

Chem. J. Chinese Universities ›› 2017, Vol. 38 ›› Issue (3): 413.doi: 10.7503/cjcu20160546
• Organic Chemistry • Previous Articles Next Articles
MU Boshuai1, CHANG Suna2, BIAN Yanqing2,*( ), LI Yuan1,*(
), LI Yuan1,*( )
)
Received:2016-07-28
Online:2017-03-10
Published:2017-02-23
Contact:
BIAN Yanqing,LI Yuan
E-mail:bianyangqing151@sohu.com;liyuanhbsd@163.com
Supported by:CLC Number:
TrendMD:
MU Boshuai, CHANG Suna, BIAN Yanqing, LI Yuan. Asymmetric Synthesis and Antibacterial Activity of Chiral 2-Methoxycarbonyl-4-fluorophenyl-1,5-benzothiazepines†[J]. Chem. J. Chinese Universities, 2017, 38(3): 413.
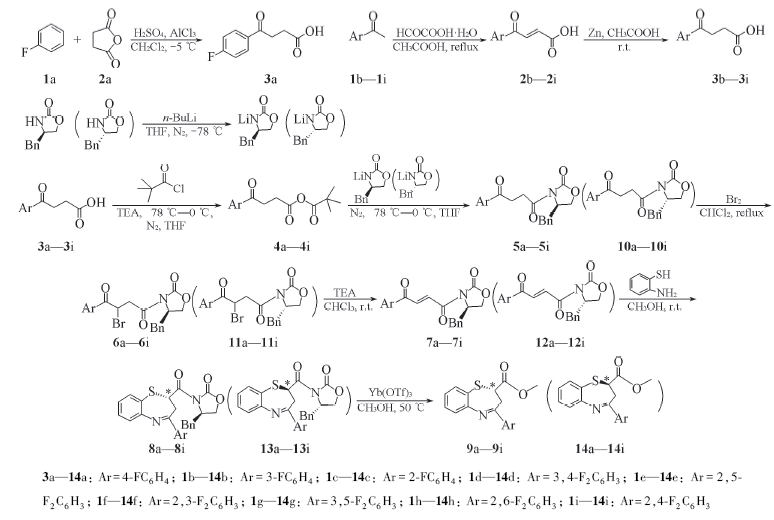
Scheme 1 Synthesis route of 1,5-benzothiazepines(9a—9i and 14a—14i) 3a—14a: Ar=4-FC6H4; 1b—14b: Ar=3-FC6H4; 1c—14c: Ar=2-FC6H4; 1d—14d: Ar=3,4-F2C6H3; 1e—14e: Ar=2,5-F2C6H3; 1f—14f: Ar=2,3-F2C6H3; 1g—14g: Ar=3,5-F2C6H3; 1h—14h: Ar=2,6-F2C6H3; 1i—14i: Ar=2,4-F2C6H3
| Compd. | Isolated yield(%) | m. p./℃ | HRMS(calcd., [M+Na]+), m/z | IR(KBr), | e.e.(%) |
|---|---|---|---|---|---|
| 9a | 32.0 | 93—96 | 338.0625(338.0621) | 1611, 1717 | 84 |
| 9b | 28.2 | 102—103 | 338.0623(338.0621) | 1610, 1733 | 65 |
| 9c | 23.5 | 83—85 | 338.0625(338.0621) | 1603, 1739 | 35 |
| 9d | 35.0 | 98—100 | 356.0531(356.0527) | 1598, 1733 | 93 |
| 9e | 24.8 | 111—113 | 356.0531(356.0527) | 1616, 1737 | 76 |
| 9f | 29.0 | 105—107 | 356.0528(356.0527) | 1607, 1728 | 67 |
| 9g | 36.3 | 86—88 | 356.0533(356.0527) | 1610, 1721 | 65 |
| 9h | 34.3 | 95—97 | 356.0532(356.0527) | 1615, 1728 | 71 |
| 9i | 25.7 | 76—78 | 356.0527(356.0527) | 1600, 1728 | 40 |
| 14a | 37.0 | 94—96 | 338.0629(338.0621) | 1595, 1708 | 60 |
| 14b | 25.8 | 102—103 | 338.0625(338.0621) | 1615, 1728 | 70 |
| 14c | 29.5 | 83—86 | 338.0625(338.0621) | 1621, 1746 | 34 |
| 14d | 33.4 | 98—99 | 356.0534(356.0527) | 1597, 1731 | 95 |
| 14e | 36.2 | 111—113 | 356.0534(356.0527) | 1605, 1731 | 70 |
| 14f | 38.2 | 106—108 | 356.0528(356.0527) | 1597, 1731 | 54 |
| 14g | 36.2 | 86—89 | 356.0525(356.0527) | 1601, 1729 | 73 |
| 14h | 42.1 | 95—97 | 356.0532(356.0527) | 1627, 1727 | 71 |
| 14i | 25.9 | 77—79 | 356.0534(356.0527) | 1611, 1731 | 40 |
Table 1 Yields, melting points, HRMS and IR data for compounds 9a—9i and 14a—14i
| Compd. | Isolated yield(%) | m. p./℃ | HRMS(calcd., [M+Na]+), m/z | IR(KBr), | e.e.(%) |
|---|---|---|---|---|---|
| 9a | 32.0 | 93—96 | 338.0625(338.0621) | 1611, 1717 | 84 |
| 9b | 28.2 | 102—103 | 338.0623(338.0621) | 1610, 1733 | 65 |
| 9c | 23.5 | 83—85 | 338.0625(338.0621) | 1603, 1739 | 35 |
| 9d | 35.0 | 98—100 | 356.0531(356.0527) | 1598, 1733 | 93 |
| 9e | 24.8 | 111—113 | 356.0531(356.0527) | 1616, 1737 | 76 |
| 9f | 29.0 | 105—107 | 356.0528(356.0527) | 1607, 1728 | 67 |
| 9g | 36.3 | 86—88 | 356.0533(356.0527) | 1610, 1721 | 65 |
| 9h | 34.3 | 95—97 | 356.0532(356.0527) | 1615, 1728 | 71 |
| 9i | 25.7 | 76—78 | 356.0527(356.0527) | 1600, 1728 | 40 |
| 14a | 37.0 | 94—96 | 338.0629(338.0621) | 1595, 1708 | 60 |
| 14b | 25.8 | 102—103 | 338.0625(338.0621) | 1615, 1728 | 70 |
| 14c | 29.5 | 83—86 | 338.0625(338.0621) | 1621, 1746 | 34 |
| 14d | 33.4 | 98—99 | 356.0534(356.0527) | 1597, 1731 | 95 |
| 14e | 36.2 | 111—113 | 356.0534(356.0527) | 1605, 1731 | 70 |
| 14f | 38.2 | 106—108 | 356.0528(356.0527) | 1597, 1731 | 54 |
| 14g | 36.2 | 86—89 | 356.0525(356.0527) | 1601, 1729 | 73 |
