

Chem. J. Chinese Universities ›› 2014, Vol. 35 ›› Issue (1): 37.doi: 10.7503/cjcu20130721
• Analytical Chemistry • Previous Articles Next Articles
WEI Wanghui1, WANG Qing1, CHU Yanqiu1,*( ), WANG Rizhi2,*(
), WANG Rizhi2,*( ), DING Chuanfan1
), DING Chuanfan1
Received:2013-07-25
Online:2014-01-10
Published:2013-12-13
Contact:
CHU Yanqiu,WANG Rizhi
E-mail:chuyq@fudan.edu.cn;rizhi9752@hotmail.com
Supported by:CLC Number:
TrendMD:
WEI Wanghui, WANG Qing, CHU Yanqiu, WANG Rizhi, DING Chuanfan. Fragmentation Reactions of Complexes of Alkali Metal Ions with Pentaserine, Pentaleucine and Pentalysine in Gas Phase†[J]. Chem. J. Chinese Universities, 2014, 35(1): 37.
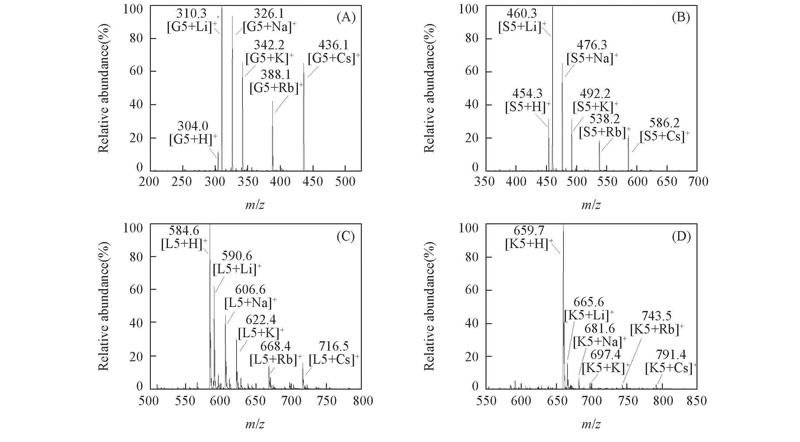
Fig.1 Mass spectra for competition reaction of pentapeptides with alkali metal ions in a molar ratio of 1∶1∶1∶1∶1∶1∶1(A) G5; (B) S5; (C) L5; (D) K5. c(Pentapeptide)=c(Alkali metal ion)=1.0×10-4 mol/L.
| 105 [G | 105 [K+]0/(mol·L-1) | IP | IPM | IPM2 | a1 | a2 | 10-5 Ka1/(L·mol-1) |
|---|---|---|---|---|---|---|---|
| 5.00 | 10.00 | 7.54 | 100.00 | 97.94 | 0.487 | 0.477 | 4.84 |
| 5.00 | 15.00 | 3.17 | 100.00 | 98.03 | 0.497 | 0.487 | 4.06 |
| 5.00 | 20.00 | 1.92 | 100.00 | 98.35 | 0.499 | 0.491 | 3.96 |
| 5.00 | 25.00 | 1.68 | 100.00 | 98.42 | 0.499 | 0.492 | 3.15 |
| 5.00 | 30.00 | 1.32 | 100.00 | 99.31 | 0.498 | 0.495 | 3.15 |
| Average | 3.83 | ||||||
| SD | 0.70 | ||||||
| RSD(%) | 18.2 |
Table 1 Ka1 values of the complexes of G5 with K+
| 105 [G | 105 [K+]0/(mol·L-1) | IP | IPM | IPM2 | a1 | a2 | 10-5 Ka1/(L·mol-1) |
|---|---|---|---|---|---|---|---|
| 5.00 | 10.00 | 7.54 | 100.00 | 97.94 | 0.487 | 0.477 | 4.84 |
| 5.00 | 15.00 | 3.17 | 100.00 | 98.03 | 0.497 | 0.487 | 4.06 |
| 5.00 | 20.00 | 1.92 | 100.00 | 98.35 | 0.499 | 0.491 | 3.96 |
| 5.00 | 25.00 | 1.68 | 100.00 | 98.42 | 0.499 | 0.492 | 3.15 |
| 5.00 | 30.00 | 1.32 | 100.00 | 99.31 | 0.498 | 0.495 | 3.15 |
| Average | 3.83 | ||||||
| SD | 0.70 | ||||||
| RSD(%) | 18.2 |
| 105 [S | 105 [K+]0/(mol·L-1) | IP | IPM | IPM2 | a1 | a2 | 10-4 |
|---|---|---|---|---|---|---|---|
| 5.00 | 10.00 | 23.99 | 100.00 | 51.09 | 0.571 | 0.292 | 9.86 |
| 5.00 | 15.00 | 12.80 | 100.00 | 70.47 | 0.546 | 0.384 | 9.25 |
| 5.00 | 20.00 | 8.15 | 100.00 | 97.10 | 0.487 | 0.473 | 9.49 |
