

Chem. J. Chinese Universities ›› 2018, Vol. 39 ›› Issue (11): 2534.doi: 10.7503/cjcu20180260
• Physical Chemistry • Previous Articles Next Articles
Received:2018-04-04
Online:2018-11-10
Published:2018-08-23
Contact:
DONG Xiaoyan
E-mail:d_xy@tju.edu.cn
Supported by:CLC Number:
TrendMD:
ZHANG Huan, DONG Xiaoyan. Effects of EGCG on Amyloid β-Protein Fibrillogenesis and Cytotoxicity at Different pH Values†[J]. Chem. J. Chinese Universities, 2018, 39(11): 2534.
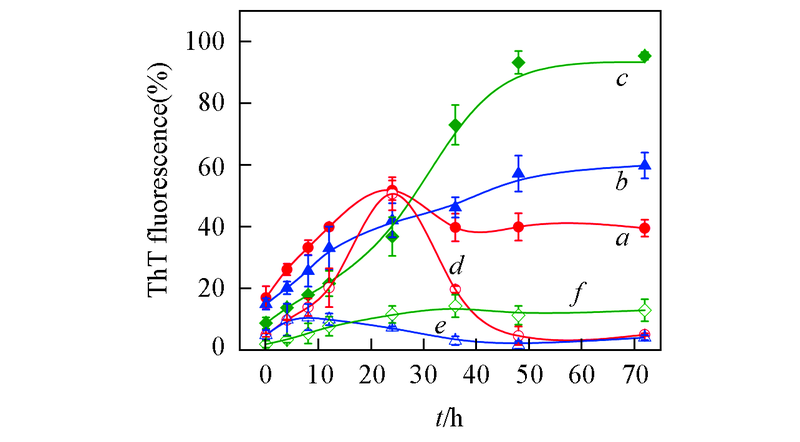
Fig.2 Aggregation kinetics of Aβ42 incubated in the absence and presence of equimolar EGCG under different pH valuesa―c. Aβ42 under pH=5.0(a), 6.0(b), 7.4(c), respectively; d―f. Aβ42 with equimolar EGCG under pH=5.0(d), 6.0(e), 7.4(f). The incubations were done at 37 ℃, 150 r/min. Concentrations of Aβ42 and EGCG were 25 μmol/L.
| t/h | pH=5.0 | pH=6.0 | pH=7.4 | |||
|---|---|---|---|---|---|---|
| — | EGCG | — | EGCG | — | EGCG | |
| 0 | 29.5±5.2 | 27.0±4.4 | 24.7±1.8 | 22.8±1.5 | 23.0±2.2 | 18.4±2.6 |
| 6 | 41.5±3.7 | 38.4±2.8 | 33.5±3.4 | 23.4±2.3 | 27.9±1.7 | 25.3±2.7 |
| 12 | 44.9±7.3 | 35.6±3.2 | 42.0±3.9 | 23.7±5.6 | 38.5±1.6 | 30.9±2.6 |
| 24 | 39.7±1.8 | 35.1±2.2 | 45.9±5.7 | 23.8±2.4 | 49.2±4.2 | 33.9±2.2 |
| 48 | 41.8±4.9 | 30.5±5.4 | 40.9±2.0 | 19.1±0.6 | 70.9±5.8 | 39.3±2.8 |
Table 1 β-Sheet content(%) of Aβ42 in absence and presence of EGCG under different pH values
| t/h | pH=5.0 | pH=6.0 | pH=7.4 | |||
|---|---|---|---|---|---|---|
| — | EGCG | — | EGCG | — | EGCG | |
| 0 | 29.5±5.2 | 27.0±4.4 | 24.7±1.8 | 22.8±1.5 | 23.0±2.2 | 18.4±2.6 |
| 6 | 41.5±3.7 | 38.4±2.8 | 33.5±3.4 | 23.4±2.3 | 27.9±1.7 | 25.3±2.7 |
