

Chem. J. Chinese Universities ›› 2017, Vol. 38 ›› Issue (5): 778.doi: 10.7503/cjcu20160752
• Organic Chemistry • Previous Articles Next Articles
LI Xiao1,2,*( ), GAO Liguo1, GONG Ying1, MA Yajun1, MA Xiangrong1
), GAO Liguo1, GONG Ying1, MA Yajun1, MA Xiangrong1
Received:2016-10-31
Online:2017-05-10
Published:2017-03-22
Contact:
LI Xiao
E-mail:lixiaoylxy2010@163.com
Supported by:CLC Number:
TrendMD:
LI Xiao, GAO Liguo, GONG Ying, MA Yajun, MA Xiangrong. Direct Asymmetric Aldol Reaction of Acetophenones and Aromatic Aldehydes Catalyzed by Chiral Al/Zn Heterobimetallic Compounds ZABDP†[J]. Chem. J. Chinese Universities, 2017, 38(5): 778.
| Compd. | Appearance | m. p./℃ | [α |
|---|---|---|---|
| 1 | White solid | 51—52(50—52.5[ | 26(c 1.6) |
| 2 | White solid | 43—45 | 61(c 1.55) |
| 3 | Pale yellow oil | 8(c 8.0) | |
| 4 | Pale yellow oil | 26(c 3.45) | |
| 5 | White solid | 115—117(115—116[ | 65(c 2.0) |
| 6 | White solid | 85—86(85—87[ | 123(c 0.7) |
| 7 | White solid | 107—109 | -44(c 0.5) |
| 8 | White solid | 100—102(101—102[ | 38(c 7.2) |
| 9 | White solid | 47—48.5(47—49[ | 70(c 1.35) |
| 10 | White solid | 82—84(82—84[ | 11(c 8.5) |
| 11 | White solid | 39—41(39—42[ | 27(c 2.03) |
| 12 | White solid | 68—69(67—69[ | 90(c 1.4) |
| 13 | White solid | 95—96(94—96[ | 155(c 2.0) |
| 14 | White solid | 82—83(82—84[ | 33(c 3.2) |
| 15 | White solid | 78—79.5(78—79[ | 54(c 5.6) |
| 16 | White solid | 61(60—61[ | 10(c 9.6) |
| 17 | White solid | 68—71 | 28(c1.6) |
| 18 | Pale yellow oil | 4(c 0.8) | |
| 19 | Pale yellow oil | 4(c 1.0) | |
| 20 | White solid | 107(106—107[ | 35(c 4.25) |
| 21 | White solid | 116—117(116—117[ | 42(c 1.4) |
Table 1 Appearance, melting points and optical rotation for compounds 1—21
| Compd. | Appearance | m. p./℃ | [α |
|---|---|---|---|
| 1 | White solid | 51—52(50—52.5[ | 26(c 1.6) |
| 2 | White solid | 43—45 | 61(c 1.55) |
| 3 | Pale yellow oil | 8(c 8.0) | |
| 4 | Pale yellow oil | 26(c 3.45) | |
| 5 | White solid | 115—117(115—116[ | 65(c 2.0) |
| 6 | White solid | 85—86(85—87[ | 123(c 0.7) |
| 7 | White solid | 107—109 | -44(c 0.5) |
| 8 | White solid | 100—102(101—102[ | 38(c 7.2) |