| 14h | 42.1 | 95—97 | 356.0532(356.0527) | 1627, 1727 | 71 |
| 14i | 25.9 | 77—79 | 356.0534(356.0527) | 1611, 1731 | 40 |
| Compd. | 1H NMR(500 MHz, CDCl3), δ | 13C NMR(125 MHz, CDCl3), δ |
|---|---|---|
| 9a | 7.09—8.06(m, 8H, PhH), 4.34(dd, 1H, SCH, J=12.00, 5.00 Hz), 3.75(s, 3H, OCH3), 3.09—3.20(m, 2H, CH2) | 170.7, 168.1, 165.9, 163.9, 152.1, 135.4, 130.6, 129.6(d, JC—F=8.7 Hz), 125.5, 125.0, 121.0, 118.3, 115.8(d, JC—F=21.6 Hz), 125.2, 55.4, 52.6, 34.9 |
| 9b | 7.10—7.80(m, 8H, PhH), 4.34(dd, 1H, SCH, J=12.00, 6.00 Hz), 3.76(s, 3H, OCH3), 3.10—3.19(m, 2H, CH2) | 170.6, 168.0, 164.1, 162.1, 152.1 135.4, 130.5, 130.3(d, JC—F=8.0 Hz), 125.7, 125.0, 123.0, 121.0, 118.3, 114.2(d, JC—F=22.6 Hz), 55.8, 52.7, 31.6 |
| 9c | 7.10—8.08(m, 8H, PhH), 4.52(dd, 1H, SCH, J=11.50, 6.50 Hz), 3.73(s, 3H, OCH3), 3.09—3.18(m, 2H, CH2) | 170.9, 168.0, 162.3, 160.4, 151.5 135.5, 132.6(d, JC—F=8.7 Hz), 130.8, 130.5, 125.7, 124.9, 124.6, 121.5, 116.4(d, JC—F=23.1 Hz) 55.4, 52.6, 34.9 |
| 9d | 7.12—7.81(m, 7H, PhH), 4.51(dd, 1H, SCH, J=12.00, 5.50 Hz), 3.74(s, 3H, OCH3), 3.17(t, 1H, CH2, J=12.50 Hz), 3.08(dd, 1H, CH2, J=13.00, 5.50 Hz) | 170.7, 166.8, 151.3(dd, JC—F=145.5, 13.5 Hz), 151.2, 149.3(dd, JC—F=149.3, 13.5 Hz), 135.5, 130.5, 128.9(d, JC—F=8.1 Hz), 126.0, 125.2(d, JC—F=1.6 Hz), 125.0, 124.4(d, JC—F=6.8 Hz), 121.5, 119.4(d, JC—F=17.1 Hz), 55.5, 52.6, 34.9 |
| 9e | 7.12—7.82(m, 7H, PhH), 4.51(dd, 1H, SCH, J=11.50, 6.50 Hz), 3.75(s, 3H, OCH3), 3.08—3.16(m, 2H, CH2) | 170.7, 166.7, 159.7(dd, JC—F=164.37, 1.50 Hz), 157.1(dd, JC—F=167.00, 1.62 Hz), 151.1, 135.5, 130.5, 127.9(dd, JC—F=13.50, 7.38 Hz), 126.0, 125.0, 121.6, 119.2(dd, JC—F=24.38, 9.25 Hz), 117.7(dd, JC—F=26.50, 8.37 Hz), 116.7(dd, JC—F=25.37, 3.62 Hz), 55.5, 52.6, 34.7 |
| 9f | 7.13—7.82(m, 7H, PhH), 4.51(dd, 1H, SCH, J=12.00, 5.50 Hz), 3.74(s, 3H, OCH3), 3.17(t, 1H, CH2, J=12.75 Hz), 3.08(dd, 1H, CH2, J=13.00, 5.50 Hz) | 170.7, 167.0, 151.9, 151.3(dd, JC—F=145.5, 13.5 Hz), 149.8(dd, JC—F=149.3, 13.5 Hz), 135.5, 130.6, 128.8(d, JC—F=8.2 Hz), 126.0, 125.3(d, JC—F=1.6 Hz), 125.0, 124.4(d, JC—F=6.8 Hz), 121.5, 119.4(d, JC—F=17.1 Hz), 55.5, 52.6, 34.9 |
| 9g | 6.94—7.58(m, 7H, PhH), 4.35(dd, 1H, SCH, J=12.00, 6.00 Hz), 3.77(s, 3H, OCH3), 3.06—3.16(m, 2H, CH2) | 170.5, 166.7, 163.2(dd, JC—F=247.6, 12.5 Hz), 151.9, 140.8(t, JC—F=8.6 Hz), 135.5, 130.6, 125.9, 125.0, 120.9, 110.3(dd, JC—F=20.6, 6.1 Hz), 106.4(t, JC—F=25.7 Hz), 55.8, 52.7, 31.5 |
| 9h | 7.01—7.60(m, 7H, PhH), 4.49(dd, 1H, SCH, J=12.0, 5.5 Hz), 3.73(s, 3H, OCH3), 3.23(t, 1H, CH2, J=12.5 Hz), 2.87(dd, 1H, CH2, J=13.0, 5.0 Hz) | 170.6, 163.8, 160.3(dd, JC—F=250.2, 6.7 Hz), 151.4, 135.4, 131.1(t, JC—F=10.1 Hz), 130.4, 126.1, 125.3, 121.5, 118.1(t, JC—F=18.1 Hz), 112.1(dd, JC—F=20.2, 4.8 Hz), 55.6, 52.6, 35.8 |
| 9i | 6.84—8.15(m, 7H, PhH), 4.48(dd, 1H, SCH, J=12.00, 5.50 Hz), 3.74(s, 3H, OCH3), 3.06—3.17(m, 2H, CH2) | 170.7, 167.0, 164.7(dd, JC—F=253.2, 12.5 Hz), 161.7(dd, JC—F=251.6, 12.2 Hz), 151.0, 135.5, 132.3(dd, JC—F=9.8, 4.5 Hz), 130.6, 127.9, 127.3, 126.0, 125.0, 112.3(dd, JC—F=21.0, 3.0 Hz), 104.6(t, JC—F=26.1 Hz), 55.4, 52.6, 34.8 |
| 14a | 7.09—8.06(m, 8H, PhH), 4.34(dd, 1H, SCH, J=12.00, 5.50 Hz), 3.75(s, 3H, OCH3), 3.09—3.20(m, 2H, CH2) | 170.7, 168.1, 165.9, 163.9, 152.1, 135.4, 130.6, 129.6(d, JC—F=8.7 Hz), 125.5, 125.0, 121.0, 118.3, 115.8(d, JC—F=21.2 Hz) , 125.2, 55.6, 52.7, 31.5 |