| 12 | 44.9±7.3 | 35.6±3.2 | 42.0±3.9 | 23.7±5.6 | 38.5±1.6 | 30.9±2.6 |
| 24 | 39.7±1.8 | 35.1±2.2 | 45.9±5.7 | 23.8±2.4 | 49.2±4.2 | 33.9±2.2 |
| 48 | 41.8±4.9 | 30.5±5.4 | 40.9±2.0 | 19.1±0.6 | 70.9±5.8 | 39.3±2.8 |
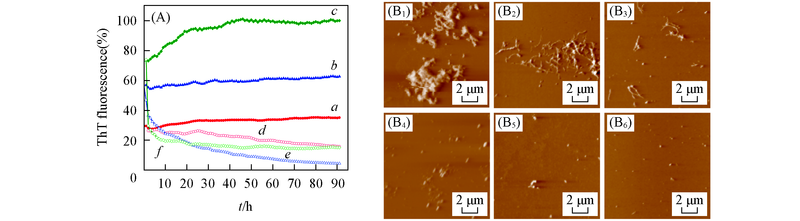
Fig.3 EGCG-induced remodeling of Aβ42 mature fibrils under different pH values(A) Loss of ThT fluorescence of Aβ42 fibrils measured in the absence and presence of EGCG at different conditions. a―c. Aβ42 aggregates under pH=5.0, 6.0, 7.4; d―f. Aβ42 aggregates with equimolar EGCG under pH=5.0, 6.0, 7.4. (B1—B6): AFM images of Aβ42 aggregates in the absence and presence of EGCG under different pH. (B1―B3) Aβ42 aggregates under pH=5.0, 6.0, 7.4, (B4―B6) Aβ42 aggregates with equimolar EGCG under pH=5.0, 6.0, 7.4. The samples were incubated at 37 ℃ for 4 d. Concentrations of Aβ42 aggregates and EGCG were 25 μmol/L.
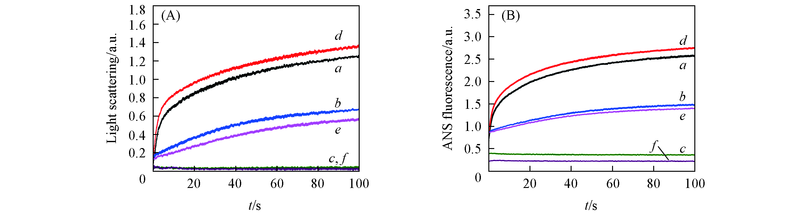
Fig.4 Effect of EGCG on Aβ42 rapid aggregation kinetics under different pH values(A) Rayleigh light scatting; (B) ANS fluorescence. a.―c. Aβ42 under pH=5.0, 6.0, 7.4; d.―f. Aβ42 with equimolar EGCG under pH=5.0, 6.0, 7.4. The detections were performed at 37 ℃. Final concentrations of Aβ42 and EGCG were 10 μmol/L.
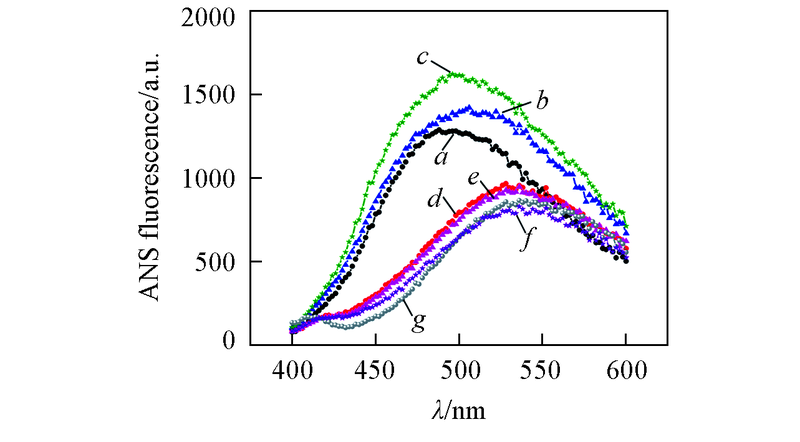
Fig.5 ANS fluorescence spectra of Aβ42 species in the absence and presence of equimolar EGCG under different pHa—c. Aβ42 under pH=5.0, 6.0, 7.4; d—f. Aβ42 with equimolar EGCG under pH=5.0, 6.0, 7.4; g. ANS control. The samples were incubated at 37 ℃, 150 r/min for 48 h. Concentrations of Aβ42 and EGCG were 25 μmol/L.
