | 10 | 360 | 113.3 | [ |
| NRs/NF | 1.0 mol/L PBS | | 10 | 204 | 81.8 | [ |
| Ni0.1Co0.9P/CC | 1.0 mol/L PBS | 0.58 | 10 | 125 | 103 | [ |
| Mo2C/MoO2 | 0.1 mol/L PBS | 0.285 | 10 | 290 | | [ |
| Co2N/TM | 1.0 mol/L PBS | 1.08 | 10 | 290 | 138 | [ |
| Syn-MoSx | 1.0 mol/L PBS | 0.523 | Onset | 94 | 111 | [ |
| Co-P-B/rGO | 0.1 mol/L PBS | 0.28 | 5 | 418 | 82 | [ |
| SiO2 PPy NTs-CFs | 1.0 mol/L PBS | 0.335 | 10 | 183 | 100.2 | [ |
| [1] | Dinh C. T., Jain A., de Arquer F. P. G., de Luna P. , Nature Energy, 2019,4, 107— 114 |
| [2] | Chen Z. L., Ha Y., Jia H. X., Yan X. X. , Adv. Energy Mater, 2019,9, 1803918 |
| [3] | Chen Z. L., Xu H. B., Ha Y., Li X. Y. , Applied Catalysis B: Environmental, 2019,250, 213— 223 |
| [4] | Sui C., Chen K., Zhao L., Zhou L. , Nanoscale, 2018,10, 15324— 15331 |
| [5] | Shang Z. J., Yan L. L., Rao G. S., Xiong T., Tian W., Lin X., Zhong Q. L., Ren B. , Chem. J. Chinese Universities, 2014,35(12), 2688— 2693 |
| ( 商中瑾, 颜亮亮, 饶贵仕, 熊婷, 田伟, 林璇, 钟起玲, 任斌 . 高等学校化学学报, 2014,35(12), 2688— 2693) | |
| [6] | Peng Z. K., Wang H. Y., Zhou L. L., Wang Y. B. , J. Mater. Chem. A, 2019,7, 6676— 6685 |
| [7] | Ren X., Wu D., Ge R., Sun X. , Nano Research, 2018,11, 2024— 2033 |
| [8] | Inami Y., Ogihara H., Nagamatsu S., Asakura K. , ACS Catalysis, 2019,9, 2448— 2457 |
| [9] | Han W., Kuepper K., Hou P., Akram W ., ChemSusChem, 2018,11, 3661— 3671 |
| [10] | Zhou Y., Li T., Xi S., He C ., ChemCatChem, 2018,10, 5487— 5495 |
| [11] | Xu T. Y., Wei S. T., Zhang X. L., Zhang D. T. , Materials Research Experss, 2019,7, 075501 |
| [12] | Cai P., Huang J., Chen J., Wen Z ., Angew Chem. Int. Ed. Engl., 2017,56, 4858— 4861 |
| [13] | Ren X., Wang W., Ge R., Hao S. , Chem. Commun.(Camb) 2017,53, 9000— 9003 |
| [14] | Qian X., Xu C., Jiang Y. Q., Zhang J. , Chemical Engineering Journal, 2019,368, 202— 211 |
| [15] | Qi K., Xie Y., Wang R., Liu S. Y. , Applied Surface Science, 2019,466, 847— 853 |
| [16] | Wang X., Li W., Xiong D., Petrovykh D. Y. , Advanced Functional Materials, 2016,26, 4067— 4077 |
| [17] | Hoa V. H., Tran D. T., Le H. T. , Applied Catalysis B: Environmental, 2019,253, 235— 245 |
| [18] | Huang Y. C., Hu L., Liu R., Hu Y. W. , Applied Catalysis B: Environmental, 2019,251, 181— 194 |
| [19] | Ge R., Wang S., Su J., Dong Y ., Nanoscale, 2018,10, 13930— 13935 |
| [20] | Pu Z., Zhao J., Amiinu I. S., Li W. , Energy & Environmental Science, 2019,12, 952— 957 |
| [21] | Li Y. H., Chen B. X., Duan X. Z., Chen S. M. , Applied Catalysis B: Environmental, 2019,249, 306— 315 |
| [22] | Wang K., Huang B., Lin F., Lv F ., Advanced Energy Materials, 2018,8, 1801891 |
| [23] | Hao W. J., Wu R. B., Zhang R. Q., Ha Y. , Adv. Energy Mater., 2018,8, 1801372 |