| 14b | 7.11—7.81(m, 8H, PhH), 4.36(dd, 1H, SCH, J=12.00, 5.50 Hz), 3.76(s, 3H, OCH3), 3.10—3.20(m, 2H, CH2) | 170.6, 168.1, 164.1, 162.1, 152.0, 135.0, 130.6, 130.3(d, JC—F=7.2 Hz), 125.7, 125.0, 123.0, 121.0, 118.3, 114.2(d, JC—F=22.7 Hz) 55.8, 52.7, 31.6 |
| 14c | 7.12—8.08(m, 8H, PhH), 4.51(dd, 1H, SCH, J=12.00, 6.00 Hz), 3.73(s, 3H, OCH3), 3.09—3.18(m, 2H, CH2) | 170.8, 168.1, 162.3, 160.4, 151.4, 135.5, 132.70(d, JC—F=8.8 Hz), 130.8, 130.5, 125.8, 125.0, 124.6, 121.5, 116.4(d, JC—F=23.1 Hz) 55.4, 52.6, 34.9 |
| 14d | 7.13—8.01(m, 7H, PhH), 4.50(dd, 1H, SCH, J=12.00, 5.50 Hz), 3.74(s, 3H, OCH3), 3.16(t, 1H, CH2, J=12.50 Hz), 3.07(dd, 1H, CH2, J=13.00, 5.50 Hz) | 170.6, 166.7, 152.5(dd, JC—F=145.7, 12.8 Hz), 152.1, 150.5(dd, JC—F=149.1, 13.5 Hz), 135.5, 134.6(d, JC—F=8.1 Hz), 130.6, 125.7, 125.0, 123.8(d, JC—F=6.5 Hz), 120.8, 117.4(d, JC—F=17.5 Hz), 116.5(d, JC—F=18.5 Hz), 55.7, 52.7, 31.3 |
| 14e | 7.12—7.84(m, 7H, PhH), 4.52(dd, 1H, SCH, J=11.00, 7.00 Hz), 3.75(s, 3H, OCH3), 3.12—3.18(m, 2H, CH2) | 170.8, 166.7, 159.7(dd, JC—F=164.4, 1.5 Hz), 157.1(dd, JC—F=166.7, 1.6 Hz), 151.1, 135.5, 130.5, 127.9(dd, JC—F=13.5, 7.3 Hz), 126.0, 125.0, 121.6, 119.2(dd, JC—F=24.3, 9.2 Hz), 117.7(dd, JC—F=26.5, 8.2 Hz), 116.7(dd, JC—F=25.3, 3.6 Hz), 55.4, 52.6, 34.7 |
| 14f | 7.12—7.81(m, 7H, PhH), 4.50(dd, 1H, SCH, J=12.00, 5.50 Hz), 3.74(s, 3H, OCH3), 3.17(t, 1H, CH2, J=12.75 Hz), 3.08(dd, 1H, CH2, J=13.00, 5.50 Hz) | 170.7, 166.9, 151.4, 151.3(dd, JC—F=144.6, 13.5 Hz), 149.3(dd, JC—F=153.0, 13.5 Hz), 135.5, 130.6, 128.8(d, JC—F=8.1 Hz), 126.0, 125.3(d, JC—F=1.6 Hz), 125.0, 124.4(d, JC—F=6.8 Hz), 121.5, 119.4(d, JC—F=17.0 Hz), 55.5, 52.6, 34.9 |
| 14g | 6.94—7.58(m, 7H, PhH), 4.35(dd, 1H, SCH, J=12.00, 6.00 Hz), 3.77(s, 3H, OCH3), 3.07—3.16(m, 2H, CH2) | 170.5, 166.7, 163.2(dd, JC—F=247.2, 12.6 Hz), 151.9, 140.8(t, JC—F=8.8 Hz), 135.5, 130.6, 125.9, 125.0, 120.9, 110.3(dd, JC—F=20.6, 6.1 Hz), 106.4(t, JC—F=25.7 Hz), 55.8, 52.7, 31.5 |
| 14h | 7.00—7.60(m, 7H, PhH), 4.49(dd, 1H, SCH, J=12.0, 5.5 Hz), 3.73(s, 3H, OCH3), 3.23(t, 1H, CH2, J=12.5 Hz), 2.87(dd, 1H, CH2, J=13.0, 5.0 Hz) | 170.7, 163.9, 160.3(dd, JC—F=250.1, 6.7 Hz), 151.3, 135.4, 131.1(t, JC—F=10.1 Hz), 130.4, 126.1, 125.3, 121.5, 118.1(t, JC—F=18.1 Hz), 112.1(dd, JC—F=20.2, 4.8 Hz), 55.6, 52.6, 35.8 |
| 14i | 6.86—8.19(m, 7H, PhH), 4.49(dd, 1H, SCH, J=12.00, 6.50 Hz), 3.74(s, 3H, OCH3), 3.10—3.20(m, 2H, CH2) | 170.7, 167.1, 164.7(dd, JC—F=246.7, 12.5 Hz), 161.7(dd, JC—F=251.6, 12.2 Hz), 151.3, 135.6, 132.2(dd, JC—F=9.8, 4.5 Hz), 130.5, 127.9, 127.3, 126.7, 125.0, 112.3(dd, JC—F=21.0, 3.0 Hz), 104.3(t, JC—F=26.1 Hz), 55.4, 52.6, 34.8 |
Table 2 1H NMR and 13C NMR data for compounds 9a—9i and 14a—14i
| Compd. | 1H NMR(500 MHz, CDCl3), δ | 13C NMR(125 MHz, CDCl3), δ |
|---|---|---|
| 9a | 7.09—8.06(m, 8H, PhH), 4.34(dd, 1H, SCH, J=12.00, 5.00 Hz), 3.75(s, 3H, OCH3), 3.09—3.20(m, 2H, CH2) | 170.7, 168.1, 165.9, 163.9, 152.1, 135.4, 130.6, 129.6(d, JC—F=8.7 Hz), 125.5, 125.0, 121.0, 118.3, 115.8(d, JC—F=21.6 Hz), 125.2, 55.4, 52.6, 34.9 |
| 9b | 7.10—7.80(m, 8H, PhH), 4.34(dd, 1H, SCH, J=12.00, 6.00 Hz), 3.76(s, 3H, OCH3), 3.10—3.19(m, 2H, CH2) | 170.6, 168.0, 164.1, 162.1, 152.1 135.4, 130.5, 130.3(d, JC—F=8.0 Hz), 125.7, 125.0, 123.0, 121.0, 118.3, 114.2(d, JC—F=22.6 Hz), 55.8, 52.7, 31.6 |