| 9 | White solid | 47—48.5(47—49[ | 70(c 1.35) |
| 10 | White solid | 82—84(82—84[ | 11(c 8.5) |
| 11 | White solid | 39—41(39—42[ | 27(c 2.03) |
| 12 | White solid | 68—69(67—69[ | 90(c 1.4) |
| 13 | White solid | 95—96(94—96[ | 155(c 2.0) |
| 14 | White solid | 82—83(82—84[ | 33(c 3.2) |
| 15 | White solid | 78—79.5(78—79[ | 54(c 5.6) |
| 16 | White solid | 61(60—61[ | 10(c 9.6) |
| 17 | White solid | 68—71 | 28(c1.6) |
| 18 | Pale yellow oil | 4(c 0.8) | |
| 19 | Pale yellow oil | 4(c 1.0) | |
| 20 | White solid | 107(106—107[ | 35(c 4.25) |
| 21 | White solid | 116—117(116—117[ | 42(c 1.4) |
| Compd. | 1H NMR(CDCl3), δ | 13C NMR(CDCl3), δ |
|---|---|---|
| 2 | (300 MHz)7.96—7.93(m, 2H), 7.60—7.55(m, 1H), 7.48—7.43(m, 2H), 7.33(d, J=8.1 Hz, 2H), 7.18(d, J=8.1 Hz, 2H), 5.33—5.29(m, 1H), 3.58(s, 1H), 3.37—3.35(m, 2H), 2.35(s,3H) | (300 MHz) 200.3, 140.0, 137.4, 136.6, 133.6, 129.2, 128.7, 128.2, 125.7, 69.9, 47.4, 21.2 |
| 3 | (400 MHz) 7.96(d,J=7.6 Hz, 2H), 7.59(t, J=7.6 Hz, 1H), 7.47(t, J=8.0 Hz, 2H), 7.29—7.21(m, 3H), 7.12(d, J=7.2 Hz, 1H), 5.32(t, J=6.0 Hz, 1H), 3.37(d, J=5.6 Hz, 2H)[ | |
| 4 | (300 MHz) 7.98—7.95(m, 2H), 7.62—7.57(m, 2H), 7.50—7.45(m, 2H), 7.30—7.16(m, 3H), 5.58(t, J=5.7 Hz, 1H), 3.52(d, J=1.8 Hz, 1H), 3.33—3.31(m 2H), 2.36(s, 3H)[ | (300 MHz) 200.31, 140.90, 136.53, 134.06, 133.69, 130.45, 128.74, 128.17, 127.47, 125.50, 66.49, 46.49, 46.09, 19.14 |
| 7 | (300 MHz) 8.01—7.96(m, 4H), 7.73—7.68(m, 1H), 7.63—7.58(m, 1H), 7.50—7.44(m, 3H), 5.88—5.84(m, 1H), 4.02(s, 1H), 3.78—3.71(dd, J=2.1, 15.6 Hz ,1H), 3.26—3.17(dd, J=9.3, 8.4 Hz,1H) | (300 MHz) 199.96, 138.6, 136.3, 133.9, 128.8, 128.4, 128.3, 128.2, 124.5, 65.9, 46.5 |
| 17 | (300 MHz) 7.96(d, J=7.2 Hz, 2H), 7.62—7.57(m, 1H), 7.50—7.45(m, 2H), 7.34—7.29(m, 2H), 7.15—7.13(m, 1H), 5.46—5.41(dd, J=5.7, 3.0 Hz, 1H), 3.63(d, J=3.3 Hz 1H), 3.42(d, J=6.0 Hz, 2H) | (200 MHz) 200.1, 144.2, 136.5, 133.7, 128.7, 128.2, 126.3, 125.6, 120.9, 66.6, 46.5 |
| 18 | (400 MHz) 7.96(d, J=7.2 Hz, 2H), 7.60(t, J=7.2 Hz, 1H), 7.48(t, J=7.6 Hz, 2H), 4.35—4.29(m, 1H), 3.16(dd, J=2.8, 17.4 Hz, 1H), 3.04(dd, J=8.8, 17.8 Hz, 1H), 1.91—1.85(m, 1H), 1.64—1.57(m, 1H), 1.29—1.22(m, 1H), 0.96(dd, J=3.2, 6.8 Hz, 6H)[ | |
