

Chem. J. Chinese Universities ›› 2017, Vol. 38 ›› Issue (2): 238.doi: 10.7503/cjcu20160544
• Physical Chemistry • Previous Articles Next Articles
ZHANG Jingjing1,2, LI Li1,2,3,*( ), HAO Yuting2, SUN Leilei2, ZHANG Xinyue2
), HAO Yuting2, SUN Leilei2, ZHANG Xinyue2
Received:2016-07-28
Online:2017-02-10
Published:2017-01-17
Contact:
LI Li
E-mail:qqhrll@163.com
Supported by:CLC Number:
TrendMD:
ZHANG Jingjing, LI Li, HAO Yuting, SUN Leilei, ZHANG Xinyue. Preparation and Photocatalytic Hydrogen Evolution Performance of In2O3/ZrO2-TiO2 Hollow Spheres†[J]. Chem. J. Chinese Universities, 2017, 38(2): 238.
| Sample | SBET/(m2·g-1) | D/nm | Vtotal/(cm3·g-1) | D/nm | Eg/eV | Crystal parameter/nm | |
|---|---|---|---|---|---|---|---|
| a | c | ||||||
| In2O3/ZrO2-TiO2 | 57.45 | 10.78 | 0.155 | 19.16 | 3.09 | 0.3822 | 0.9735 |
| In2O3/ZrO2-TiO2-H | 66.92 | 11.24 | 0.188 | 24.10 | 3.15 | 0.3802 | 0.9661 |
Table 1 BET surface areas(SBET), average pore diameters(D), pore volumes(Vtotal ), crystallite size(D), band-gap energy(Eg) and cell parameters of In2O3/ZrO2-TiO2 and In2O3/ZrO2-TiO2-H
| Sample | SBET/(m2·g-1) | D/nm | Vtotal/(cm3·g-1) | D/nm | Eg/eV | Crystal parameter/nm | |