| [24] | Zhang P., Wang M., Yang Y., Yao T ., Nano Energy, 2016,19, 98— 107 |
| [25] | Kim J., Kim H., Kim S. K., Ahn S. H. , Journal of Materials Chemistry A, 2018,6, 6282— 6288 |
| [26] | Hao W. J., Wu R.B., Yang H.Y., Guo Y.H. , J. Mater. Chem. A, 2019,7, 12440— 12445 |
| [27] | Li Q., Zou X., Ai X., Chen H ., Advanced Energy Materials, 2018,9, 1803369 |
| [28] | Menezes P. W., Indra A., Zaharieva I., Walter C. , Energy & Environmental Science, 2019,3, 988— 999 |
| [29] | Xie L., Zhang R., Cui L., Liu D ., Angew Chem. Int. Ed. Engl., 2017,56, 1064— 1068 |
| [30] | Lu X., Pan J., Lovell E., Tan T. H. , Energy & Environmental Science, 2018,11, 1898— 1910 |
| [31] | Li H., Wen P., Li Q., Dun C ., Advanced Energy Materials, 2017,7, 1700513 |
| [32] | Li K. D., Zhang J. F., Wu R., Yu Y.F. , Advanced Science, 2016,3, 1500426 |
| [33] | Zou X. X., Huang X. X., Goswami A., Silva R. , Angewandte Chemie-International Edition, 2014,53, 4372— 4376 |
| [34] | Feng L. L., Li G. D., Liu Y. P., Wu Y. Y. , ACS Applied Materials & Interfaces, 2015,7, 980— 988 |
| [35] | Gu H. H., Huang Y. P., Zuo L. Z., Fan W. , Inorganic Chemistry Frontiers, 2016,3, 1280— 1288 |
| [36] | Wu R., Xiao B., Gao Q., Zheng Y. R. , Angew. Chem. Inter. Ed. Engl., 2018,57, 15445— 15449 |
| [37] | Liu M., Yang Y., Luan X., Dai X ., Acs Sustainable Chemistry & Engineering, 2018,6, 14356— 14364 |
| [38] | Zhang L., Xie L., Ma M., Qu F ., Catalysis Science & Technology, 2017,7, 2689— 2694 |
| [39] | Li Y., Nakamura R ., Chinese Journal of Catalysis, 2018,39, 401— 406 |
| [40] | Li P., Jin Z., Xiao D. , J. Mater. Chem. A, 2014,2, 18420— 18427 |
| [41] | Feng J. X., Xu H., Ye S. H., Ouyang G. , Angew Chem. Int. Ed. Engl., 2017,56, 8120— 8124 |
| [1] | YANG Lijun, YU Yang, ZHANG Lei. Construction of Dual-functional 2D/3D Hydrid Co2P-CeO x Heterostructure Integrated Electrode for Electrocatalytic Urea Oxidation Assisted Hydrogen Production [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220082. |
| [2] | WANG Zumin, MENG Cheng, YU Ranbo. Doping Regulation in Transition Metal Phosphides for Hydrogen Evolution Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220544. |
| [3] | WU Yaqiang, LIU Siming, JIN Shunjin, YAN Yongqing, WANG Zhao, CHEN Lihua, SU Baolian. Synthesis of Zn-Doped NiCoP Catalyst with Porous Double-layer Nanoarray Structure and Its Electrocatalytic Properties for Hydrogen Evolution [J]. Chem. J. Chinese Universities, 2021, 42(8): 2483. |
| [4] | ZHANG Nan, HAN Kuo, LI Yue, WANG Chunru, ZHAO Feng, HAN Dongxue, NIU Li. Core-shell Heterostructure Construction Between Thiospinel CuCo2S4 and MoS2 for Improved Hydrogen Evolution Electrocatalytic Performance [J]. Chem. J. Chinese Universities, 2021, 42(4): 1307. |