| 5.00 | 25.00 | 6.97 | 100.00 | 97.57 | 0.489 | 0.477 | 8.09 |
| 5.00 | 30.00 | 5.47 | 100.00 | 98.15 | 0.491 | 0.482 | 8.00 |
| Average | 8.94 | ||||||
| SD | 0.84 | ||||||
| RSD(%) | 9.4 |
Table 2 Ka1 values of the complexes of S5 with K+
| 105 [S | 105 [K+]0/(mol·L-1) | IP | IPM | IPM2 | a1 | a2 | 10-4 |
|---|---|---|---|---|---|---|---|
| 5.00 | 10.00 | 23.99 | 100.00 | 51.09 | 0.571 | 0.292 | 9.86 |
| 5.00 | 15.00 | 12.80 | 100.00 | 70.47 | 0.546 | 0.384 | 9.25 |
| 5.00 | 20.00 | 8.15 | 100.00 | 97.10 | 0.487 | 0.473 | 9.49 |
| 5.00 | 25.00 | 6.97 | 100.00 | 97.57 | 0.489 | 0.477 | 8.09 |
| 5.00 | 30.00 | 5.47 | 100.00 | 98.15 | 0.491 | 0.482 | 8.00 |
| Average | 8.94 | ||||||
| SD | 0.84 | ||||||
| RSD(%) | 9.4 |
| 105 [L | 105 [K+]0/(mol·L-1) | IP | IPM | IPM2 | a1 | a2 | 10-4 |
|---|---|---|---|---|---|---|---|
| 5.00 | 10.00 | 51.03 | 100.00 | 8.19 | 0.628 | 0.051 | 3.08 |
| 5.00 | 15.00 | 33.63 | 100.00 | 13.53 | 0.680 | 0.092 | 2.79 |
| 5.00 | 20.00 | 21.85 | 100.00 | 15.31 | 0.729 | 0.112 | 3.01 |
| 5.00 | 25.00 | 18.68 | 100.00 | 17.09 | 0.737 | 0.126 | 2.68 |
| 5.00 | 30.00 | 15.71 | 100.00 | 20.75 | 0.733 | 0.152 | 2.57 |
| Average | 2.83 | ||||||
| SD | 0.21 | ||||||
| RSD(%) | 7.4 |
Table 3 Ka1 values of the complexes of L5 with K+
| 105 [L | 105 [K+]0/(mol·L-1) | IP | IPM | IPM2 | a1 | a2 | 10-4 |
|---|---|---|---|---|---|---|---|
| 5.00 | 10.00 | 51.03 | 100.00 | 8.19 | 0.628 | 0.051 | 3.08 |
| 5.00 | 15.00 | 33.63 | 100.00 | 13.53 | 0.680 | 0.092 | 2.79 |
| 5.00 | 20.00 | 21.85 | 100.00 | 15.31 | 0.729 | 0.112 | 3.01 |
| 5.00 | 25.00 | 18.68 | 100.00 | 17.09 | 0.737 | 0.126 | 2.68 |
| 5.00 | 30.00 | 15.71 | 100.00 | 20.75 | 0.733 | 0.152 | 2.57 |
| Average | 2.83 | ||||||
| SD | 0.21 | ||||||
| RSD(%) | 7.4 |
| 105 [K | 105 [K+]0/(mol·L-1) | IP | IPM | IPM2 | a1 | a2 | 10-3 |
|---|---|---|---|---|---|---|---|
| 5.00 | 10.00 | 100.00 | 22.88 | 7.29 | 0.176 | 0.056 | 2.68 |
| 5.00 | 15.00 | 100.00 | 34.33 | 14.11 | 0.231 | 0.095 | 2.66 |
| 5.00 | 20.00 | 100.00 | 42.90 | 22.47 | 0.259 | 0.136 | 2.47 |
| 5.00 | 25.00 | 100.00 | 51.93 | 31.90 | 0.282 | 0.174 | 2.37 |
| 5.00 | 30.00 | 100.00 | 61.13 | 40.60 | 0.303 | 0.201 | 2.31 |
| Average | 2.50 | ||||||
| SD | 0.17 | ||||||
| RSD(%) | 6.8 |