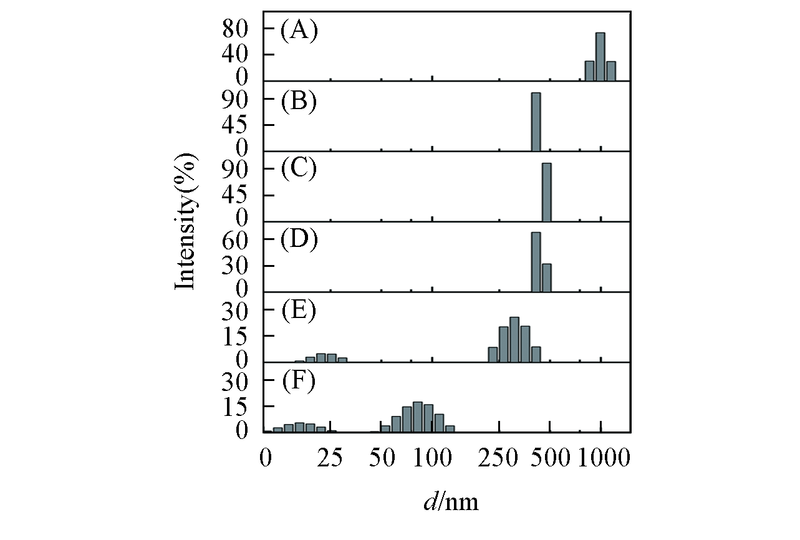
Fig.6 DLS analysis of the Aβ42 species in the absence and presence of equimolar EGCG under different pH values(A), (C), (E): Aβ42 under pH=5.0, 6.0, 7.4; (B), (D), (F): Aβ42 with equimolar EGCG under pH=5.0, 6.0, 7.4. The samples were incubated at 37 ℃, 150 r/min for 48 h. Aβ42 concentration was 25 μmol/L.
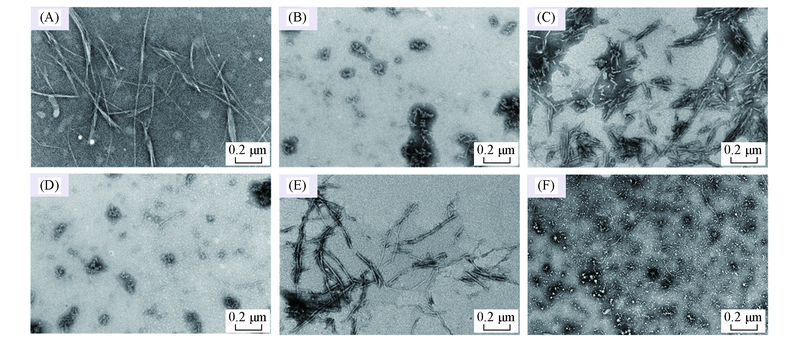
Fig.7 TEM images of Aβ42 species in the absence and presence of EGCG under different pH values(A), (C), (E) Aβ42 under pH=5.0, 6.0, 7.4; (B), (D), (F) Aβ42 with equimolar EGCG under pH=5.0, 6.0, 7.4.The samples were incubated at 37 ℃, 150 r/min for 48 h. Aβ42 concentration was 25 μmol/L.
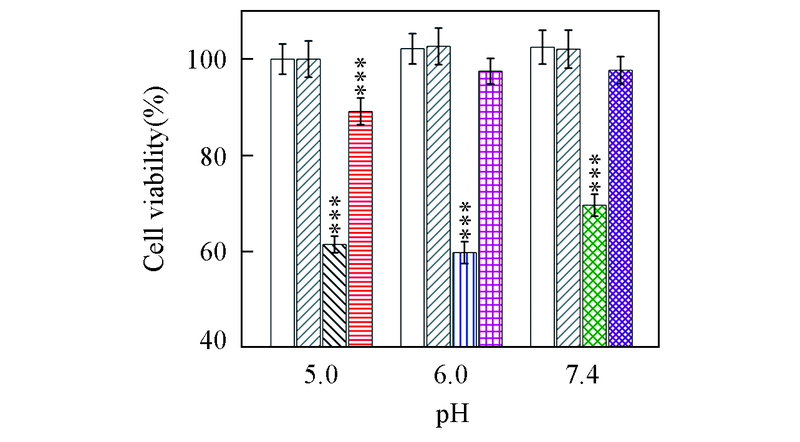
Fig.8 Viability of SH-SY5Y cells incubated with Aβ42 species in the absence and presence of equimolar EGCG under different pH valuesBuffer-treated group in the absence() and presence() of EGCG; Aβ42 under pH=5.0(), 6.0(), 7.4(); Aβ42 with equimolar EGCG under pH 5.0(), 6.0(), 7.4(). The incubations were performed at 37 ℃, 150 r/min. The aged-concentration of Aβ42 was 25 μmol/L, and the final concentration of Aβ42 was 5 μmol/L in cells. *** P<0.001 as compared to buffer-treated group.