| 9c | 7.10—8.08(m, 8H, PhH), 4.52(dd, 1H, SCH, J=11.50, 6.50 Hz), 3.73(s, 3H, OCH3), 3.09—3.18(m, 2H, CH2) | 170.9, 168.0, 162.3, 160.4, 151.5 135.5, 132.6(d, JC—F=8.7 Hz), 130.8, 130.5, 125.7, 124.9, 124.6, 121.5, 116.4(d, JC—F=23.1 Hz) 55.4, 52.6, 34.9 |
| 9d | 7.12—7.81(m, 7H, PhH), 4.51(dd, 1H, SCH, J=12.00, 5.50 Hz), 3.74(s, 3H, OCH3), 3.17(t, 1H, CH2, J=12.50 Hz), 3.08(dd, 1H, CH2, J=13.00, 5.50 Hz) | 170.7, 166.8, 151.3(dd, JC—F=145.5, 13.5 Hz), 151.2, 149.3(dd, JC—F=149.3, 13.5 Hz), 135.5, 130.5, 128.9(d, JC—F=8.1 Hz), 126.0, 125.2(d, JC—F=1.6 Hz), 125.0, 124.4(d, JC—F=6.8 Hz), 121.5, 119.4(d, JC—F=17.1 Hz), 55.5, 52.6, 34.9 |
| 9e | 7.12—7.82(m, 7H, PhH), 4.51(dd, 1H, SCH, J=11.50, 6.50 Hz), 3.75(s, 3H, OCH3), 3.08—3.16(m, 2H, CH2) | 170.7, 166.7, 159.7(dd, JC—F=164.37, 1.50 Hz), 157.1(dd, JC—F=167.00, 1.62 Hz), 151.1, 135.5, 130.5, 127.9(dd, JC—F=13.50, 7.38 Hz), 126.0, 125.0, 121.6, 119.2(dd, JC—F=24.38, 9.25 Hz), 117.7(dd, JC—F=26.50, 8.37 Hz), 116.7(dd, JC—F=25.37, 3.62 Hz), 55.5, 52.6, 34.7 |
| 9f | 7.13—7.82(m, 7H, PhH), 4.51(dd, 1H, SCH, J=12.00, 5.50 Hz), 3.74(s, 3H, OCH3), 3.17(t, 1H, CH2, J=12.75 Hz), 3.08(dd, 1H, CH2, J=13.00, 5.50 Hz) | 170.7, 167.0, 151.9, 151.3(dd, JC—F=145.5, 13.5 Hz), 149.8(dd, JC—F=149.3, 13.5 Hz), 135.5, 130.6, 128.8(d, JC—F=8.2 Hz), 126.0, 125.3(d, JC—F=1.6 Hz), 125.0, 124.4(d, JC—F=6.8 Hz), 121.5, 119.4(d, JC—F=17.1 Hz), 55.5, 52.6, 34.9 |
| 9g | 6.94—7.58(m, 7H, PhH), 4.35(dd, 1H, SCH, J=12.00, 6.00 Hz), 3.77(s, 3H, OCH3), 3.06—3.16(m, 2H, CH2) | 170.5, 166.7, 163.2(dd, JC—F=247.6, 12.5 Hz), 151.9, 140.8(t, JC—F=8.6 Hz), 135.5, 130.6, 125.9, 125.0, 120.9, 110.3(dd, JC—F=20.6, 6.1 Hz), 106.4(t, JC—F=25.7 Hz), 55.8, 52.7, 31.5 |
| 9h | 7.01—7.60(m, 7H, PhH), 4.49(dd, 1H, SCH, J=12.0, 5.5 Hz), 3.73(s, 3H, OCH3), 3.23(t, 1H, CH2, J=12.5 Hz), 2.87(dd, 1H, CH2, J=13.0, 5.0 Hz) | 170.6, 163.8, 160.3(dd, JC—F=250.2, 6.7 Hz), 151.4, 135.4, 131.1(t, JC—F=10.1 Hz), 130.4, 126.1, 125.3, 121.5, 118.1(t, JC—F=18.1 Hz), 112.1(dd, JC—F=20.2, 4.8 Hz), 55.6, 52.6, 35.8 |
| 9i | 6.84—8.15(m, 7H, PhH), 4.48(dd, 1H, SCH, J=12.00, 5.50 Hz), 3.74(s, 3H, OCH3), 3.06—3.17(m, 2H, CH2) | 170.7, 167.0, 164.7(dd, JC—F=253.2, 12.5 Hz), 161.7(dd, JC—F=251.6, 12.2 Hz), 151.0, 135.5, 132.3(dd, JC—F=9.8, 4.5 Hz), 130.6, 127.9, 127.3, 126.0, 125.0, 112.3(dd, JC—F=21.0, 3.0 Hz), 104.6(t, JC—F=26.1 Hz), 55.4, 52.6, 34.8 |
| 14a | 7.09—8.06(m, 8H, PhH), 4.34(dd, 1H, SCH, J=12.00, 5.50 Hz), 3.75(s, 3H, OCH3), 3.09—3.20(m, 2H, CH2) | 170.7, 168.1, 165.9, 163.9, 152.1, 135.4, 130.6, 129.6(d, JC—F=8.7 Hz), 125.5, 125.0, 121.0, 118.3, 115.8(d, JC—F=21.2 Hz) , 125.2, 55.6, 52.7, 31.5 |
| 14b | 7.11—7.81(m, 8H, PhH), 4.36(dd, 1H, SCH, J=12.00, 5.50 Hz), 3.76(s, 3H, OCH3), 3.10—3.20(m, 2H, CH2) | 170.6, 168.1, 164.1, 162.1, 152.0, 135.0, 130.6, 130.3(d, JC—F=7.2 Hz), 125.7, 125.0, 123.0, 121.0, 118.3, 114.2(d, JC—F=22.7 Hz) 55.8, 52.7, 31.6 |
| 14c | 7.12—8.08(m, 8H, PhH), 4.51(dd, 1H, SCH, J=12.00, 6.00 Hz), 3.73(s, 3H, OCH3), 3.09—3.18(m, 2H, CH2) | 170.8, 168.1, 162.3, 160.4, 151.4, 135.5, 132.70(d, JC—F=8.8 Hz), 130.8, 130.5, 125.8, 125.0, 124.6, 121.5, 116.4(d, JC—F=23.1 Hz) 55.4, 52.6, 34.9 |
