

Chem. J. Chinese Universities ›› 2019, Vol. 40 ›› Issue (11): 2340.doi: 10.7503/cjcu20190308
• Physical Chemistry • Previous Articles Next Articles
TANG Jiayi1,YAO Junjie1,ZHANG Xiaojun2,MA Liang2,ZHANG Tingmei3,NIU Zheng1,CHEN Xiangzhen1,ZHAO Liang1,JIANG Lin3,SUN Yinghui1,*( )
)
Received:2019-05-28
Online:2019-11-10
Published:2019-08-20
Contact:
SUN Yinghui
E-mail:yinghuisun@suda.edu.cn
Supported by:CLC Number:
TrendMD:
TANG Jiayi,YAO Junjie,ZHANG Xiaojun,MA Liang,ZHANG Tingmei,NIU Zheng,CHEN Xiangzhen,ZHAO Liang,JIANG Lin,SUN Yinghui. Multi-shell Hollow FeP Microspheres as Efficient Electrocatalyst for Hydrogen Evolution at All pH Values †[J]. Chem. J. Chinese Universities, 2019, 40(11): 2340.
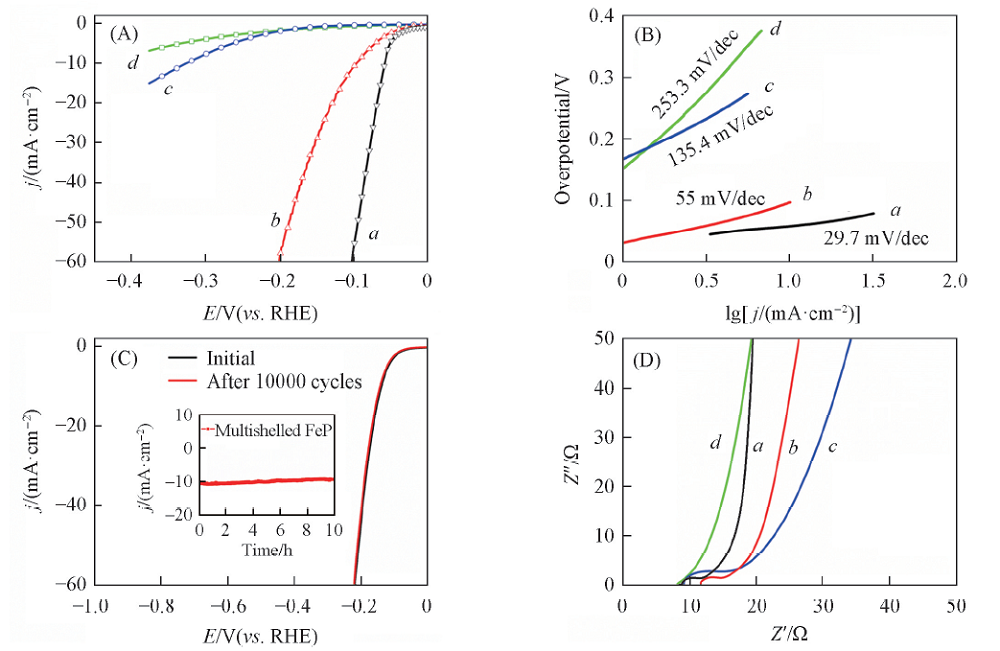
Fig.5 HER performances in 0.5 mol/L H2SO4 solution (A) Polarization curves(scan rate: 10 mV/s); (B) Tafel plots; (C) polarization stability plot of FeP/CNT(scan rate 500 mV/s); (D) impedance plots. a. Pt/C; b. FeP/CNT; c. FeP; d. CNT. Inset is current density curves for 10 h under constant overpotential of 97 mV.