| 19 | (400 MHz) 7.97(d, J=7.2 Hz, 2H), 7.60(t, J=7.6 Hz, 1H), 7.48(t, J=7.6 Hz, 2H), 4.24—4.22(m, 1H), 3.18(dd, J=2.8, 17.6 Hz, 1H), 3.05(dd, J=4.4, 17.6 Hz, 1H), 1.65—1.43(m, 4H), 0.97(t, J=7.2 Hz, 3H)[ |
Table 2 1H NMR, 13C NMR data for new or pale yellow oil compounds
| Compd. | 1H NMR(CDCl3), δ | 13C NMR(CDCl3), δ |
|---|---|---|
| 2 | (300 MHz)7.96—7.93(m, 2H), 7.60—7.55(m, 1H), 7.48—7.43(m, 2H), 7.33(d, J=8.1 Hz, 2H), 7.18(d, J=8.1 Hz, 2H), 5.33—5.29(m, 1H), 3.58(s, 1H), 3.37—3.35(m, 2H), 2.35(s,3H) | (300 MHz) 200.3, 140.0, 137.4, 136.6, 133.6, 129.2, 128.7, 128.2, 125.7, 69.9, 47.4, 21.2 |
| 3 | (400 MHz) 7.96(d,J=7.6 Hz, 2H), 7.59(t, J=7.6 Hz, 1H), 7.47(t, J=8.0 Hz, 2H), 7.29—7.21(m, 3H), 7.12(d, J=7.2 Hz, 1H), 5.32(t, J=6.0 Hz, 1H), 3.37(d, J=5.6 Hz, 2H)[ | |
| 4 | (300 MHz) 7.98—7.95(m, 2H), 7.62—7.57(m, 2H), 7.50—7.45(m, 2H), 7.30—7.16(m, 3H), 5.58(t, J=5.7 Hz, 1H), 3.52(d, J=1.8 Hz, 1H), 3.33—3.31(m 2H), 2.36(s, 3H)[ | (300 MHz) 200.31, 140.90, 136.53, 134.06, 133.69, 130.45, 128.74, 128.17, 127.47, 125.50, 66.49, 46.49, 46.09, 19.14 |
| 7 | (300 MHz) 8.01—7.96(m, 4H), 7.73—7.68(m, 1H), 7.63—7.58(m, 1H), 7.50—7.44(m, 3H), 5.88—5.84(m, 1H), 4.02(s, 1H), 3.78—3.71(dd, J=2.1, 15.6 Hz ,1H), 3.26—3.17(dd, J=9.3, 8.4 Hz,1H) | (300 MHz) 199.96, 138.6, 136.3, 133.9, 128.8, 128.4, 128.3, 128.2, 124.5, 65.9, 46.5 |
| 17 | (300 MHz) 7.96(d, J=7.2 Hz, 2H), 7.62—7.57(m, 1H), 7.50—7.45(m, 2H), 7.34—7.29(m, 2H), 7.15—7.13(m, 1H), 5.46—5.41(dd, J=5.7, 3.0 Hz, 1H), 3.63(d, J=3.3 Hz 1H), 3.42(d, J=6.0 Hz, 2H) | (200 MHz) 200.1, 144.2, 136.5, 133.7, 128.7, 128.2, 126.3, 125.6, 120.9, 66.6, 46.5 |
| 18 | (400 MHz) 7.96(d, J=7.2 Hz, 2H), 7.60(t, J=7.2 Hz, 1H), 7.48(t, J=7.6 Hz, 2H), 4.35—4.29(m, 1H), 3.16(dd, J=2.8, 17.4 Hz, 1H), 3.04(dd, J=8.8, 17.8 Hz, 1H), 1.91—1.85(m, 1H), 1.64—1.57(m, 1H), 1.29—1.22(m, 1H), 0.96(dd, J=3.2, 6.8 Hz, 6H)[ | |
| 19 | (400 MHz) 7.97(d, J=7.2 Hz, 2H), 7.60(t, J=7.6 Hz, 1H), 7.48(t, J=7.6 Hz, 2H), 4.24—4.22(m, 1H), 3.18(dd, J=2.8, 17.6 Hz, 1H), 3.05(dd, J=4.4, 17.6 Hz, 1H), 1.65—1.43(m, 4H), 0.97(t, J=7.2 Hz, 3H)[ |