Table 4 Ka1 values of the complexes of K5 with K+
| 105 [K | 105 [K+]0/(mol·L-1) | IP | IPM | IPM2 | a1 | a2 | 10-3 |
|---|---|---|---|---|---|---|---|
| 5.00 | 10.00 | 100.00 | 22.88 | 7.29 | 0.176 | 0.056 | 2.68 |
| 5.00 | 15.00 | 100.00 | 34.33 | 14.11 | 0.231 | 0.095 | 2.66 |
| 5.00 | 20.00 | 100.00 | 42.90 | 22.47 | 0.259 | 0.136 | 2.47 |
| 5.00 | 25.00 | 100.00 | 51.93 | 31.90 | 0.282 | 0.174 | 2.37 |
| 5.00 | 30.00 | 100.00 | 61.13 | 40.60 | 0.303 | 0.201 | 2.31 |
| Average | 2.50 | ||||||
| SD | 0.17 | ||||||
| RSD(%) | 6.8 |
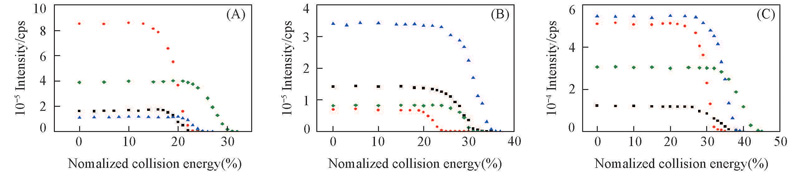
Fig.2 Relationship between intensity of mass spectrometric peak and normalized collision energy The precursors are the complexes of G5(■), S5(●), L5(▲) or K5(◆) with H+(A), Li+(B) and 2Li+(C).
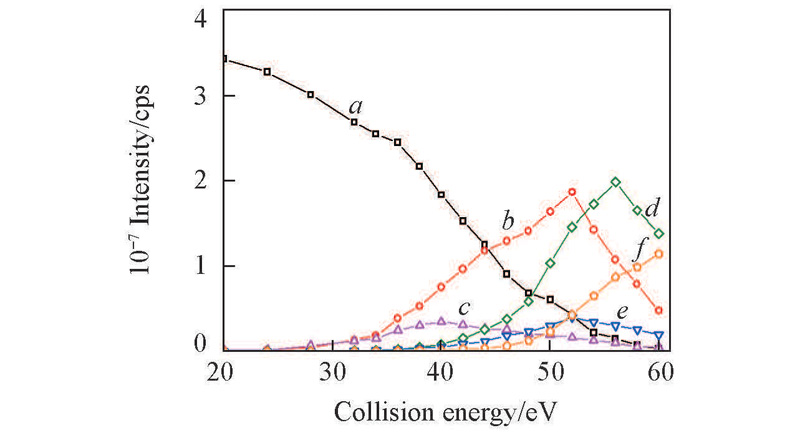
Fig.4 CID breakdown curves for the complexes of Na+ with pentaleucinea. Precursor ion [L5+Na]+; b. [b4+Na+OH]+; c. [b4+Na-H]+; d. [y3+Na+H]+; e. [b3+Na-H]+; f. [y2+Na+H]+.
| Precursor ion(#) | an/an*/an** | Other | ||
|---|---|---|---|---|
| [S5+H]+ | a4, a3, a2, a1, a4-H2O, a4-2H2O, a3-H2O, a3-2H2O, a2-H2O | b4, b3, b2, b4-H2O, b3-H2O, b2-H2O, b3-2H2O | y3, y2, y1 | [#-H2O]+, [#-2H2O]+, [#-3H2O]+, [#-4H2O]+ |
| [S5+Li]+ | a4*, a4*-CH2O, a4*-H2O, a3*, a3*-CH2O, a3*-H2O, a2*, a2*-CH2O, a2*-H2O, a1* | [b4+Li+OH]+, [b4+Li+OH- CH2O]+, b4*-H2O, b4*-CO2, [b3+Li+OH]+, b4*-H2O-CH2O, b3*-H2O, b4*, b3*-CO2, [b3+ Li+OH-CH2O]+, b3*, b2*, b3*-H2O-CH2O, b2*-H2O, b2*-H2O-CH2O | y2*, y2*-H2O, y1* | [#-H2O]+, [#-CH2O]+, [#-H2O-CH2O]+ , [#-H2O-CO2]+, [#-H2O-2CH2O]+, [#-2H2O-CH2O]+ |
| [S5+Na]+ | a4*, a4*-CH2O, a3*, a1* | [b4+Na+OH]+, [b4+Na+OH- CH2O]+, b4*, b4*-H2O, [b3+ Na+OH]+, b3*, b2*, [b3+Na+ OH-CH2O]+ | y2* | [#-H2O]+, [#-CH2O]+, [#-H2O-CH2O]+, [#-H2O-CO2]+ |
| [S5+K]+ | a4*, a4*-CH2O | [b4+K+OH]+, [b4+K+OH- CH2O]+, b4*, b4*-H2O, [b3+ K+OH]+, [b3+K+OH-CH2O]+, b3* | K+, [#-H2O]+, [#-CH2O]+, [#-2CH2O]+ | |
| [S5+Rb]+ | Rb+ | |||
| [S5+Cs]+ | Cs+ | |||
| [S5+2Li-H]+ | a4**, a3**, a4*, a3* | b3**, b3**-CH2O, b2**, b2**-CH2O | y4**, y3**, y2**, y1**, y4**-CH2O, y3**-CH2O, y2**-CH2O, y1**-CH2O, y4**-2CH2O, y3**-CH2O | [#-H2O]+, [#-CH2O]+, [#-2CH2O]+, [#-3CH2O]+, [#-4CH2O]+, [#-H2O-CH2O]+, [#-H2O-2CH2O]+, [#-H2O-3CH2O]+, [#-2H2O-3CH2O]+ |