| Electrolyte | a/mV | b /(mV·dec-1) | |
|---|---|---|---|
| 0.5 mol/L H2SO4 | 220 | 55.0 | 0.266 |
| 1.0 mol/L KOH | 320 | 64.9 | 0.021 |
| 1.0 mol/L Na2SO4 | 920 | 163.2 | 0.012 |
| Electrolyte | a/mV | b /(mV·dec-1) | |
|---|---|---|---|
| 0.5 mol/L H2SO4 | 220 | 55.0 | 0.266 |
| 1.0 mol/L KOH | 320 | 64.9 | 0.021 |
| 1.0 mol/L Na2SO4 | 920 | 163.2 | 0.012 |
| Catalyst | Electrolyte | η10/mA | Tafel slope/(mV·dec-1) | Ref. |
|---|---|---|---|---|
| FeP/CNT | 0.5 mol/L H2SO4 | 97 | 55 | This work |
| 1.0 mol/L KOH | 169 | 64.9 | This work | |
| 1.0 mol/L Na2SO4 | 495 | 163.2 | This work | |
| FeP powder/carbon | 0.5 mol/L H2SO4 | 110 | 57 | [ |
| 1.0 mol/L KOH | 185 | 93 | [ | |
| FeP bond carbon nanowires networks | 0.5 mol/L H2SO4 | 256 | 75.8 | [ |
| Nanoporous FeP nanosheets | 0.5 mol/L H2SO4 | 230 | 67 | [ |
| FeP nanowires | 0.5 mol/L H2SO4 | 96 | 37 | [ |
| 1.0 mol/L KOH | 194 | 67 | [ | |
| FeP NPs@NPC | 0.5 mol/L H2SO4 | 130 | 67 | [ |
| 1.0 mol/L KOH | 214 | 82 | [ | |
| 1.0 mol/L PBS | 386 | 136 | [ | |
| FeP NPs @hollow carbon nanobox | 0.5 mol/L H2SO4 | 88 | 49 | [ |
| 1.0 mol/L KOH | 180 | 71 | [ | |
| Ni-doped FeP/C hollow nanorods | 0.5 mol/L H2SO4 | 72 | 54 | [ |
| 1.0 mol/L KOH | 95 | 72 | [ | |
| 1.0 mol/L PBS | 117 | 70 | [ | |
| FeP NCs@NCNs | 0.5 mol/L H2SO4 | 114 | 84 | [ |
| 1.0 mol/L KOH | 205 | 70 | [ | |
| 1.0 mol/L PBS | 409 | 92 | [ | |
| CoP/Co2P NPs@NC | 0.5 mol/L H2SO4 | 126 | 79 | [ |
| 1.0 mol/L KOH | 198 | 82 | [ | |
| 1.0 mol/L PBS | 459 | —— | [ | |
| NiP NPs | 0.5 mol/L H2SO4 | 115 | 46 | [ |
| Catalyst | Electrolyte | η10/mA | Tafel slope/(mV·dec-1) | Ref. |
|---|---|---|---|---|
| FeP/CNT | 0.5 mol/L H2SO4 | 97 | 55 | This work |