|---|---|---|---|---|---|---|---|
| a | c | ||||||
| In2O3/ZrO2-TiO2 | 57.45 | 10.78 | 0.155 | 19.16 | 3.09 | 0.3822 | 0.9735 |
| In2O3/ZrO2-TiO2-H | 66.92 | 11.24 | 0.188 | 24.10 | 3.15 | 0.3802 | 0.9661 |
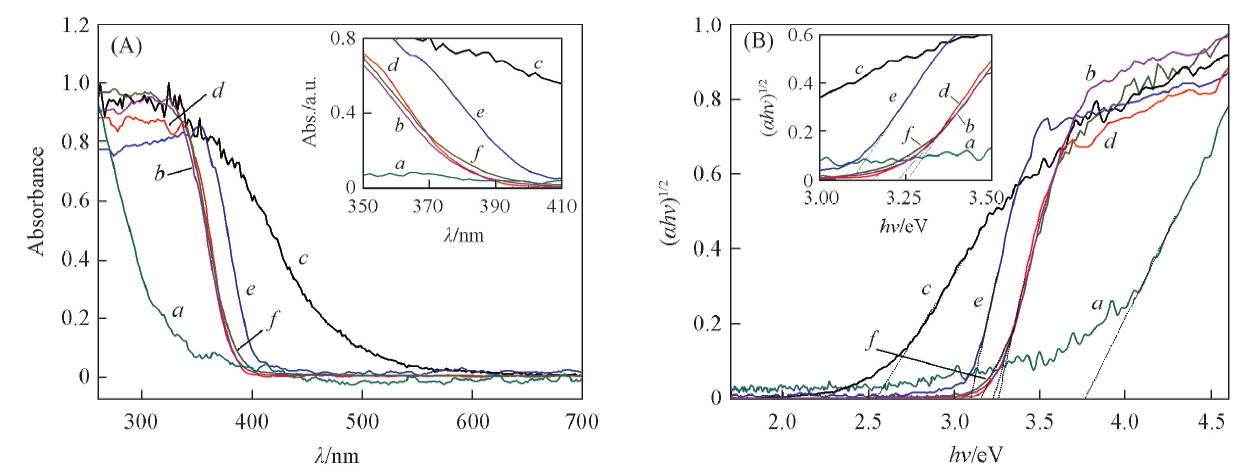
Fig.4 UV-Vis DRS spectra(A) and Kubelka-Munk energy curve plots(B) of ZrO2(a), TiO2(b), In2O3(c), ZrO2-TiO2-H(d), In2O3/ZrO2-TiO2(e) and In2O3/ZrO2-TiO2-H(f)
| Semiconductor | X/eV | Eg/eV | ECB/eV | EVB/eV |
|---|---|---|---|---|
| In2O3 | 5.28 | 2.53 | -0.48 | 2.05 |
| ZrO2 | 5.91 | 3.75 | -0.46 | 3.30 |
| TiO2 | 5.81 | 3.22 | -0.30 | 2.92 |
Table 2 Absolute electronegativity(X/eV), energy band gaps(Eg/eV), conduction band potential(ECB/eV) and valence band potential(EVB/eV) of In2O3, ZrO2 and TiO2
| Semiconductor | X/eV | Eg/eV | ECB/eV | EVB/eV |
|---|---|---|---|---|
| In2O3 | 5.28 | 2.53 | -0.48 | 2.05 |
| ZrO2 | 5.91 | 3.75 | -0.46 | 3.30 |
| TiO2 | 5.81 | 3.22 | -0.30 | 2.92 |
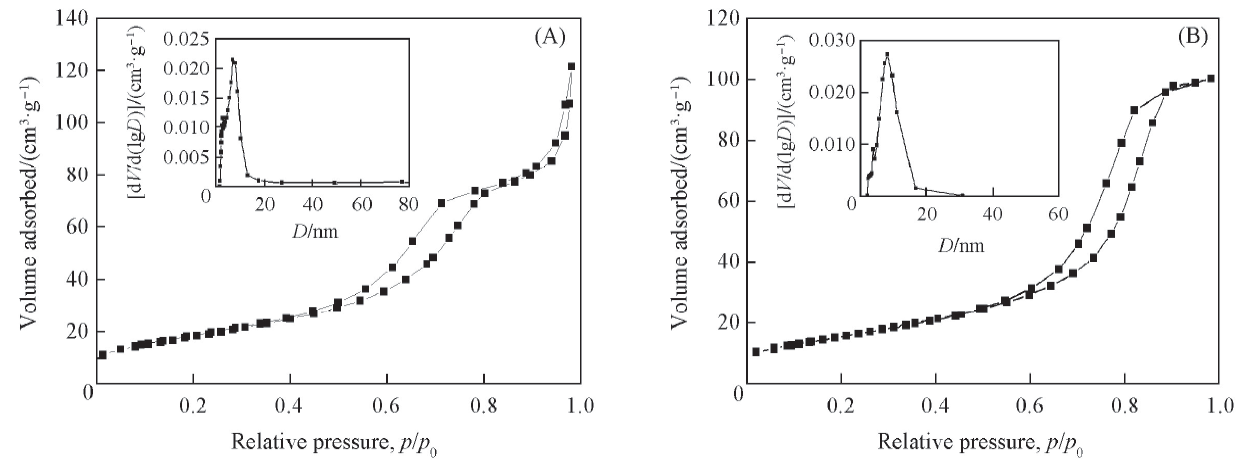
Fig.5 N2 adsorption-desorption isotherms of In2O3/ZrO2-TiO2-H(A) and In2O3/ZrO2-TiO2(B) The insets show the BJH pore size distribution of the corresponding samples.