| 14d | 7.13—8.01(m, 7H, PhH), 4.50(dd, 1H, SCH, J=12.00, 5.50 Hz), 3.74(s, 3H, OCH3), 3.16(t, 1H, CH2, J=12.50 Hz), 3.07(dd, 1H, CH2, J=13.00, 5.50 Hz) | 170.6, 166.7, 152.5(dd, JC—F=145.7, 12.8 Hz), 152.1, 150.5(dd, JC—F=149.1, 13.5 Hz), 135.5, 134.6(d, JC—F=8.1 Hz), 130.6, 125.7, 125.0, 123.8(d, JC—F=6.5 Hz), 120.8, 117.4(d, JC—F=17.5 Hz), 116.5(d, JC—F=18.5 Hz), 55.7, 52.7, 31.3 |
| 14e | 7.12—7.84(m, 7H, PhH), 4.52(dd, 1H, SCH, J=11.00, 7.00 Hz), 3.75(s, 3H, OCH3), 3.12—3.18(m, 2H, CH2) | 170.8, 166.7, 159.7(dd, JC—F=164.4, 1.5 Hz), 157.1(dd, JC—F=166.7, 1.6 Hz), 151.1, 135.5, 130.5, 127.9(dd, JC—F=13.5, 7.3 Hz), 126.0, 125.0, 121.6, 119.2(dd, JC—F=24.3, 9.2 Hz), 117.7(dd, JC—F=26.5, 8.2 Hz), 116.7(dd, JC—F=25.3, 3.6 Hz), 55.4, 52.6, 34.7 |
| 14f | 7.12—7.81(m, 7H, PhH), 4.50(dd, 1H, SCH, J=12.00, 5.50 Hz), 3.74(s, 3H, OCH3), 3.17(t, 1H, CH2, J=12.75 Hz), 3.08(dd, 1H, CH2, J=13.00, 5.50 Hz) | 170.7, 166.9, 151.4, 151.3(dd, JC—F=144.6, 13.5 Hz), 149.3(dd, JC—F=153.0, 13.5 Hz), 135.5, 130.6, 128.8(d, JC—F=8.1 Hz), 126.0, 125.3(d, JC—F=1.6 Hz), 125.0, 124.4(d, JC—F=6.8 Hz), 121.5, 119.4(d, JC—F=17.0 Hz), 55.5, 52.6, 34.9 |
| 14g | 6.94—7.58(m, 7H, PhH), 4.35(dd, 1H, SCH, J=12.00, 6.00 Hz), 3.77(s, 3H, OCH3), 3.07—3.16(m, 2H, CH2) | 170.5, 166.7, 163.2(dd, JC—F=247.2, 12.6 Hz), 151.9, 140.8(t, JC—F=8.8 Hz), 135.5, 130.6, 125.9, 125.0, 120.9, 110.3(dd, JC—F=20.6, 6.1 Hz), 106.4(t, JC—F=25.7 Hz), 55.8, 52.7, 31.5 |
| 14h | 7.00—7.60(m, 7H, PhH), 4.49(dd, 1H, SCH, J=12.0, 5.5 Hz), 3.73(s, 3H, OCH3), 3.23(t, 1H, CH2, J=12.5 Hz), 2.87(dd, 1H, CH2, J=13.0, 5.0 Hz) | 170.7, 163.9, 160.3(dd, JC—F=250.1, 6.7 Hz), 151.3, 135.4, 131.1(t, JC—F=10.1 Hz), 130.4, 126.1, 125.3, 121.5, 118.1(t, JC—F=18.1 Hz), 112.1(dd, JC—F=20.2, 4.8 Hz), 55.6, 52.6, 35.8 |
| 14i | 6.86—8.19(m, 7H, PhH), 4.49(dd, 1H, SCH, J=12.00, 6.50 Hz), 3.74(s, 3H, OCH3), 3.10—3.20(m, 2H, CH2) | 170.7, 167.1, 164.7(dd, JC—F=246.7, 12.5 Hz), 161.7(dd, JC—F=251.6, 12.2 Hz), 151.3, 135.6, 132.2(dd, JC—F=9.8, 4.5 Hz), 130.5, 127.9, 127.3, 126.7, 125.0, 112.3(dd, JC—F=21.0, 3.0 Hz), 104.3(t, JC—F=26.1 Hz), 55.4, 52.6, 34.8 |
| Empirical formula | C17H13F2NO2S | T/K | 298(2) |
|---|---|---|---|
| Formula mass | 333.34 | F(000) | 688 |
| Crystal system | Monoclinic | θ range for data collection/(°) | 2.83—25.02 |
| Space group | P21/n | Limiting indices | -8≤h≤9, -16≤k≤25, -11≤l≤10 |
| a/nm | 7.6497(7) | Reflections collected/unique | 7578/2704(Rint=0.0294) |
| b/nm | 21.5632(19) | Completeness to θ=25.02°(%) | 99.80 |
| c/nm | 9.3170(8) | Absorption correction | Semi-empirical from equivalents |
| α/(°) | 90 | Max. and min. transmission | 0.9249 and 0.9037 |
| β/(°) | 90.9530(10) | Refinement method | Full-matrix least-squares on F2 |
| γ/(°) | 90 | Data/restraints/parameters | 2704/0/209 |
| Volume/nm3 | 1536.6(2) | Goodness-of-fit on F2 | 1.07 |
| Z | 4 | FinalR indices [I>2σ(I)] | R1=0.0447, wR2=0.1060 |
| Calculated density/(g·cm-3) | 1.441 | R indices(all data) | R1=0.0686, wR2=0.1164 |
| Absorption coefficient/mm-1 | 0.240 | Largest diff. peak and hole/(e·nm-3) | 0.218 and -0.225 |
Table 3 X-ray crystallographic data of compound 9h
| Empirical formula | C17H13F2NO2S | T/K | 298(2) |
|---|---|---|---|
| Formula mass | 333.34 | F(000) | 688 |
| Crystal system | Monoclinic | θ range for data collection/(°) | 2.83—25.02 |
| Space group | P21/n | Limiting indices | -8≤h≤9, -16≤k≤25, -11≤l≤10 |
| a/nm | 7.6497(7) | Reflections collected/unique | 7578/2704(Rint=0.0294) |
| b/nm | 21.5632(19) | Completeness to θ=25.02°(%) | 99.80 |