| 1.0 mol/L KOH | 169 | 64.9 | This work | |
| 1.0 mol/L Na2SO4 | 495 | 163.2 | This work | |
| FeP powder/carbon | 0.5 mol/L H2SO4 | 110 | 57 | [ |
| 1.0 mol/L KOH | 185 | 93 | [ | |
| FeP bond carbon nanowires networks | 0.5 mol/L H2SO4 | 256 | 75.8 | [ |
| Nanoporous FeP nanosheets | 0.5 mol/L H2SO4 | 230 | 67 | [ |
| FeP nanowires | 0.5 mol/L H2SO4 | 96 | 37 | [ |
| 1.0 mol/L KOH | 194 | 67 | [ | |
| FeP NPs@NPC | 0.5 mol/L H2SO4 | 130 | 67 | [ |
| 1.0 mol/L KOH | 214 | 82 | [ | |
| 1.0 mol/L PBS | 386 | 136 | [ | |
| FeP NPs @hollow carbon nanobox | 0.5 mol/L H2SO4 | 88 | 49 | [ |
| 1.0 mol/L KOH | 180 | 71 | [ | |
| Ni-doped FeP/C hollow nanorods | 0.5 mol/L H2SO4 | 72 | 54 | [ |
| 1.0 mol/L KOH | 95 | 72 | [ | |
| 1.0 mol/L PBS | 117 | 70 | [ | |
| FeP NCs@NCNs | 0.5 mol/L H2SO4 | 114 | 84 | [ |
| 1.0 mol/L KOH | 205 | 70 | [ | |
| 1.0 mol/L PBS | 409 | 92 | [ | |
| CoP/Co2P NPs@NC | 0.5 mol/L H2SO4 | 126 | 79 | [ |
| 1.0 mol/L KOH | 198 | 82 | [ | |
| 1.0 mol/L PBS | 459 | —— | [ | |
| NiP NPs | 0.5 mol/L H2SO4 | 115 | 46 | [ |
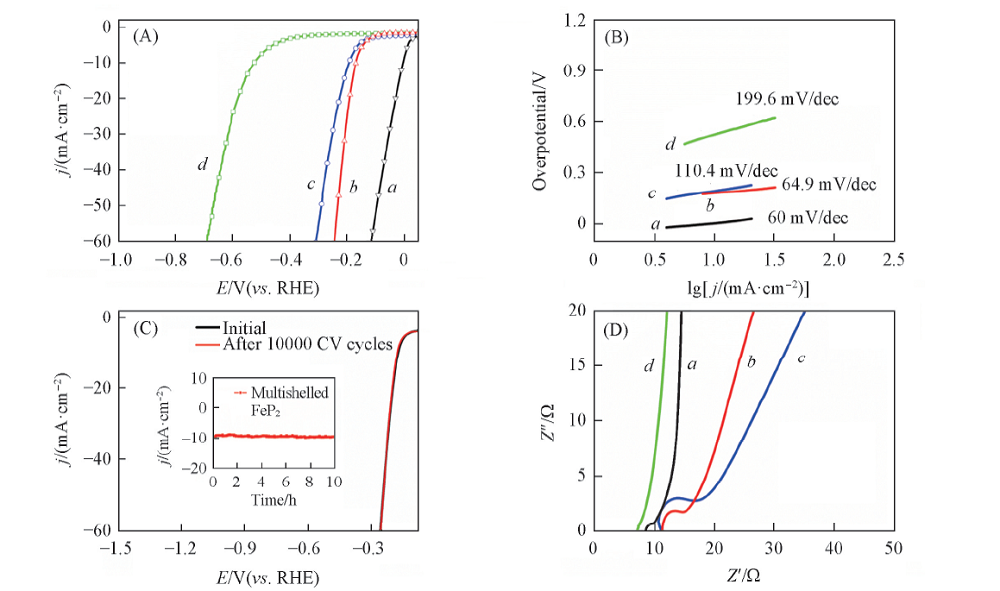
Fig.6 HER performances in 1.0 mol/L KOH solution (A) Polarization curves(scan rate: 10 mV/s); (B) Tafel plot; (C) polarization stability plot of FeP(scan rate 500 mV/s); (D) impedance plots. a. Pt/C; b. FeP/CNT; c. FeP; d. CNT. The inset is current density curves for 10 h under constant overpotential of 169 mV.
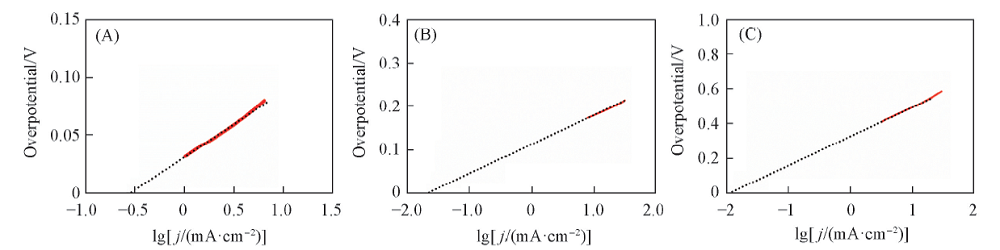
Fig.8 Exchange current densities of FeP/CNT electrocatalyst in different electrolytes calculated by the extrapolation of Tafel curves Electrolyte: (A) 0.5 mol/L H2SO4; (B) 1.0 mol/L KOH; (C) 1.0 mol/L Na2SO4 solution.