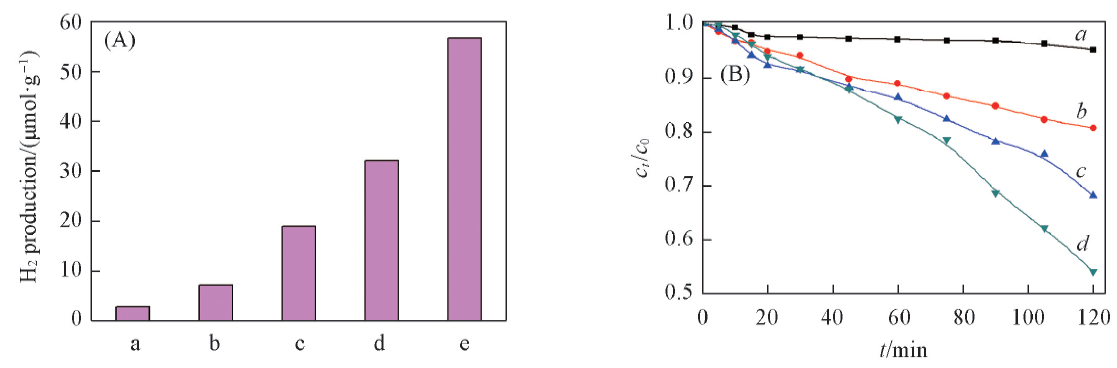
Fig.6 Hydrogen production amount of different photocatalysts(t=8 h)(A) and capture test results for UV photocatalytic degradation of MO using In2O3/ZrO2-TiO2-H(B) (A) a. P25; b. ZrO2; c. ZrO2-TiO2-H; d. In2O3/ZrO2-TiO2; e. In2O3/ZrO2-TiO2-H. (B) a. Benzoquinone; b. triethanolamine; c. tert-butanol; d. In2O3/ZrO2-TiO2-H.
| [1] | Zhong Y., Wang Z. X., Zhang R. F., Bai F., Wu H. M., Raid H., Fan H. Y., ACS Nano,2014, 8(1), 827—833 |
| [2] | Yang Y., Wang G. Z., Deng Q., Dickon H. L. N., Zhao H. J., ACS Appl. Mater. Interfaces,2014, 6, 3008—3015 |
| [3] | Xiong J. Y., Li Z., Chen J., Zhang S. Q., Wang L. Z., Dou S. X., ACS Appl. Mater. Interfaces,2014, 6, 15716—15725 |
| [4] | Samson K., liwa M. S, Socha R. P., Gora-Marek K., Mucha D., Rutkowska-Zbik D., Paul J. F., Ruggiero-Mikołajczyk M., Grabowski R., Słoczynski J., ACS Catal., 2014, 4, 3730—3741 |
| [5] | Zhang D., Qiu R., Song L., J. Hazard Mater,2009, 163(2/3), 843—847 |
| [6] | Wang X. Y., Wang H., Yang X. T., Su X. G., Chem. Res. Chinese Universities,2016, 32(4), 661—664 |
| [7] | Shi Y. K., Hu X. J., Zhu B. L., Zhang S. M., Huang W. P., Chem. Res. Chinese Universities,2015, 31(5), 851—857 |
| [8] | Wang H. Z., Liu N., Lu J., Chem. Res. Chinese Universities,2015, 31(5), 846—850 |
| [9] | Kim J. H., Kang S. B., Lee J. H., Organic Electronics,2013, 14(5), 1305—1312 |
| [10] | Zhang T., Gu F., Han D., Sensors and Actuators B: Chemical,2013, 177, 1180—1188 |
| [11] | Galazka Z., Uecker R., Irmscher K., J. Crystal Growth,2013, 362, 349—352 |
| [12] | Shao D., Qin L., Sawyer S., Optical Materials,2013, 35(3), 563—566 |
| [13] | Sun J. H., Zhang J. S., Zhang M. W., Markus A., Fu X. Z., Wang X. C., Nature Commun., 2012, 3, 1139 |
| [14] | Zheng D. D., Huang C. J., Wang X. C., Nanoscale,2015, 7, 465—470 |
| [15] | Zheng D. D., Pang C. Y., Liu Y. X., Wang X. C., Chem. Commun., 2015, 51, 9706—9709 |
| [16] | Zheng D. D., Pang C. Y., Wang X. C., Chem. Commun., 2015, 51, 17467—17470 |
| [17] | Chinh C. N., Nhu N. V., Trong-On D., J. Mater. Chem. A,2015, 3, 18345—18359 |
| [18] | Min Y. L., Wan Y., Liu R., Yu S. H., Chinese J. Inorg. Chem., 2008, 24(7), 1172—1176 |
| (闵宇霖, 万勇, 刘蓉, 俞书宏. 无机化学学报, 2008, 24(7), 1172—1176) | |
| [19] | Cai J. J., Wu X. Q., Li S. X., Zheng F. Y., Zhu L. C., Lai Z. H., ACS Appl. Mater. Interfaces,2015, 7, 3764—3772 |
| [20] | Wang C., Geng A., Guo Y., J. Colloid Interface Sci., 2006, 301(1), 236—247 |
| [21] | Li L., Lu D., Ji Y., Zhao Y. H., Acta Physico-Chimica Sinica,2010, 26(5), 1323—1329 |