| c/nm | 9.3170(8) | Absorption correction | Semi-empirical from equivalents |
| α/(°) | 90 | Max. and min. transmission | 0.9249 and 0.9037 |
| β/(°) | 90.9530(10) | Refinement method | Full-matrix least-squares on F2 |
| γ/(°) | 90 | Data/restraints/parameters | 2704/0/209 |
| Volume/nm3 | 1536.6(2) | Goodness-of-fit on F2 | 1.07 |
| Z | 4 | FinalR indices [I>2σ(I)] | R1=0.0447, wR2=0.1060 |
| Calculated density/(g·cm-3) | 1.441 | R indices(all data) | R1=0.0686, wR2=0.1164 |
| Absorption coefficient/mm-1 | 0.240 | Largest diff. peak and hole/(e·nm-3) | 0.218 and -0.225 |
| Compd. | e.e.(%) | C. Neofonmans | Compd. | e.e.(%) | C. Neofonmans | ||
|---|---|---|---|---|---|---|---|
| ATCC 34874 | Clinical isolate | ATCC 34874 | Clinical isolate | ||||
| 9a, 14a(Raceme) | 0 | 18.30 | 19.30 | 14e | 70 | 21.47 | 21.90 |
| 9a | 84 | 12.03 | 12.97 | 9f, 14f(Raceme) | 0 | 18.23 | 18.87 |
| 14a | 60 | 21.60 | 22.70 | 9f | 67 | 15.30 | 15.23 |
| 9b, 14b(Raceme) | 0 | 18.57 | 19.23 | 14f | 54 | 20.07 | 19.70 |
| 9b | 65 | 13.20 | 12.60 | 9g, 14g(Raceme) | 0 | 14.67 | 17.53 |
| 14b | 70 | 21.37 | 21.63 | 9g | 65 | 13.23 | 16.20 |
| 9c, 14c(Raceme) | 0 | 20.07 | 22.50 | 14g | 73 | 15.23 | 17.50 |
| 9c | 35 | 18.73 | 20.13 | 9h, 14h(Raceme) | 0 | 6.00 | 6.00 |
| 14c | 34 | 21.83 | 24.10 | 9h | 71 | 6.00 | 6.00 |
| 9d, 14d(Raceme) | 0 | 19.47 | 18.67 | 14h | 40 | 6.00 | 6.00 |
| 9d | 93 | 12.90 | 13.03 | 9i, 14i(Raceme) | 0 | 11.70 | 10.57 |
| 14d | 95 | 23.33 | 20.47 | 9i | 71 | 6.00 | 6.00 |
| 9e, 14e(Raceme) | 0 | 17.60 | 18.33 | 14i | 40 | 13.83 | 14.20 |
| 9e | 76 | 16.73 | 17.03 | DMSO | 6.00 | 6.00 | |
Table 4 Zone(mm) of growth inhibition of compounds 9a—9i and 14a—14i*
| Compd. | e.e.(%) | C. Neofonmans | Compd. | e.e.(%) | C. Neofonmans | ||
|---|---|---|---|---|---|---|---|
| ATCC 34874 | Clinical isolate | ATCC 34874 | Clinical isolate | ||||
| 9a, 14a(Raceme) | 0 | 18.30 | 19.30 | 14e | 70 | 21.47 | 21.90 |
| 9a | 84 | 12.03 | 12.97 | 9f, 14f(Raceme) | 0 | 18.23 | 18.87 |
| 14a | 60 | 21.60 | 22.70 | 9f | 67 | 15.30 | 15.23 |
| 9b, 14b(Raceme) | 0 | 18.57 | 19.23 | 14f | 54 | 20.07 | 19.70 |
| 9b | 65 | 13.20 | 12.60 | 9g, 14g(Raceme) | 0 | 14.67 | 17.53 |
| 14b | 70 | 21.37 | 21.63 | 9g | 65 | 13.23 | 16.20 |
| 9c, 14c(Raceme) | 0 | 20.07 | 22.50 | 14g | 73 | 15.23 | 17.50 |
| 9c | 35 | 18.73 | 20.13 | 9h, 14h(Raceme) | 0 | 6.00 | 6.00 |
| 14c | 34 | 21.83 | 24.10 | 9h | 71 | 6.00 | 6.00 |
| 9d, 14d(Raceme) | 0 | 19.47 | 18.67 | 14h | 40 | 6.00 | 6.00 |
| 9d | 93 | 12.90 | 13.03 | 9i, 14i(Raceme) | 0 | 11.70 | 10.57 |
| 14d | 95 | 23.33 | 20.47 | 9i | 71 | 6.00 | 6.00 |
| 9e, 14e(Raceme) | 0 | 17.60 | 18.33 | 14i | 40 | 13.83 | 14.20 |
| 9e | 76 | 16.73 | 17.03 | DMSO | 6.00 | 6.00 | |
| Compd. | MIC(C. Neofonmans) | MFC(C. Neofonmans) | ||
|---|---|---|---|---|
| ATCC 34874 | Clinical isolate | ATCC 34874 | Clinical isolate | |
| 14a | 10.0 | 4.0 | 22.0 | 20.0 |
| 14b | 8.0 | 8.0 | 22.0 | 22.0 |
| 14c | 10.0 | 8.0 | 22.0 | 22.0 |
| 14d | 8.0 | 6.0 | 20.0 | 22.0 |
| 14e | 10.0 | 8.0 | 20.0 | 24.0 |
| 14f | 10.0 | 4.0 | 22.0 | 20.0 |
| A | 20.0 | 22.0 | >128.0 | >128.0 |
Table 5 MIC and MFC values(μg/mL) for compounds 14a—14f and fluconazole
| Compd. | MIC(C. Neofonmans) | MFC(C. Neofonmans) | ||
|---|---|---|---|---|
| ATCC 34874 | Clinical isolate | ATCC 34874 | Clinical isolate | |