| [1] | Feng X. L., Qu Z. K., Chen J., Wang D. D., Chen X., Yang W. S., Chem. J. Chinese Universities, 2017,38(11), 1999— 2005 |
| ( 冯晓磊, 曲宗凯, 陈俊, 王登登, 陈旭, 杨文胜 . 高等学校化学学报, 2017,38(11), 1999— 2005) | |
| [2] | Jian J., Yuan L., Li H., Liu H. H., Zhang X. J., Sun X. J., Yuan H. M., Feng S. H., Chem. Res. Chinese Universities, 2019,35(2), 179— 185 |
| [3] | Pu Z., Amiinu I. S., Kou Z., Li W., Mu S ., Angew. Chem. Int. Ed., 2017,56(38), 11559— 11564 |
| [4] | Zheng Y., Jiao Y., Zhu Y., Li L. H., Han Y., Chen Y., Jaroniec M., Qiao S. Z., J. Am. Chem. Soc., 2016,138(49), 16174— 16181 |
| [5] | Han L., Dong S. J., Wang E. K ., Adv. Mater., 2016,28(42), 9266— 9291 |
| [6] | Tang C., Gan L. F., Zhang R., Lu W. B., Jiang X. E., Asiri A. M., Sun X. P., Wang J., Chen L ., Nano Lett., 2016,16(10), 6617— 6621 |
| [7] | Li D., Baydoun H., Kulikowski B., Brock S. L ., Chem. Mater., 2017,29(7), 3048— 3054 |
| [8] | Zhang T. Q., Liu J., Huang L. B., Zhang X. D., Sun Y. G., Liu X. C., Bin D. S., Chen X., Cao A. M., Hu J. S., Wan L. J., J. Am. Chem. Soc., 2017,139(32), 11248— 11253 |
| [9] | Jiang P., Liu Q., Liang Y. H., Tian J. Q., Asiri A. M., Sun X. P ., Angew. Chem. Int. Ed., 2014,53(47), 12855— 12859 |
| [10] | Lv C. C., Peng Z., Zhao Y. X., Huang Z. P., Zhang C., J. Mater. Chem. A, 2016,4(4), 1454— 1460 |
| [11] | Yan Y., Zhao B., Yi S. C., Wang X., J. Mater. Chem. A, 2016,4(33), 13005— 13010 |
| [12] | Popczun E. J., Read C. G., Roske C. W., Lewis N. S., Schaak R. E ., Angew. Chem. Int. Ed., 2014,53(21), 5427— 5430 |
| [13] | Chung D. Y., Jun S. W., Yoon G., Kim H., Yoo J. M., Lee K. S., Kim T., Shin H., Sinha A. K., Kwon S. G., Kang K., Hyeon T., Sung Y. E., J. Am. Chem. Soc., 2017,139(19), 6669— 6674 |
| [14] | Ran J. R., Ma T. Y., Gao G. P., Du X. W., Qiao S. Z ., Energy Environ. Sci., 2015,8(12), 3708— 3717 |
| [15] | Lu Q. P., Yu Y. F., Ma Q. L., Chen B., Zhang H ., Adv. Mater., 2016,28(10), 1917— 1933 |
| [16] | Sun H. M., Xu X. B., Yan Z. H., Chen X., Cheng F. Y., Weiss P. S., Chen J ., Chem. Mater., 2017,29(19), 8539— 8547 |
| [17] | Gao W., Yan M., Cheung H. Y., Xia Z. M., Zhou X. M., Qin Y. B., Wong C. Y., Ho J. C., Chang C. R., Qu Y. Q., Nano Energy, 2017,38, 290— 296 |
| [18] | Xiao P., Alam Sk M., Thia L., Ge X. M., Lim R. J., Wang J. Y., Lim K. H., Wang X ., Energy Environ. Sci., 2014,7(8), 2624— 2629 |
| [19] | Yan Y., Shi X. R., Miao M., He T., Dong Z. H., Zhan K., Yang J. H., Zhao B., Xia B. Y ., Nano Res., 2018,11(7), 3537— 3547 |
| [20] | Wang F. L., Yang X. D., Dong B. X., Yu X., Xue H. G., Feng L. G ., Electrochem. Commun., 2018,92, 33— 38 |
| [21] | Lu X. F., Yu L., Lou X. W., Sci. Adv., 2019, 5(2), eaav6009 |
| [22] | Wang X. H., Feng J., Bai Y. C., Zhang Q., Yin Y. D ., Chem. Rev., 2016,116(18), 10983— 11060 |
| [23] | Zeng Y., Luo J. Z., Wang Y. Z., Qiao L., Zou B., Zheng W. T., Nanoscale, 2017,9(44), 17576— 17584 |
