

Chem. J. Chinese Universities ›› 2015, Vol. 36 ›› Issue (6): 1187.doi: 10.7503/cjcu20140931
• Physical Chemistry • Previous Articles Next Articles
CAO Xiaolu, WANG Longlong, WANG Yajun, XU Qunjie, LI Qiaoxia*( )
)
Received:2014-10-22
Online:2015-06-10
Published:2015-05-22
Contact:
LI Qiaoxia
E-mail:liqiaoxia@ shiep. edu. cn
Supported by:CLC Number:
TrendMD:
CAO Xiaolu, WANG Longlong, WANG Yajun, XU Qunjie, LI Qiaoxia. Facile Preparation of Amino-modified Pd/TiO2/C Nanocatalyst and Its Electrocatalytic Performance for Ethanol Oxidation in Alkaline Solution†[J]. Chem. J. Chinese Universities, 2015, 36(6): 1187.
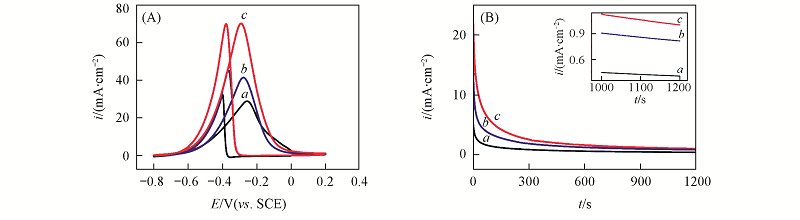
Fig.7 Cyclic voltammograms on Pd/C(a), Pd/TiO2/C(b) and Pd/TiO2/C-APTMS(c) catalysts in 1.0 mol/L NaOH+1.0 mol/L H3CH2OH solution at a scan rate of 50 mV/s(A) and corresponding chronoamperometric i-t curves at -0.2 V(B)
| [1] | Bianchini C., Shen P. K., Chemical Reviews, 2009, 109(9), 4183—4206 |
| [2] | Wang Y., Shi F. F., Yang Y. Y., Cai W. B., J. Power Sources, 2013, 243, 369—373 |
| [3] | Wang Y., Tan D., Chem. Res. Chinese Universities, 2014, 30(2), 320—325 |
| [4] | Li H., Sun G., Jiang Q., Zhu M., Sun S., Qin X., Electrochem. Commun., 2007, 9(6), 1410—1415 |
| [5] | Gattia D. M., Antisari M. V., Giorgi L., Marazzi R., Piscopiello E., Montone A., Bellitto S., Lococcia S., Traversa E., J. Power Sources, 2009, 194(1), 243—251 |
| [6] | Antolini E., Appl. Catal. B: Environ., 2009, 88(1), 1—24 |
| [7] | Han J., Zhou Z. Y., Wang Q., Lü M. Q., Chen C., Sun S. G., J. Electrochem., 2014, 20(2), 110—115 |
| (韩金, 周志有, 王强, 吕妙强, 陈驰, 孙世刚.电化学, 2014,20(2), 110—115) | |
| [8] | He W., Zou L. L., Zhou Y., Lu X. J., Li Y., Zhang X. G., Yang H., Chem. J. Chinese Universities, 2012, 33(1), 133—138 |
| (何卫, 邹亮亮, 周毅, 卢向军, 李媛, 张校刚, 杨辉.高等学校化学学报, 2012,33(1), 133—138) | |
| [9] | Qin Y. H., Yang H. H., Lv R. L., Wang W. G., Wang C. W., Electrochimica Acta, 2013, 106, 372—377 |
| [10] | Tiido K., Alexeyeva N., Couillard M., Bock C. R., MacDougall B., Tammeveski K., Electrochimica Acta, 2013, 107, 509—517 |
| [11] | Huang S. Y., Ganesan P., Popov B. N., ACS Catalysis, 2012, 2(5), 825—831 |
| [12] | Qi L., Yin Y., Tu W. G., Wu B. B., Wang Z. S., Liu J. G., Gu J., Zou Z. G., J. Electrochem., 2014, 20(4), 377—381 |
| (戚利, 殷瑛, 涂文广, 吴兵兵, 王兆生, 刘建国, 顾军, 邹志刚.电化学, 2014,20(4), 377—381) | |
| [13] | Cao C., Hu C., Tian J., Shen W., Wang S., Liu H., J. Electrochem. Soc., 2013, 160(11), 793—799 |