| [S5+2Na-H]+ | a4**-CH2O, a4**-2CH2O, a4**-3CH2O, a3**-CH2O | b2**, b4*, b4*-CH2O | y4**, y3**, y2**, y1**, y4**-CH2O, y3**-CH2O, y2**-CH2O, y1**-CH2O, y3**-2CH2O, y2**-2CH2O | [#-H2O]+, [#-CH2O]+, [#-2CH2O]+, [#- 3CH2O]+, [#-4CH2O]+, [#-5CH2O]+, [#-H2O- CH2O]+, [#-H2O- 2CH2O]+, [#-H2O- 3CH2O]+, [#-H2O- 4CH2O]+ |
| [S5+2K-H]+ | a4**-H2O | b3**-CH2O, b3**-2CH2O, b2**-CH2O | y4**, y3**, y2**, y1**, y3**-CH2O, y2**-CH2O, y1**-CH2O, y2**-2CH2O | K+, [#-H2O]+, [#-2H2O]+, [#-CH2O]+, [#-2CH2O]+, [#-3CH2O]+, [#-4CH2O]+, [#-H2O-CH2O]+, [#-H2O-2CH2O]+, [#-2H2O- 2CH2O]+, [#-H2O- 3CH2O]+, [#-2H2O- 3CH2O]+, [#-H2O- 4CH2O]+ |
| [S5+2Rb-H]+ | a4**, a4**-H2O | b3**, b1**, b3**-2H2O | y4**, y3**, y2**, y1** | Rb+, [#-H2O]+, [#-CH2O]+, [#-2CH2O]+, [#-3CH2O]+, [#-H2O- CH2O]+, [#-H2O-2CH2O]+ |
| [S5+2Cs-H]+ | a4**-H2O | b3** | y4**, y3**, y1**, y1**-CH2O | Cs+, [#-H2O]+, [#-CH2O]+, [#-2CH2O]+, [#-H2O-CH2O]+, [#-H2O-2CH2O]+, [#-3CH2O]+ |
Table 5 Main fragment ions of protonated and alkali metal cationized S5 complexes obtained with CID
| Precursor ion(#) | an/an*/an** | Other | ||
|---|---|---|---|---|
| [S5+H]+ | a4, a3, a2, a1, a4-H2O, a4-2H2O, a3-H2O, a3-2H2O, a2-H2O | b4, b3, b2, b4-H2O, b3-H2O, b2-H2O, b3-2H2O | y3, y2, y1 | [#-H2O]+, [#-2H2O]+, [#-3H2O]+, [#-4H2O]+ |
| [S5+Li]+ | a4*, a4*-CH2O, a4*-H2O, a3*, a3*-CH2O, a3*-H2O, a2*, a2*-CH2O, a2*-H2O, a1* | [b4+Li+OH]+, [b4+Li+OH- CH2O]+, b4*-H2O, b4*-CO2, [b3+Li+OH]+, b4*-H2O-CH2O, b3*-H2O, b4*, b3*-CO2, [b3+ Li+OH-CH2O]+, b3*, b2*, b3*-H2O-CH2O, b2*-H2O, b2*-H2O-CH2O | y2*, y2*-H2O, y1* | [#-H2O]+, [#-CH2O]+, [#-H2O-CH2O]+ , [#-H2O-CO2]+, [#-H2O-2CH2O]+, [#-2H2O-CH2O]+ |
| [S5+Na]+ | a4*, a4*-CH2O, a3*, a1* | [b4+Na+OH]+, [b4+Na+OH- CH2O]+, b4*, b4*-H2O, [b3+ Na+OH]+, b3*, b2*, [b3+Na+ OH-CH2O]+ | y2* | [#-H2O]+, [#-CH2O]+, [#-H2O-CH2O]+, [#-H2O-CO2]+ |
| [S5+K]+ | a4*, a4*-CH2O | [b4+K+OH]+, [b4+K+OH- CH2O]+, b4*, b4*-H2O, [b3+ K+OH]+, [b3+K+OH-CH2O]+, b3* | K+, [#-H2O]+, [#-CH2O]+, [#-2CH2O]+ | |
| [S5+Rb]+ | Rb+ | |||
| [S5+Cs]+ | Cs+ | |||
| [S5+2Li-H]+ | a4**, a3**, a4*, a3* | b3**, b3**-CH2O, b2**, b2**-CH2O | y4**, y3**, y2**, y1**, y4**-CH2O, y3**-CH2O, y2**-CH2O, y1**-CH2O, y4**-2CH2O, y3**-CH2O | [#-H2O]+, [#-CH2O]+, [#-2CH2O]+, [#-3CH2O]+, [#-4CH2O]+, [#-H2O-CH2O]+, [#-H2O-2CH2O]+, [#-H2O-3CH2O]+, [#-2H2O-3CH2O]+ |