| [24] | Zhang T. M., Zheng J. Z., Liang Z. Q., Zhao B., Zeng H. J., Guo W., Zhao L., Sun Y. H., Abdulhalim I., Jiang L., Electrochim. Acta, 2019,306, 151— 158 |
| [25] | Grosvenor A. P., Wik S. D., Cavell R. G., Mar A ., Inorg. Chem., 2005,44(24), 8988— 8998 |
| [26] | Tian J. Q., Liu Q., Liang Y. H., Xing Z. C., Asiri A. M., Sun X. P., ACS Appl. Mater. Interfaces, 2014,6(23), 20579— 20584 |
| [27] | Jiang P., Liu Q., Sun X. P., Nanoscale, 2014,6(22), 13440— 13445 |
| [28] | Li J. S., Wang Y., Liu C. H., Li S. L., Wang Y. G., Dong L. Z., Dai Z. H., Li Y. F., Lan Y. Q ., Nat Commun., 2016,7, 11204— 11211 |
| [29] | Li F., Zhao X. L., Mahmood J., Okyay M. S., Jung S. M., Ahmad I., Kim S. J., Han G. F., Park N., Baek J. B., ACS Nano, 2017,11(7), 7527— 7533 |
| [30] | Li M., Liu T. T., Bo X. J., Zhou M., Guo L. P., Guo S. J., Nano Energy, 2017,33, 221— 228 |
| [31] | Xu Y., Wu R., Zhang J. F., Shi Y. M., Zhang B ., Chem. Commun., 2013,49(59), 6656— 6658 |
| [32] | Son C. Y., Kwak I. H., Lim Y. R., Park J., Chem. Commun., 2016,52(13), 2819— 2822 |
| [33] | Pu Z. H., Amiinu I. S., Zhang C. T., Wang M., Kou Z. K., Mu S. C., Nanoscale, 2017,9(10), 3555— 3560 |
| [34] | Peng Z., Qiu X. Y., Yu Y., Jiang D., Wang H. T., Cai G. Y., Zhang X. X., Dong Z. H., Carbon, 2019,152, 16— 23 |
| [35] | Yu Y., Peng Z., Asif M., Wang H. T., Wang W., Wu Z. X., Wang Z. Y., Qiu X. Y., Tan H., Liu H. F ., ACS Sustainable Chem. Eng., 2018,6(9), 11587— 11594 |
| [36] | Lv X. W., Ren J. T., Wang Y. S., Liu Y. P., Yuan Z. Y., ACS Sustainable Chem. Eng., 2019,7(9), 8993— 9001 |
| [37] | Popczun E. J., McKone J. R., Read C. G., Biacchi A. J., Wiltrout A. M., Lewis N. S., Schaak R. E., J. Am. Chem. Soc., 2013,135(25), 9267— 9270 |
| [38] | Kucernak A. R., Zalitis C., J. Phys. Chem.C, 2016,120, 10721— 10745 |
| [39] | Watzele S., Fichtner J., Garlyyev B., Schwämmlein J. N., Bandarenka A. S., ACS Catal., 2018,8, 9456— 9462 |
| [1] | FAN Jianling, TANG Hao, QIN Fengjuan, XU Wenjing, GU Hongfei, PEI Jiajing, CEHN Wenxing. Nitrogen Doped Ultra-thin Carbon Nanosheet Composited Platinum-ruthenium Single Atom Alloy Catalyst for Promoting Electrochemical Hydrogen Evolution Process [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220366. |
| [2] | YANG Lijun, YU Yang, ZHANG Lei. Construction of Dual-functional 2D/3D Hydrid Co2P-CeO x Heterostructure Integrated Electrode for Electrocatalytic Urea Oxidation Assisted Hydrogen Production [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220082. |
| [3] | WONG Honho, LU Qiuyang, SUN Mingzi, HUANG Bolong. Rational Design of Graphdiyne-based Atomic Electrocatalysts: DFT and Self-validated Machine Learning [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220042. |
| [4] | JIN Xiangyuan, ZHANG Libing, SUN Xiaofu, HAN Buxing. Electrocatalytic CO2 Reduction over Single-atom Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220035. |