| [14] | Wang X. M., Xia Y. Y., Electrochimica Acta, 2010, 55(3), 851—856 |
| [15] | Liu C.P., Yang H., Xing W., Lu T.H., Chem. J. Chinese Universities, 2002, 23(7), 1367—1370 |
| (刘长鹏, 杨辉, 邢巍, 陆天虹.高等学校化学学报, 2002,23(7), 1367—1370) | |
| [16] | Hu F., Ding F., Song S., Shen P., J. Power Sources, 2006, 163, 415—419 |
| [17] | Jiang K., Cai W. B., Appl. Catal. B: Environ., 2014, 147, 185—192 |
| [18] | Photong S., Boonamnuayvitaya V., Water, Air Soil Poll., 2010, 210(1—4), 453—461 |
| [19] | Wang J. Y., Huo S J., Cai W. B., Xu Q. J., Chem. Lett., 2006, 35(6), 582—583 |
| [20] | Etgar L., Zhang W., Gabriel S. G., Hickey S. K., Nazeeruddin M., Eychmüller A., Liu B., Grätzel M., Adv. Mater., 2012, 24(16), 2202—2206 |
| [21] | Yu X., Pickup P. G., Electrochem. Commun., 2010, 12, 800—803 |
| [22] | Zhang S., Shao Y. Y., Yin G.P., Lin Y. H., J. Mater. Chem., 2009, 19(42), 7995—8001 |
| [23] | Lv Q., Zhao X., Zhao X., Li C., Liu C., Xing W., J. Power Sources, 2012, 218, 93—99 |
| [24] | Li Q. X., Yang Y. C., Liu M. S., Mao H. M., Xu Q. J., J. Electrochem., 2014, 20(1), 85—88 |
| (李巧霞, 杨意超, 刘明爽, 毛宏敏, 徐群杰.电化学, 2014,20(1), 85—88) | |
| [25] | Mao H., Wang L., Zhu P., Xu Q., Li Q., Inter. J. Hydrogen Energy, 2014, 39(31), 17583—17588 |
| [26] | Chu D. B., Yin X. J., Feng D. X., Lin H. S., Tian Z. W., Acta Physico-Chimica Sinica, 2006, 22(10), 1238—1242 |
| (褚道葆, 尹晓娟, 冯德香, 林华水, 田昭武.物理化学学报, 2006,22(10), 1238—1242) | |
| [27] | Zana A., Rüdiger C., Kunze-Liebhäuser J., Granozzi G., Reeler N. E. A., Vosch T., Kirkensgaard J. J. K., Arenz M., Electrochimica Acta, 2014, 139, 21—28 |
| [1] | ZHAO Sheng, HUO Zhipeng, ZHONG Guoqiang, ZHANG Hong, HU Liqun. Preparation of Modified Gadolinium/Boron/Polyethylene Nanocomposite and Its Radiation Shielding Performance for Neutron and Gamma-ray [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220039. |
| [2] | XU Xiaolong, FANG Lining, LIU Changyu, LIU Minchao, JIA Jianbo. Preparation of Z-type g-C3N4/Pt/TiO2 Nanotube Array Composite Electrode and Its Performance of Photoelectric Oxidation of Methanol [J]. Chem. J. Chinese Universities, 2021, 42(9): 2926. |
| [3] | SHI Ying, HU Guangjian, WU Minjie, LI Feng. Applications of Low Temperature Plasma for the Materials in Li-ion Batteries [J]. Chem. J. Chinese Universities, 2021, 42(5): 1315. |
| [4] | JI Shaobo, CHEN Xiaodong. Surface and Interface Chemistry in Flexible Electronics [J]. Chem. J. Chinese Universities, 2021, 42(4): 1074. |