| [S5+2Na-H]+ | a4**-CH2O, a4**-2CH2O, a4**-3CH2O, a3**-CH2O | b2**, b4*, b4*-CH2O | y4**, y3**, y2**, y1**, y4**-CH2O, y3**-CH2O, y2**-CH2O, y1**-CH2O, y3**-2CH2O, y2**-2CH2O | [#-H2O]+, [#-CH2O]+, [#-2CH2O]+, [#- 3CH2O]+, [#-4CH2O]+, [#-5CH2O]+, [#-H2O- CH2O]+, [#-H2O- 2CH2O]+, [#-H2O- 3CH2O]+, [#-H2O- 4CH2O]+ |
| [S5+2K-H]+ | a4**-H2O | b3**-CH2O, b3**-2CH2O, b2**-CH2O | y4**, y3**, y2**, y1**, y3**-CH2O, y2**-CH2O, y1**-CH2O, y2**-2CH2O | K+, [#-H2O]+, [#-2H2O]+, [#-CH2O]+, [#-2CH2O]+, [#-3CH2O]+, [#-4CH2O]+, [#-H2O-CH2O]+, [#-H2O-2CH2O]+, [#-2H2O- 2CH2O]+, [#-H2O- 3CH2O]+, [#-2H2O- 3CH2O]+, [#-H2O- 4CH2O]+ |
| [S5+2Rb-H]+ | a4**, a4**-H2O | b3**, b1**, b3**-2H2O | y4**, y3**, y2**, y1** | Rb+, [#-H2O]+, [#-CH2O]+, [#-2CH2O]+, [#-3CH2O]+, [#-H2O- CH2O]+, [#-H2O-2CH2O]+ |
| [S5+2Cs-H]+ | a4**-H2O | b3** | y4**, y3**, y1**, y1**-CH2O | Cs+, [#-H2O]+, [#-CH2O]+, [#-2CH2O]+, [#-H2O-CH2O]+, [#-H2O-2CH2O]+, [#-3CH2O]+ |
| Precursor ion(#) | an/ | Other | ||
|---|---|---|---|---|
| [L5+H]+ | a4, a2, a1, a4-NH3, a3-NH3, a4-CH2-CH-(CH3)2 | b3, b2 | y4, y3, y2 | [#-H2O]+ |
| [L5+Li]+ | a4*, a3*, a3*-(CH3)2, a4*- CH2-CH-(CH3)2, a2*, a2*-(CH3)2 | [b4+Li+OH]+, b4*-NH3, [b3+Li+OH]+, b3*-NH3, b4*, b3*, b2* | y3*, y2*, y1* | [#-H2O-NH3]+ |
| [L5+Na]+ | a4*, a3*, a1*, a3*-CH2-CH- (CH3)2, a2*-CH2-CH-(CH3)2 | [b4+Na+OH]+, b3*, [b3+Na+OH]+, b2* | y2*, y1* | |
| [L5+K]+ | a4*, a3* | [b4+K+OH]+, b4*, [b3+K+OH]+ | y2*, y2*-NH3, y4*-CO | K+, [#-CO]+, [#-CO2]+ |
| [L5+Rb]+ | [b4+Rb+OH]+ | Rb+ | ||
| [L5+Cs]+ | Cs+ | |||
| [L5+2Li-H]+ | a4**, a3**, a4*, a3*, a1*, a3*-CO2, a2*-CO2 | b2*, b4*-H2O-NH3, b3*-H2O-NH3 | y4**, y2**, y1**, y3*, y1* | [#-CO2]+, [#-H2O-NH3]+ |
| [L5+2Na-H]+ | a4**, a4*, a3*, a2*, a1*, a3*-H2O-NH3, a2*-H2O-NH3, a2*-CH2-CH-(CH3)2 | b3*, b3*-NH3 | y4**, y3**, y2**, y1**, y2**-CO2 | [#-CO2]+ |
| [L5+2K-H]+ | a4*, a3*, a2*, a1*-(CH3)2 | y4**, y3**, y2**, y1**, y1**-H2O-NH3 | K+ | |
| [L5+2Rb-H]+ | a3**-CH2-CH-(CH3)2, a2**-CH2-CH-(CH3)2 | y3**, y2**, y1**, y3**-NH3 | Rb+ | |
| [L5+2Cs-H]+ | a2**-CH2-CH-(CH3)2 | y3**, y2**, y1**, y4**-CH2-CH-(CH3)2 | Cs+ |
Table 6 Main product ions of protonated and alkali metal cationized L5 complexes obtained with CID
| Precursor ion(#) | an/ | Other | ||
|---|---|---|---|---|