| Entry | Ligand(5%, molar ratio to substrate) | Solvent | Yieldb(%) | e.e.c | Entry | Ligand(5%, molar ratio to substrate) | Solvent | Yieldb(%) | e.e.c |
|---|---|---|---|---|---|---|---|---|---|
| 1 | L-a | DMF | 13.3 | 10 | 6 | L-b | DMSO | 1.8 | 12 |
| 2 | L-b | DMF | 19.0 | 40 | 7 | L-b | ClCH2CH2Cl | 1.8 | 15 |
| 3 | L-b | THF | 2.7 | 12 | 8 | L-b | MeCN | 16.0 | 39 |
| 4 | L-b | Et2O | 5.3 | 9 | 9 | L-b | NMP | ||
| 5 | L-b | Toluene | 4.9 | 18 |
Table 3 Selection of the optimal liganda
| Entry | Ligand(5%, molar ratio to substrate) | Solvent | Yieldb(%) | e.e.c | Entry | Ligand(5%, molar ratio to substrate) | Solvent | Yieldb(%) | e.e.c |
|---|---|---|---|---|---|---|---|---|---|
| 1 | L-a | DMF | 13.3 | 10 | 6 | L-b | DMSO | 1.8 | 12 |
| 2 | L-b | DMF | 19.0 | 40 | 7 | L-b | ClCH2CH2Cl | 1.8 | 15 |
| 3 | L-b | THF | 2.7 | 12 | 8 | L-b | MeCN | 16.0 | 39 |
| 4 | L-b | Et2O | 5.3 | 9 | 9 | L-b | NMP | ||
| 5 | L-b | Toluene | 4.9 | 18 |
| Entry | Additive(%, molar ratio to substrate) | Yieldb(%) | e.e.c(%) | Entry | Additive(%, molar ratio to substrate) | Yieldb(%) | e.e.c(%) |
|---|---|---|---|---|---|---|---|
| 1 | 19.0 | 40 | 9 | Et2NH(60) | 19 | 60 | |
| 2 | Et3N(80) | 7.5 | 51 | 10 | Et2NH(100) | 22 | 61 |
| 3 | DIPEA(80) | 11.0 | 49 | 11 | Et2NH(120) | 26 | 43 |
| 4 | TMEDA(80) | 4.4 | 51 | 12d | Et2NH(80)/0.4 nm MS | 21 | 80 |
| 5 | Tetrahydropyrrole(80) | 9.3 | 60 | 13d,e | Et2NH(80)/Ph3PS/0.4 nm MS | 29 | 63 |
| 6 | 2,6-Lutidine(80) | 12.4 | 46 | 14d,e | Et2NH(80)/Ph3POP/0.4 nm MS | 23 | 70 |
| 7 | NMM(80) | 3.1 | 27 | 15d,e | Et2NH(80)/Ph3P/0.4 nm MS | 24 | 65 |
| 8 | Et2NH(80) | 23.0 | 66 |
Table 4 Selection of the optimal additivea
| Entry | Additive(%, molar ratio to substrate) | Yieldb(%) | e.e.c(%) | Entry | Additive(%, molar ratio to substrate) | Yieldb(%) | e.e.c(%) |
|---|---|---|---|---|---|---|---|
| 1 | 19.0 | 40 | 9 | Et2NH(60) | 19 | 60 | |
| 2 | Et3N(80) | 7.5 | 51 | 10 | Et2NH(100) | 22 | 61 |
| 3 | DIPEA(80) | 11.0 | 49 | 11 | Et2NH(120) | 26 | 43 |
| 4 | TMEDA(80) | 4.4 | 51 | 12d | Et2NH(80)/0.4 nm MS | 21 | 80 |
| 5 | Tetrahydropyrrole(80) | 9.3 | 60 | 13d,e | Et2NH(80)/Ph3PS/0.4 nm MS | 29 | 63 |
| 6 | 2,6-Lutidine(80) | 12.4 | 46 | 14d,e | Et2NH(80)/Ph3POP/0.4 nm MS | 23 | 70 |