| [1] | Chiti F., Dobson C. M., Annu. Rev. Biochem., 2017, 86(1), 27—68 |
| [2] | Walsh D. M., Lomakin A., Benedek G. B., Condron M. M., Teplow D. B., J. Biol. Chem., 1999, 274(36), 25945—25952 |
| [3] | Jakob-Roetne R., Jacobsen H., Angew. Chem. Int. Ed. Engl., 2009, 48(17), 3030—3059 |
| [4] | Zheng L., Cedazo-Minguez A., Hallbeck M., Jerhammar F., Marcusson J., Terman A., Transl. Neurodegener., 2012, 1(1), 1—7 |
| [5] | Tam J. H., Seah C., Pasternak S. H., Mol. Brain, 2014, 7(1), 54 |
| [6] | Peralvarez-Marin A., Barth A., Graslund A., J. Mol. Biol., 2008, 379(3), 589—596 |
| [7] | Brännström K., Öhman A., Nilsson L., Pihl M., Sandblad L., Olofsson A., J. Am. Chem. Soc., 2014, 136(31), 10956—10964 |
| [8] | Gorman P. M., Yip C. M., Fraser P. E., Chakrabartty A. P., J. Mol. Biol., 2003, 325(4), 743—757 |
| [9] | Khandogin J., Brooks C. L., Proc. Natl. Acad. Sci. USA, 2007, 104(43), 16880—16885 |
| [10] | Su Y., Chang P. T., Brain Res., 2001, 893(1/2), 287—291 |
| [11] | Du W. J., Guo J. J., Gao M. T., Hu S. Q., Dong X. Y., Han Y. F., Liu F. F., Jiang S., Sun Y., Sci. Rep., 2015, 5, 7992 |
| [12] | Song S. M., Ma X. W., Zhou Y. H., Xu M. T., Shuang S. M., Dong C., Chem. Res. Chinese Universities, 2016, 32(2), 172—177 |
| [13] | Xiong N., Dong X. Y., Zheng J., Liu F. F., Sun Y., ACS Appl. Mater. Interfaces, 2015, 7(10), 5650—5662 |
| [14] | Takahashi T., Mihara H. T., Acc. Chem. Res., 2008, 41(10), 1309—1318 |
| [15] | Xie B. L., Li X., Dong X. Y., Sun Y., Langmuir, 2014, 30(32), 9789—9796 |
| [16] | Kerr M. L., Gasperini R., Gibbs M. E., Hou X., Shepherd C. E., Strickland D. K., Foa L., Lawen A., Small D. H., J. Neurochem., 2010, 112(5), 1199—1209 |
| [17] | Linse S., Cabaleiro-Lago C., Xue W. F., Lynch I., Lindman S., Thulin E., Radford S. E., Dawson K. A., Proc. Natl. Acad. Sci. USA, 2007, 104(21), 8691—8696 |
| [18] | Xie L., Luo Y., Lin D., Xi W., Yang X., Wei G., Nanoscale, 2014, 6(16), 9752—9762 |
| [19] | Mak J. C., Clin. Exp. Pharmacol. Physiol., 2012, 39(3), 265—273 |
| [20] | Nagle D. G., Ferreira D., Zhou Y. D., Phytochemistry, 2006, 67(17), 1849—1855 |
| [21] | Weinreb O., Amit T., Mandel S., Youdim M. B., Genes Nutr., 2009, 4(4), 283—296 |
| [22] | Ehrnhoefer D. E., Bieschke J., Boeddrich A., Herbst M., Masino L., Lurz R., Engemann S., Pastore A., Wanker E. E., Nat. Struct. Mol. Biol., 2008, 15(6), 558—566 |
| [23] | Liu Y., Liu Y., Wang S. H., Dong S. Z., Chang P., Jiang Z. F., RSC Adv., 2015, 5(77), 62402—62413 |
| [24] | Bieschke J., Russ J., Friedrich R. P., Ehrnhoefer D. E., Wobst H., Neugebauer K., Wanker E. E., Proc. Natl. Acad. Sci. USA, 2010, 107(17), 7710—7715 |
| [25] | Palhano F. L., Lee J., Grimster N. P., Kelly J. W., J. Am. Chem. Soc., 2013, 135(20), 7503—7510 |
| [26] | Wang S. H., Liu F. F., Dong X. Y., Sun Y., J. Phys. Chem. B, 2010, 114(35), 11576—11583 |
| [27] | Wang Q., Shah N., Zhao J., Wang C., Zhao C., Liu L., Li L., Zhou F., Zheng J., Phys. Chem. Chem. Phys., 2011, 13(33), 15200—15210 |
| [28] | Tomaselli S., Esposito V., Vangone P., van Nuland N. A., Bonvin A. M., Guerrini R., Tancredi T., Temussi P. A., Picone D., Chem. Bio. Chem., 2006, 7(2), 257—267 |
| [29] | LeVine H., Amyloid, 1995, 2(1), 1—6 |