| [L5+H]+ | a4, a2, a1, a4-NH3, a3-NH3, a4-CH2-CH-(CH3)2 | b3, b2 | y4, y3, y2 | [#-H2O]+ |
| [L5+Li]+ | a4*, a3*, a3*-(CH3)2, a4*- CH2-CH-(CH3)2, a2*, a2*-(CH3)2 | [b4+Li+OH]+, b4*-NH3, [b3+Li+OH]+, b3*-NH3, b4*, b3*, b2* | y3*, y2*, y1* | [#-H2O-NH3]+ |
| [L5+Na]+ | a4*, a3*, a1*, a3*-CH2-CH- (CH3)2, a2*-CH2-CH-(CH3)2 | [b4+Na+OH]+, b3*, [b3+Na+OH]+, b2* | y2*, y1* | |
| [L5+K]+ | a4*, a3* | [b4+K+OH]+, b4*, [b3+K+OH]+ | y2*, y2*-NH3, y4*-CO | K+, [#-CO]+, [#-CO2]+ |
| [L5+Rb]+ | [b4+Rb+OH]+ | Rb+ | ||
| [L5+Cs]+ | Cs+ | |||
| [L5+2Li-H]+ | a4**, a3**, a4*, a3*, a1*, a3*-CO2, a2*-CO2 | b2*, b4*-H2O-NH3, b3*-H2O-NH3 | y4**, y2**, y1**, y3*, y1* | [#-CO2]+, [#-H2O-NH3]+ |
| [L5+2Na-H]+ | a4**, a4*, a3*, a2*, a1*, a3*-H2O-NH3, a2*-H2O-NH3, a2*-CH2-CH-(CH3)2 | b3*, b3*-NH3 | y4**, y3**, y2**, y1**, y2**-CO2 | [#-CO2]+ |
| [L5+2K-H]+ | a4*, a3*, a2*, a1*-(CH3)2 | y4**, y3**, y2**, y1**, y1**-H2O-NH3 | K+ | |
| [L5+2Rb-H]+ | a3**-CH2-CH-(CH3)2, a2**-CH2-CH-(CH3)2 | y3**, y2**, y1**, y3**-NH3 | Rb+ | |
| [L5+2Cs-H]+ | a2**-CH2-CH-(CH3)2 | y3**, y2**, y1**, y4**-CH2-CH-(CH3)2 | Cs+ |
| Precursor ion(#) | an/ | Other | ||
|---|---|---|---|---|
| [K5+H]+ | a4-(CH2)4-NH2, a3-(CH2)4-NH2, a1, a1-NH3 | b4, b3, b2, b1, b4-H2O, b3-H2O, b2-H2O, b4-H2O-NH3, b4-H2O-2NH3, b3-H2O-NH3, b3-H2O-2NH3, b2-H2O-NH3, b2-H2O-2NH3 | y4, y3, y2, y1 | [#-H2O]+ |
| [K5+Li]+ | a1*, a2*- (CH2)4-NH2 | [b4+Li+OH]+, b4*, b3*, b2*, b1*, b4*-H2O, b4*-H2O-NH3, [b3+Li+OH]+, b3*-H2O, b3*-H2O-NH3, b2*-H2O, b2*-H2O-NH3 | y2*, y1* | [#-H2O]+ |
| [K5+Na]+ | a1*, a3*- (CH2)4-NH2 | [b4+Na+OH]+, b4*, b3*, b2*, b1*, b4*-H2O, b2*-H2O-NH3, [b3+Na+OH]+, b3*-H2O | y4*-CO2, y2*, y1* | [#-H2O]+ |
| [K5+K]+ | a2* | [b4+K+OH]+, b4*, b3*, b2*, b1*, b3*-H2O, b2*-H2O, [b3+K+OH]+ | y2*, y1*, y1*-(CH2)4 | K+, [#-H2O]+, [#-CO2]+ |
| [K5+Rb]+ | [b4+Rb+OH]+ | Rb+ | ||
| [K5+Cs]+ | [b4+Cs+OH]+ | Cs+ | ||
| [K5+2Li-H]+ | a4**, a3**, a2**, a2**-H2O-NH3, a1* | b3**, b2**, b4*, b3*, b1* | y4**, y3**, y2**, y1**, y4*, y1* | [#-CO2]+, [#-CO2-CH2-NH2]+ |
| [K5+2Na-H]+ | a4**, a4**-4CH2 | b4**, b4**-CO2, b4*, b3*, b2* | y4**, y3**, y2**, y1**, y3**-CO2, y2**-CO2, y4*, y3*, y2*, y1*, y1**-4CH2 | [#-CO2]+ |
| [K5+2K-H]+ | a2**, a2**- (CH2)4-NH2 | b4* | y4**, y3**, y2**, y1**, y4*, y3* | K+, [#-CO2]+ |