| [5] | YAN Fanyong, SUN Zhonghui, PANG Jiping, JIANG Yingxia, CHEN Yuan. Functionalized Carbon Dots of Benzothiazine Derivatives for Detection of Quercetin in Ginkgo Biloba Tea [J]. Chem. J. Chinese Universities, 2020, 41(8): 1768. |
| [6] | PENG Xinyan, LIU Yunhong, LI Jiawen, FENG Yilong, WANG Hanchun. Preparation and Characterization of Tannin/Zwitterionic Modified Oil-water Separation Membrane † [J]. Chem. J. Chinese Universities, 2020, 41(6): 1337. |
| [7] | CHEN Liangdan,ZOU Wei,WU Liang,XIA Fanjie,HU Zhiyi,LI Yu,SU Baolian. Nano-Al2O3 Coated Li-rich Cathode Material Li1. 2Ni0.13Co0.13Mn0.54O2 for Highly Improved Lithium-ion Batteries † [J]. Chem. J. Chinese Universities, 2020, 41(6): 1329. |
| [8] | LIU Shuaizhuo,ZHANG Qian,LIU Ning,XIAO Wenyan,FAN Leiyi,ZHOU Ying. One-step Synergistic Hydrophobic Modification of Melamine Sponge and Its Application † [J]. Chem. J. Chinese Universities, 2020, 41(3): 521. |
| [9] | ZHANG Xinyu, WANG Hong, FANG Yun, FAN Ye. Stimuli-responsive Fe3O4 Nanoparticle Modified by Conjugated Linoleic Acid [J]. Chem. J. Chinese Universities, 2020, 41(11): 2519. |
| [10] | MAO Long, LIU Yuejun, FAN Shuhong. Preparation and Properties of Polypyrrole Modified Layered Clay/poly(ε-caprolactone) Antibacterial Nanocomposites [J]. Chem. J. Chinese Universities, 2019, 40(8): 1726. |
| [11] | SUI Jiayang, LIU Xiaoyang, QIAN Miaomiao, ZHU Yanchao, XUE Beichen, FENG Yi, TIAN Yumei, WANG Xiaofeng. Surface Modification of Silica/carbon Black Derived from Rice Husks and Its Influence on Natural Rubber Composites† [J]. Chem. J. Chinese Universities, 2019, 40(7): 1561. |
| [12] | LIN Zhouchen,HUANG Qiaoxi,LEI Ming. Fabrication and Electrocatalytic Performance of Graphene-fullerene Ammonium Iodide Composite Supported Pd Nanocatalyst for Ethanol Oxidation† [J]. Chem. J. Chinese Universities, 2019, 40(5): 1013. |
| [13] | CHEN Weimin, ZHU Zhenyu. Performances of a Hybrid Carbon Material Supported Pd Catalyst for Ethanol Electrooxidation† [J]. Chem. J. Chinese Universities, 2018, 39(2): 337. |
| [14] | LIU Zile, ZENG Zequan, YANG Jieyang, CUI Yan, WU Ailian, LI Zhe, HUANG Zhanggen. Degradation of Phenol with Persulfate Activated by Surface Modified Activated Carbon† [J]. Chem. J. Chinese Universities, 2017, 38(7): 1241. |
| [15] | MA Zichuan, LI Junshu, XING Shengtao. Facile Cyclohexane-mediated Hydrothermal Synthesis of Modified δ-MnO2 with Enhanced Fenton-like Catalytic Activity† [J]. Chem. J. Chinese Universities, 2017, 38(4): 636. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||