| (李莉, 陆丹, 计远, 赵月红. 物理化学学报, 2010, 26(5), 1323—1329) | |
| [22] | Reddy B. M., Reddy G. K., Rao K. N., J. Mater. Sci., 2009, 44(18), 4874—4882 |
| [23] | Zhang P., Yu Y., Wang E., ACS Applied Materials & Interfaces,2014, 6(7), 4622—4629 |
| [24] | Wang J., Yu Y., Li S., J. Phys. Chem. C,2013, 117(51), 27120—27126 |
| [25] | Zhang J. Q., Li L., Liu D., J. Mol. Catal(Chin), 2015, 29(4), 348—358 |
| (张剑琦, 李莉, 柳迪. 分子催化, 2015, 29(4), 348—358) | |
| [26] | Grzegorz C., Radoslaw P., Izabela S., Phys. Chem. C,2007, 111(15), 5599—5604 |
| [27] | Lu L., Li L., Huang X. D., Li Z., Zhang X. L., Wang L. L., Chem. J. Chinese Universities,2013, 34(9), 2202—2209 |
| (路露, 李莉, 黄贤丹, 李召, 张秀丽, 王丽丽. 高等学校化学学报, 2013, 34(9), 2202—2209) | |
| [28] | Philip G. H., Nicholas C. L., Wayne D., J. Phys. Chem. B,1998, 102, 10672—10679 |
| [29] | Abdallah W. A., Taylor S. D., J. Phys. Chem. C,2008, 112(48), 18963—18972 |
| [30] | Li L., Zhang X. L., Zhang W. Z., Wang L. L., Chen X., Gao Y., Colloids and Surfaces A: Physicochem. Eng. Aspects,2014, 457, 134—141 |
| [31] | Zhang J. J., Li L., Wang S., Huang T. T., Hao Y. T., Qi Y. Y., RSC Adv., 2016, 6, 13991—14001 |
| [32] | Chavillon B., Renaud A., Tessier F., J. Am. Chem. Soc., 2012, 134, 464—470 |
| [33] | Geoffrey I. N. W., James B. M., Hicham I., Dong X., Chem. Mater., 2008, 20, 1183—1190 |
| [34] | Xu Y., Martin A. A. S., American Mineralogist,2000, 85, 543—556 |
| [35] | Zou S., Schatz G. C., J. Chem. Phys., 2004, 121(24), 12606—12612 |
| [36] | Wang X. H., Zhang Y. K., Hao C., Feng F., Yin H. B., Si N. C., Ind. Eng. Chem. Res., 2014, 53, 6585—6592 |
| [37] | Li L., Wang L.L., Zhang W. Z., Zhang X. L., Chen X., Dong X.,J. Nanopart Res., 2014, (16), 2753 |
| [38] | Li Y. R., Li L. L., Gong Y. Q., Bai S., Nano Res., 2015, 8, 3621 |
| [1] | LI Wei, LUO Piao, HUANG Lianzhan, CUI Zhiming. Lithium Polystyrene Sulfonate Based Interfacial Protective Layer for Lithium Metal Anodes [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220166. |
| [2] | GUI Chen, WANG Haolin, SHAO Baixuan, YANG Yujing, XU Guangqing. Molten-salt-assistance Synthesis and Photocatalytic Hydrogen Evolution Performances of g-C3N4 Nanostructures [J]. Chem. J. Chinese Universities, 2021, 42(3): 827. |
| [3] | SUN Yaguang, ZHANG Hanyan, MING Tao, XU Baotong, GAO Yu, DING Fu, XU Zhenhe. Synthesis of ZnIn2S4/g-C3N4 Nanocomposites with Efficient Photocatalytic H2 Generation Activity by a Simple Hydrothermal Method [J]. Chem. J. Chinese Universities, 2021, 42(10): 3160. |