| [K5+2Rb-H]+ | b4* | y3**, y2**, y1**, y3* | Rb+ | |
| [K5+2Cs-H]+ | b4* | y3**, y2**, y1** | Cs+ |
Table 7 Main product ions of protonated and alkali metal cationized K5 complexes obtained with CID
| Precursor ion(#) | an/ | Other | ||
|---|---|---|---|---|
| [K5+H]+ | a4-(CH2)4-NH2, a3-(CH2)4-NH2, a1, a1-NH3 | b4, b3, b2, b1, b4-H2O, b3-H2O, b2-H2O, b4-H2O-NH3, b4-H2O-2NH3, b3-H2O-NH3, b3-H2O-2NH3, b2-H2O-NH3, b2-H2O-2NH3 | y4, y3, y2, y1 | [#-H2O]+ |
| [K5+Li]+ | a1*, a2*- (CH2)4-NH2 | [b4+Li+OH]+, b4*, b3*, b2*, b1*, b4*-H2O, b4*-H2O-NH3, [b3+Li+OH]+, b3*-H2O, b3*-H2O-NH3, b2*-H2O, b2*-H2O-NH3 | y2*, y1* | [#-H2O]+ |
| [K5+Na]+ | a1*, a3*- (CH2)4-NH2 | [b4+Na+OH]+, b4*, b3*, b2*, b1*, b4*-H2O, b2*-H2O-NH3, [b3+Na+OH]+, b3*-H2O | y4*-CO2, y2*, y1* | [#-H2O]+ |
| [K5+K]+ | a2* | [b4+K+OH]+, b4*, b3*, b2*, b1*, b3*-H2O, b2*-H2O, [b3+K+OH]+ | y2*, y1*, y1*-(CH2)4 | K+, [#-H2O]+, [#-CO2]+ |
| [K5+Rb]+ | [b4+Rb+OH]+ | Rb+ | ||
| [K5+Cs]+ | [b4+Cs+OH]+ | Cs+ | ||
| [K5+2Li-H]+ | a4**, a3**, a2**, a2**-H2O-NH3, a1* | b3**, b2**, b4*, b3*, b1* | y4**, y3**, y2**, y1**, y4*, y1* | [#-CO2]+, [#-CO2-CH2-NH2]+ |
| [K5+2Na-H]+ | a4**, a4**-4CH2 | b4**, b4**-CO2, b4*, b3*, b2* | y4**, y3**, y2**, y1**, y3**-CO2, y2**-CO2, y4*, y3*, y2*, y1*, y1**-4CH2 | [#-CO2]+ |
| [K5+2K-H]+ | a2**, a2**- (CH2)4-NH2 | b4* | y4**, y3**, y2**, y1**, y4*, y3* | K+, [#-CO2]+ |
| [K5+2Rb-H]+ | b4* | y3**, y2**, y1**, y3* | Rb+ | |
| [K5+2Cs-H]+ | b4* | y3**, y2**, y1** | Cs+ |
| [1] | Li Y., Lin H. Q., Deng C. H., Yang P. Y., Zhang X. M., Proteomics, 2008, 8(2), 238—345 |
| [2] | Xu Y. W., Zhang L. J., Lu H. J., Yang P. Y., Anal. Chem., 2008, 80(21), 8324—8328 |
| [3] | Zhou M., McDonald J. F., Fernandez F. M., J. Am. Soc. Mass Spectrom., 2010, 21(1), 68—75 |
| [4] | Chen C., Chu Y. Q., Dai X. H., Fang X., Ding C. F., Acta Phys. Chim. Sin., 2013, 29(6), 1336—1343 |
| (陈琛, 储艳秋, 戴新华, 方向, 丁传凡.物理化学学报, 2013,29(6), 1336—1343) | |
| [5] | Halim V. A., Muck A., Hartl M., Ibanez A. Z., Giri A., Proteomics, 2009 , 9(1) , 171—181 |
| [6] | Wilson J.J., Brodbelt J. S., Anal.Chem.,2007, 79,2067—2077 |
| [7] | Biemann K., Methods Enzymol., 1990, 193, 455—460 |
| [8] | Good D. M., Wenger C. D., Coon J. J., Proteomics, 2010, 10(1), 164—167 |
| [9] | Spengler B., J. Mass. Spectrom., 1997, 32, 1019—1036 |