| [5] | ZHANG Xiaoyu, XUE Dongping, DU Yu, JIANG Su, WEI Yifan, YAN Wenfu, XIA Huicong, ZHANG Jianan. MOF-derived Carbon-based Electrocatalysts Confinement Catalyst on O2 Reduction and CO2 Reduction Reactions [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210689. |
| [6] | WANG Zumin, MENG Cheng, YU Ranbo. Doping Regulation in Transition Metal Phosphides for Hydrogen Evolution Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220544. |
| [7] | HE Yujing, LI Jiale, WANG Dongyang, WANG Fuling, XIAO Zuoxu, CHEN Yanli. Zinc-based Activated Fe/Co/N Doped Biomass Carbon Electrocatalysts with High Oxygen Reduction Activity [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220475. |
| [8] | WU Yaqiang, LIU Siming, JIN Shunjin, YAN Yongqing, WANG Zhao, CHEN Lihua, SU Baolian. Synthesis of Zn-Doped NiCoP Catalyst with Porous Double-layer Nanoarray Structure and Its Electrocatalytic Properties for Hydrogen Evolution [J]. Chem. J. Chinese Universities, 2021, 42(8): 2483. |
| [9] | ZHU Zhengxin, ZHANG Xiang, WANG Mingming, CHEN Wei. Lithium Intercalation Compounds-Hydrogen Gas Batteries [J]. Chem. J. Chinese Universities, 2021, 42(5): 1610. |
| [10] | YANG Tao, YAO Huiying, LI Qing, HAO Wei, CHI Lifeng, ZHU Jia. Density Functional Theoretical Studies on the Promising Electrocatalyst of M-BHT(M=Co or Cu) for CO2 Reduction to CH4 [J]. Chem. J. Chinese Universities, 2021, 42(4): 1268. |
| [11] | WANG Renheng, XIAO Zhe, LI Yan, SUN Yiling, FAN Shuting, ZHENG Junchao, QIAN Zhengfang, HE Zhenjiang. Synthesis of Li2FeP2O7 Cathode Material at Different Temperatures and Its Electrochemical Performance for Lithium Ion Batteries [J]. Chem. J. Chinese Universities, 2021, 42(4): 1299. |
| [12] | ZHANG Nan, HAN Kuo, LI Yue, WANG Chunru, ZHAO Feng, HAN Dongxue, NIU Li. Core-shell Heterostructure Construction Between Thiospinel CuCo2S4 and MoS2 for Improved Hydrogen Evolution Electrocatalytic Performance [J]. Chem. J. Chinese Universities, 2021, 42(4): 1307. |
| [13] | HE Qianqian, WANG Zhe, MENG Lingjia, CHEN Qian, GONG Yongji. Recent Advances of Hydrogen Evolution Reaction Catalysis Based on Transition Metal Dichalcogenides [J]. Chem. J. Chinese Universities, 2021, 42(2): 523. |
| [14] | ZHAO Guoqing, YUAN Zhao, WANG Lian, GUO Zhuo. Preparation of Ni2P/N, S co-Doped Reduced Graphene Oxide Composites and Their Electrocatalytic Properties for Hydrogen Evolution† [J]. Chem. J. Chinese Universities, 2020, 41(7): 1575. |
| [15] | SUN Qiangqiang, CAO Baoyue, ZHOU Chunsheng, ZHANG Guochun, WANG Zenglin. Enhancing Hydrogen Evolution Performance of a Regular Cube NiCu Nanocrystalline Electrocatalyst Fabricated by Normal Pluse Electrodeposition † [J]. Chem. J. Chinese Universities, 2020, 41(6): 1287. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||