| 14a | 10.0 | 4.0 | 22.0 | 20.0 |
| 14b | 8.0 | 8.0 | 22.0 | 22.0 |
| 14c | 10.0 | 8.0 | 22.0 | 22.0 |
| 14d | 8.0 | 6.0 | 20.0 | 22.0 |
| 14e | 10.0 | 8.0 | 20.0 | 24.0 |
| 14f | 10.0 | 4.0 | 22.0 | 20.0 |
| A | 20.0 | 22.0 | >128.0 | >128.0 |
| [1] | Morton G. C., Salvino J. M., Labaudiniere R. F., Herpin T. F., Tetrahedron Lett., 2000, 41(17), 3029—3033 |
| [2] | Geyer H. M., Watzman N., Buckley J. P., J. Pharm. Sci., 1970, 59(7), 964—968 |
| [3] | Bedos P., Amblard M., Subra G., Dodey P., Luccarini J., Paquet J., Pruneau D., Aumelas A., Martinez J., J. Med. Chem., 2000, 43(12), 2387—2394 |
| [4] | Atwal K. S., Bergey J. L., Moreland S., J. Med. Chem., 1987, 30(4), 627—635 |
| [5] | Tarabova B., Lacinova L., Engel J., Eur. J. Pharmaco., 2007, 573(1—3), 39—48 |
| [6] | Dandia A., Singh R., Sharma R., Phosphorus, Sulfur Silicon Relat. Elem., 2008, 183(12), 3116—3126 |
| [7] | Santo R. D., Costi R., Il Farmaco, 2005, 60(5), 385—392 |
| [8] | Desai M. D., Desai K. K., Asian J. Chem., 2002, 14(2), 974—978 |
| [9] | Bariwal J. B., Upadhyay K. D., Manvar A. T., Trivedia J. C., Singha J. S., Jainc K. S., Shah A. K., Eur. J. Med. Chem., 2008, 43(11), 2279—2290 |
| [10] | Shen S., Ye J., Liu F., Phosphorus, Sulfur Silicon Relat. Elem.,2010, 185(11), 2366—2374 |
| [11] | Yan Y., Yang X., Wu L., Phosphorus, Sulfur Silicon Relat. Elem.,2012, 187(5), 573—579 |
| [12] | Khouzani H. L., Tamjidi P., Mohammadpoor-Baltork I., Yaeghoobi M., Rahman N., Khosropour A., Moghadam M., Tangestaninejad S., Mirkhani V., Habibi M. H., Kashima A., Suzuki T., Heterocycl. Chem., 2014, 51(1), 138—150 |
| [13] | Kamble R. R., Sudha B. S., Phosphorus, Sulfur Silicon Relat. Elem.,2008, 183(7), 1691—1709 |
| [14] | Gan J. G., Ma D. M., Org. Lett., 2009, 11(13), 2788—2790 |
| [15] | Lancelot J.C., Letosis B., Saturnio C., Robba M., Synth. Commun., 1991, 21, 1901—1908 |
| [16] | Yaccoubi F., Efrit M. L. E., Zantour H., Phosphorus, Sulfur Silicon Relat. Elem., 2002, 177(10), 2321—2330 |
| [17] | Mohamed M. A. A., Synth. Commun., 2011, 41(3), 331—340 |
| [18] | Qiu Z. L., Li W. H., Zhu H. F., Liu Q., Li Y., Chem. J. Chinese Universities, 2013, 34(3), 579—589 |
| (邱召来, 李文红, 朱海菲, 刘倩, 李媛. 高等学校化学学报, 2013, 34(3, 579—589) | |
| [19] | Sun N., Zhang P., Li Y., Chin. J. Org. Chem., 2000, 20(5), 735—737 |
| (孙娜, 张萍, 李媛. 有机化学, 2000, 20(5),, 735—737) | |
| [20] | Fan S. L., Zhang B., Gao L. Y., Wang L. Z., Bian Y. Q., Li Y., Chem. J. Chinese Universities, 2014, 32(12), 2574—2583 |
| (范世丽, 张博, 高丽叶, 王兰芝, 边艳青, 李媛. 高等学校化学学报, 2014, 32(12), 2574—2583) | |
| [21] | Zhang J., Mu B. S., Wu M., Bian Y. Q., Li Y., Chem. J. Chinese Universities, 2015, 36(4), 687—697 |
| (张静, 穆博帅, 吴萌, 边艳青, 李媛. 高等学校化学学报, 2015, 36(4), 687—697) | |
| [22] | Lutz R. E., Scott G. W., J. Org. Chem., 1948, 13(2), 284—296 |
| [23] | Wang H. Y., Wang Z., Li S. H., Qiu Y. T., Liu B., Song Z. G., Liu Z. H., Chem. Res. Chinese Universities, 2016, 32(3), 373—379 |
| [24] | Pavel I., Couve-Bonnaire S., Jubault P., Pannecoucke X., Org. Lett., 2012, 14(19), 5130—5133 |
| [25] | Sun B., Yin X., Zhang J., Huang J., Xu Y., Zhang F., Wang J. H., Wang G. Q., Hu C., Chem. Res. Chinese Universities, 2016, 32(1), 1—7 |
| [1] | ZUO Huailong, LEI Simin, ZHANG Rui, LI Yuxin, CHEN Wei. Design, Synthesis and Antifungal Activity of Novel Isoquinoline Derivatives [J]. Chem. J. Chinese Universities, 2021, 42(9): 2766. |