| [30] | Ji S. R., Wu Y., Sui S. F., Gen. Physiol. Biophys., 2002, 21(4), 415—427 |
| [31] | Roychaudhuri R., Lomakin A., Bernstein S., Zheng X., Condron M. M., Benedek G. B., Bowers M., Teplow D. B., J. Mol. Biol., 2014, 426(13), 2422—2441 |
| [32] | Micsonai A., Wien F., Kernya L., Lee Y. H., Goto Y., Réfrégiers M., Kardos J., Proc. Natl. Acad. Sci. USA, 2015, 112(24), E3095—E3103 |
| [33] | Noy D., Solomonov I., Sinkevich O., Arad T., Kjaer K., Sagi I., J. Am. Chem. Soc., 2008, 130(4), 1376—1383 |
| [34] | Meng J., Zhang H., Dong X. Y., Liu F. F., Sun Y., J. Inorg. Biochem., 2018, 181, 56—64 |
| [35] | Fotakis G., Timbrell J. A., Toxicol. Lett., 2006, 160(2), 171—177 |
| [36] | Lin M. S., Chen L. Y., Tsai H. T., Wang S. S., Chang Y., Higuchi A., Chen W. Y., Langmuir, 2008, 24(11), 5802—5808 |
| [37] | Khan M. V., Rabbani G., Ahmad E., Khan R. H., Int. J. Biol. Macromol., 2014, 70(8), 606—614 |
| [38] | Hills R. D. Jr, Brooks Ⅲ C. L., J. Mol. Biol., 2007, 368(3), 894—901 |
| [39] | Jarrett J. T., Berger E. P., Lansbury P. T. Jr., Biochemistry, 1993, 32(18), 4693—4697 |
| [40] | Picotti P., De Franceschi G., Frare E., Spolaore B., Zambonin M., Chiti F., de Laureto P. P., Fontana A., J. Mol. Biol., 2007, 367(5), 1237—1245 |
| [41] | Guo M., Gorman P. M., Rico M., Chakrabartty A., Laurents D. V., FEBS Lett., 2005, 579(17), 3574—3578 |
| [42] | Ma K., Clancy E. L., Zhang Y. B., Ray D. G., Wollenberg K., Zagorski M. G., J. Am. Chem. Soc., 1999, 121(38), 8698—8706 |
| [43] | Bujacz A., Acta Cryst., 2012, 68(10), 1278—1289 |
| [44] | Arya P., Srivastava A., Vasaikar S. V., Mukherjee G., Mishra P., Kundu B., ACS Chem. Neurosci., 2014, 5(10), 982—992 |
| [45] | Walsh D. M., Selkoe D. J., J. Neurochem., 2007, 101(5), 1172—1184 |
| [46] | Wogulis M., Wright S., Cunningham D., Chilcote T., Powell K., Rydel R. E., J. Neurosci., 2005, 25(5), 1071—1080 |
| [47] | Tabner B. J., El-Agnaf O. M., Turnbull S., German M. J., Paleologou K. E., Hayashi Y., Cooper L. J., Fullwood N. J., Allsop D., J. Biol. Chem., 2005, 280(43), 35789—35792 |
| [48] | Christen Y., Am. J. Clin. Nutr., 2000, 71(2), 621S—629S |
| [49] | Miranda S., Opazo C., Larrondo L. F., Muñoz F. J., Ruiz F., Leighton F., Inestrosa N. C., Prog. Neurobiol., 2000, 62(6), 633—648 |
| [50] | Arispe N., Pollard H. B., Rojas E., Proc. Natl. Acad. Sci. USA, 1993, 90(22), 10573—10577 |
| [51] | Levites Y., Amit T., Mandel S., Youdim M. B., FASEB J., 2003, 17(8), 952—954 |
| [52] | Choi Y. T., Jung C. H., Lee S. R., Bae J. H., Baek W. K., Suh M. H., Park J., Park C. W., Suh S. I., Life Sci., 2001, 70(5), 603—614 |
| [53] | del Amo J. M. L., Fink U., Dasari M., Grelle G., Wanker E. E., Bieschke J., Reif B., J. Mol. Biol., 2012, 421(4/5), 517—524 |
| [54] | Zhou L., Elias R. J., Food Chem., 2013, 138(2/3), 1503—1509 |
| [55] | Li N., Taylor L. S., Ferruzzi M. G., Mauer L. J., J. Agric. Food Chem., 2012, 60(51), 12531—12539 |
| [56] | Liu F. F., Dong X. Y., He L., Middelberg A. P., Sun Y., J. Phys. Chem. B, 2011, 115(41), 11879—11887 |
| [57] | Bae M. J., Ishii T., Minoda K., Kawada Y., Ichikawa T., Mori T., Kamihira M., Nakayama T., Mol. Nutr. Food Res., 2009, 53(6), 709—715 |
| [58] | An T. T., Feng S., Zeng C. M., Redox Biol., 2017, 11(C), 315—321 |