| [4] | LI Li, LI Pengfei, WANG Bo. Photocatalytic Application of Covalent Organic Frameworks [J]. Chem. J. Chinese Universities, 2020, 41(9): 1917. |
| [5] | CAO Meng, LIU Yang, ZHANG Shangxi, WANG Zhenxi, XU Sheng. Synthesis and Photocatalytic Hydrogen Evolution Properties of Chitosan Cobalt Complex † [J]. Chem. J. Chinese Universities, 2020, 41(4): 735. |
| [6] | LUO Wei, LIANG Youcai, HU Zhicheng, TANG Haoran, LIU Xiaocheng, XING Yetong, HUANG Fei. Preparation of Novel Hydrophilic Conjugated Polymers and Their Applicationin Photocatalytic Hydrogen Evolution † [J]. Chem. J. Chinese Universities, 2020, 41(3): 456. |
| [7] | WANG Lin, ZHANG Yanhui, Arzugul Muslim, LAN Haidie. Morphology and Size Regulation of Polyaniline Induced by PS-b-P2VP as Template and Its Electrochemical Characters [J]. Chem. J. Chinese Universities, 2019, 40(8): 1748. |
| [8] | FENG Wei,WANG Bowei,JIANG Yang,LI Longyun. Design, Preparation and Surface-enhanced Raman Scattering(SERS) Spectrum of Single Ag Nanodot† [J]. Chem. J. Chinese Universities, 2019, 40(7): 1345. |
| [9] | MENG Zihui,WANG Yifei,XIE Tengsheng,CHEN Wei,QIU Lili,XUE Min. Molecularly Imprinted Hollow Spheres for the Solid Phase Extraction of Protein† [J]. Chem. J. Chinese Universities, 2019, 40(1): 62. |
| [10] | XU Dan,DING Yadan,WANG Xue,CONG Tie,LIU Junping,HONG Xia,PAN Ying. Microdroplet Detection of Protein Based on Superhydrophobic Polystyrene Film† [J]. Chem. J. Chinese Universities, 2018, 39(9): 1913. |
| [11] | FENG Wei,WANG Bowei,ZHENG Yan,JIANG Yang. Preparation and Surface-enhanced Raman Scattering(SERS) of Single Au Nanodot† [J]. Chem. J. Chinese Universities, 2018, 39(9): 1875. |
| [12] | QIN Yanli, XU Haiping, QIU Houtian, ZHAI Yue, DAI Xiujuan, YANG Dandan, WANG Jingrong. Preparation and PTC Properties of HIPS/HDPE/CB Composites† [J]. Chem. J. Chinese Universities, 2018, 39(6): 1305. |
| [13] | WANG Zhengzhou,YANG Ting. Preparation and Properties of Expanded Vermiculite Coated Expanded Polystyrene/Cement Composite Foams† [J]. Chem. J. Chinese Universities, 2018, 39(5): 1098. |
| [14] | DENG Caisong, PAN Tingting, DU Lan, WANG Ming, NI Haibin, NI Xiaoqi. Fabrication of Monolayer Crystalline Films on Optical Fiber End by Micro-flow Injection Method† [J]. Chem. J. Chinese Universities, 2018, 39(4): 708. |
| [15] | LI Aiju, WANG Yuxi, LU Shaoyong, LIU Kun. Ligand Exchange of Gold Nanoparticles with Thiol-terminated Polystyrene† [J]. Chem. J. Chinese Universities, 2018, 39(3): 552. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||