| [2] | HE Feng,BAI Jinhai,CHEN Shuxian,TAN Xiaobei. Antifungal Activity and Mechanism of an Essential Oil from Eremothecium ashbyii† [J]. Chem. J. Chinese Universities, 2019, 40(2): 272. |
| [3] | LI Xiao, GAO Liguo, GONG Ying, MA Yajun, MA Xiangrong. Direct Asymmetric Aldol Reaction of Acetophenones and Aromatic Aldehydes Catalyzed by Chiral Al/Zn Heterobimetallic Compounds ZABDP† [J]. Chem. J. Chinese Universities, 2017, 38(5): 778. |
| [4] | CHEN Wei, WEI Wei, LIU Ming, HUA Xuewen, LI Yuxin, LI Yonghong, ZHANG Xiao, LI Zhengming. Design, Synthesis and Biological Activity of Novel Sulfonylurea Derivatives Containing Dimethoxymethyl-substituted Pyrimidine Moiety† [J]. Chem. J. Chinese Universities, 2015, 36(7): 1291. |
| [5] | CHEN Wei, WEI Wei, ZHOU Sha, LI Yonghong, ZHANG Xiao, TONG Jun, LI Yuxin, LI Zhengming. Design, Synthesis and Biological Activity of Novel Sulfonylurea Derivatives Containing Phenyl-substituted Pyrimidine Moiety† [J]. Chem. J. Chinese Universities, 2015, 36(4): 672. |
| [6] | ZHANG Jing, MU Boshuai, WU Meng, BIAN Yanqing, LI Yuan. Synthesis, Antifungal Activity and Structure-activity Relationship of -Fluorophenyl-2,3-dihydro-1,5-benzothiazepines Derivatives† [J]. Chem. J. Chinese Universities, 2015, 36(4): 687. |
| [7] | KANG Wang, BU Huijuan, LI Wenhong, LI Yuan. Preliminary Structure-activity Relationship of 2-Ethoxycarbonyl-4-aryl-1,5-benzothiazepines with Antifungal Activity† [J]. Chem. J. Chinese Universities, 2014, 35(4): 766. |
| [8] | FAN Shili, ZHANG Bo, GAO Liye, WANG Lanzhi, BIAN Yanqing, LI Yuan. Synthesis, Antifungal Activity and Structure-activity Relationship of 2-Methoxycarbonyl/ethoxycarbonyl-4-fluorophenyl-1,5-benzothiazepines† [J]. Chem. J. Chinese Universities, 2014, 35(12): 2574. |
| [9] | LIU Zhuo, PAN Li, YU Shu-Jing, LI Zheng-Ming. Synthesis and Antifungal Activity of N-(4'-Substituted aromatic pyrimidin-2'-yl)-2-ethoxycarbonyl phenyl Sulfonylurea Derivatives [J]. Chem. J. Chinese Universities, 2013, 34(8): 1868. |
| [10] | QIU Zhao-Lai, LI Wen-Hong, ZHU Hai-Fei, LIU Qian, LI Yuan. Synthesis, Crystal Structure and Antifungal Activities of 3-(CH2)nCO2C2H5-1,5-Benzothiazepines [J]. Chem. J. Chinese Universities, 2013, 34(3): 579. |
| [11] | XIAO Tao, ZHOU Xuan, SONG Hao. Stereoselective Construction of the Key Skeleton of Oxindole Alkaloid Humantenine [J]. Chem. J. Chinese Universities, 2012, 33(12): 2676. |
| [12] | ZHAO Lian, YUAN Xiang-Guo, ZHAO Jian-Peng, CHEN Hua*, LIU Li, LI Xiao-Liu, CAO Ke-Qiang. Design, Synthesis and Antifungal Activity Against Valsa Mali of the Triamino Substitued Triazines Bearing Aminopyrimidine Group [J]. Chem. J. Chinese Universities, 2011, 32(12): 2795. |
| [13] | XIA Ya-Mu* , CHANG Liang. Asymmetric Synthesis of (-)-(1R,2S)-Myrislignan [J]. Chem. J. Chinese Universities, 2010, 31(9): 1780. |
| [14] | XU Bo-Yan, SONG Na, LI Wen-Ze, XIN Zhi-Jun, LI Ying*. First Asymmetric Synthesis of (7R,10S)-Boivinianin B [J]. Chem. J. Chinese Universities, 2009, 30(7): 1329. |
| [15] | SUN Qing-Yan*, CAO Yong-Bing, XU Jian-Ming, ZHANG Wan-Nian, ZHANG Jun, WU Qiu-Ye, ZHANG Da-Zhi, JIANG Yuan-Ying. Synthesis and Antifungal Activity of Novel Triazole Derivatives [J]. Chem. J. Chinese Universities, 2007, 28(9): 1707. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||