| [5] | HE Qianqian, WANG Zhe, MENG Lingjia, CHEN Qian, GONG Yongji. Recent Advances of Hydrogen Evolution Reaction Catalysis Based on Transition Metal Dichalcogenides [J]. Chem. J. Chinese Universities, 2021, 42(2): 523. |
| [6] | ZHAO Guoqing, YUAN Zhao, WANG Lian, GUO Zhuo. Preparation of Ni2P/N, S co-Doped Reduced Graphene Oxide Composites and Their Electrocatalytic Properties for Hydrogen Evolution† [J]. Chem. J. Chinese Universities, 2020, 41(7): 1575. |
| [7] | SUN Qiangqiang, CAO Baoyue, ZHOU Chunsheng, ZHANG Guochun, WANG Zenglin. Enhancing Hydrogen Evolution Performance of a Regular Cube NiCu Nanocrystalline Electrocatalyst Fabricated by Normal Pluse Electrodeposition † [J]. Chem. J. Chinese Universities, 2020, 41(6): 1287. |
| [8] | JIN E,SONG Kaixu,CUI Lili. Preparation and Electrocatalytic Performance of Carbon Material Co-doped by Bimetal Phosphide and Heteroatom † [J]. Chem. J. Chinese Universities, 2020, 41(6): 1362. |
| [9] | WANG Wenfeng, QIN Shan, ZHANG Rongrong, ZHOU Panpan, YANG Qinghua, CHEN Tianyun. Preparation of UIO-66-based Porous Nano-octahedral FeP@PC for Efficient and Durable Hydrogen Evolution † [J]. Chem. J. Chinese Universities, 2019, 40(9): 1979. |
| [10] | WANG Wen,TAO Xiafang,WU Yunyan,ZHAO Nan,CHENG Xiaonong,YANG Juan,ZHOU Yazhou. Fabrication and SERS Performance of “Sandwich” Structured Silver Nanoparticles/Graphene Oxide Substrates† [J]. Chem. J. Chinese Universities, 2019, 40(4): 667. |
| [11] | TANG Jiayi,YAO Junjie,ZHANG Xiaojun,MA Liang,ZHANG Tingmei,NIU Zheng,CHEN Xiangzhen,ZHAO Liang,JIANG Lin,SUN Yinghui. Multi-shell Hollow FeP Microspheres as Efficient Electrocatalyst for Hydrogen Evolution at All pH Values † [J]. Chem. J. Chinese Universities, 2019, 40(11): 2340. |
| [12] | WANG Jin-Yi1, LIU Ran2, CAI Wen-Bin1*. Selective Deposition of Metal Patterns on Glass Based on Self-assembly and Inkjet Printing [J]. Chem. J. Chinese Universities, 2008, 29(8): 1644. |
| [13] | TAN Kai*, LU Xin*, LIN Meng-Hai, ZHANG Qian-Er. Density Functional Study of the Structural and Electronic Properties of TiP10+/0/- Clusters [J]. Chem. J. Chinese Universities, 2008, 29(12): 2350. |
| [14] | HUANG Jin-Zhao1, XU Zheng 1,3* , LI Hai-Ling2, KANG Guo-Hu2, WANG Wen-Jing2. Preparation and Characterization of Nanocrystalline Ni-Mo as Cathode Catalyst via RF Magnetron Sputtering [J]. Chem. J. Chinese Universities, 2006, 27(5): 909. |
| [15] | YUE Bin, JIANG Lei, KONG Zu-Ping, JIAO Feng, LIN Xin-Rong, JIN Song-Lin . Synthesis and Characterization of Sandwich Rare Earth Metal Mono-substituted Polyoxometalates with γ-[SiW10O36]8- as Ligand [J]. Chem. J. Chinese Universities, 2004, 25(2): 199. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||