| [1] | CAO Shujie, LI Hongjun, GUAN Wenli, REN Mengtian, ZHOU Chuanzheng. Progress on the Stereocontrolled Synthesis of Phosphorothioate Oligonucleotides [J]. Chem. J. Chinese Universities, 2022, 43(Album-4): 20220304. |
| [2] | TENG Zhenyuan, ZHANG Qitao, SU Chenliang. Charge Separation and Surface Reaction Mechanisms for Polymeric Single-atom Photocatalysts [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220325. |
| [3] | YANG Jingyi, SHI Siqi, PENG Huaitao, YANG Qihao, CHEN Liang. Integration of Atomically Dispersed Ga Sites with C3N4 Nanosheets for Efficient Photo-driven CO2 Cycloaddition [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220349. |
| [4] | QIN Yongji, LUO Jun. Applications of Single-atom Catalysts in CO2 Conversion [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220300. |
| [5] | LIN Zhi, PENG Zhiming, HE Weiqing, SHEN Shaohua. Single-atom and Cluster Photocatalysis: Competition and Cooperation [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220312. |
| [6] | LI Lei, FANG Yun, XIA Yongmei, FAN Mengqi, FAN Ye. Effects of Amine Structures on the pH Window of Oleic Acid Vesicle [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220144. |
| [7] | LI Lin, QI Fenglian, QIU Lili, MENG Zihui. Dynamic Amorphous Photonic Structure Patterns Assembled by Hexagonal Magnetic Nanosheets [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220123. |
| [8] | WANG Ruina, SUN Ruifen, ZHONG Tianhua, CHI Yuwu. Fabrication of a Dispersible Large-sized Graphene Quantum Dot Assemblies from Graphene Oxide and Its Electrogenerated Chemiluminescence Behaviors [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220161. |
| [9] | LIU Shuwei, JIN Hao, YIN Wanzhong, ZHANG Hao. Gemcitabine/polypyrrole Composite Nanoparticles for Chemo-photothermal Combination Ovarian Cancer Therapy [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220345. |
| [10] | JIANG Shenghan, CAO Changlin, XIAO Liren, YANG Tang, QIAN Qingrong, CHEN Qinghua. Preparation of Composite Semiconductor Micro-sheets with UV Shielding Performance and Its Application in Polypropylene [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220071. |
| [11] | LIU Suyu, DING Fei, LI Qian, FAN Chunhai, FENG Jing. Azobenzene-integrated DNA Nanomachine [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220122. |
| [12] | WANG Xuebin, XUE Yuan, MAO Hua’nyu, XIANG Yanxin, BAO Chunyan. Preparation of Photo/reduction Dual-responsive Hydrogel Microspheres and Their Application in Three-dimensional Cell Culture [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220116. |
| [13] | JIN Ruiming, MU Xiaoqing, XU Yan. Bio-chemical Synthesis of Melanin Precursor—— 5,6-Dihydroxyindole(DHI) [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220134. |
| [14] | GAO Jian, FENG Yiyu, FANG Wenyu, WANG Hui, GE Jing, FENG Wei. Alkane Grafted Phase Change Azobenzene Materials Based on Low Temperature Heat Release [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220146. |
| [15] | TAN Yan, YU Shen, LYU Jiamin, LIU Zhan, SUN Minghui, CHEN Lihua, SU Baolian. Efficient Preparation of Mesoporous γ-Al2O3 Microspheres and Performance of Pd-loaded Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220133. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