| [10] | Qin Y. J., Wei S. G., Wang X. L., Yang F., Wang B., Guo X. H., Chem. J. Chinese Universities, 2011, 32(8), 2748—2756 |
| (秦玉娇, 魏士刚, 王晓录, 杨帆, 汪兵, 国新华.高等学校化学学报, 2011,32(8), 2748—2756) | |
| [11] | Wysocki V. H., Smith L. L., Breci L. A., J. Mass Spectrom., 2000, 35, 1399—1406 |
| [12] | Harrison A. G., Mass Spectrom. Rev., 2009, 28(4), 640—654 |
| [13] | Knapp-Mohammady M., Young A. B., Paizs B., Harrison A.G., J. Am. Soc. Mass Spectrom., 2009, 20(11), 2135—2143 |
| [14] | Paizs B., Suhai S., Mass Spectrom. Rev., 2005, 24(4), 508—548 |
| [15] | Bythell B. J., Somogyi A., Paizs B., J. Am. Soc. Mass Spectrom., 2009, 20(4), 618—624 |
| [16] | Cydzik M., Rudowska M., Stefanowicz P., Szewczuk Z., J. Am. Soc. Mass Spectrom., 2011, 22(12), 2013—2107 |
| [17] | Shields S. L., Bluhm B. K., Russell D. H., J. Am. Soc. Mass Spectrom., 2000, 11, 626—633 |
| [18] | Pingitore F., Wesdemiotis C., Anal. Chem., 2005, 77, 1796—1803 |
| [19] | Russell D. H., Mass Spectrom. Rev., 1986, 5, 167—189 |
| [20] | Tang X., Ens W., Standing K. G., Westmore J. B., Anal. Chem., 1988, 60, 1791—1799 |
| [21] | Teesch L. M., Adams J., J. Am. Chem. Soc., 1990, 112, 4110—4120 |
| [22] | Teesch L. M., Orlando R. C., Adams J., J. Am. Chem. Soc., 1991, 113, 3668—3675 |
| [23] | Crizer D. M., Xia Y., McLuckey S. A., J. Am. Soc. Mass Spectrom., 2009, 20(9), 1718—1722 |
| [24] | Zhang H. R., Chen G., Wang L., Ding L., Tian Y., Jin W. J., Zhang H. Q., Int. J. Mass Spectrom., 2006, 252(1), 1—10 |
| [25] | Chu Y. Q., Dai X. H., Jiang D., Fang X., Ding C. F., Rapid Commun. Mass Spectrom., 2010, 24, 2255—2262 |
| [26] | Guo C., Hu N., Jiang K. Z., Chen W. X., Wang X. X., Pan Y. J., Rapid Commun. Mass Spectrom., 2010, 24, 409—414 |
| [27] | Dunbar R. C., Polfer N. C., Berden G., Oomens J. , Int. J. Mass Spectrom., 2012, 330, 71—77 |
| [28] | Dunbar R. C., Steill J. D., Polfer N. C., Oomens J., J. Phys. Chem. A, 2013, 117(6), 1094—1101 |
| [29] | Pu D., Vincent J. B., Cassady C. J., J. Mass Spectrom., 2008, 43, 773—781 |
| [30] | Wang Q., Chu Y. Q., Zhang K., Dai X. H., Fang X., Ding C. F., Acta Phys. Chim. Sin., 2012, 28, 971—978 |
| (王青, 储艳秋, 张开, 戴新华, 方向, 丁传凡.物理化学学报, 2012, 28, 971—978) |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||