| 7 | NMM(80) | 3.1 | 27 | 15d,e | Et2NH(80)/Ph3P/0.4 nm MS | 24 | 65 |
| 8 | Et2NH(80) | 23.0 | 66 |
| Entry | n(Me3Al)/mmol | n(Et2Zn)/mmol | Yieldb(%) | e.e.c(%) |
|---|---|---|---|---|
| 1 | 0.01 | 0 | 11 | 20 |
| 2 | 0.005 | 0.0025 | 16 | 56 |
| 3 | 0.005 | 0.0038 | 17 | 63 |
| 4 | 0.005 | 0.005 | 23 | 56 |
| 5 | 0.005 | 0.006 | 21 | 80 |
| 6 | 0.005 | 0.075 | 15 | 75 |
| 7 | 0.005 | 0.0088 | 35 | 64 |
Table 5 Selection of the optimal ratio of Al and Zna
| Entry | n(Me3Al)/mmol | n(Et2Zn)/mmol | Yieldb(%) | e.e.c(%) |
|---|---|---|---|---|
| 1 | 0.01 | 0 | 11 | 20 |
| 2 | 0.005 | 0.0025 | 16 | 56 |
| 3 | 0.005 | 0.0038 | 17 | 63 |
| 4 | 0.005 | 0.005 | 23 | 56 |
| 5 | 0.005 | 0.006 | 21 | 80 |
| 6 | 0.005 | 0.075 | 15 | 75 |
| 7 | 0.005 | 0.0088 | 35 | 64 |
| Entry | Me3Al(%, molar ratio to substrate) | Et2Zn(%, molar ratio) | T/℃ | L-b(%, molar ratio to substrate) | Yieldb(%) | e.e.c(%) |
|---|---|---|---|---|---|---|
| 1 | 5 | 6 | r. t. | 5 | 31 | 24 |
| 2 | 5 | 6 | 0 | 5 | 26 | 35 |
| 3 | 5 | 6 | -20 | 5 | 21 | 80 |
| 4 | 5 | 6 | -78 | 5 | 18 | 66 |
| 5 | 20 | 24 | -20 | 20 | 50 | 80 |
| 6d | 20 | 24 | -20 | 20 | 74 | 82 |
Table 6 Further optimization of reaction conditionsa
| Entry | Me3Al(%, molar ratio to substrate) | Et2Zn(%, molar ratio) | T/℃ | L-b(%, molar ratio to substrate) | Yieldb(%) | e.e.c(%) |
|---|---|---|---|---|---|---|
| 1 | 5 | 6 | r. t. | 5 | 31 | 24 |
| 2 | 5 | 6 | 0 | 5 | 26 | 35 |
| 3 | 5 | 6 | -20 | 5 | 21 | 80 |
| 4 | 5 | 6 | -78 | 5 | 18 | 66 |
| 5 | 20 | 24 | -20 | 20 | 50 | 80 |
| 6d | 20 | 24 | -20 | 20 | 74 | 82 |
| Entry | R | Yieldb(%) | e.e.c(%) | Entry | R | Yieldb(%) | e.e.c(%) |
|---|---|---|---|---|---|---|---|
| 1 | Ph | 74 | 82 | 11 | 3-MeOC6H4 | 99 | 85 |
| 2 | 4-MeC6H4 | 23 | 71 | 12 | 2-MeOC6H4 | 48 | 56 |
| 3 | 3-MeC6H4 | 77 | 80 | 13 | 1-Napth | 57 | 80 |
| 4 | 2-MeC6H4 | 60 | 76 | 14 | 2-Napth | 80 | 80 |
| 5 | 4-NO2C6H4 | 90 | 80 | 15 | 2-Furyl | 63 | 90 |
| 6 | 3-NO2C6H4 | 35 | 75 | 16 | 2-Thiophene | 85 | 77 |
| 7 | 2-NO2C6H4 | 94 | 71 | 17 | 3-Thiophene | 33 | 73 |
| 8 | 4-ClC6H4 | 65 | 73 | 18 | Isovaleric | 64 | 83 |
| 9 | 3-ClC6H4 | 72 | 82 | 19 | n-Butyl | 68 | 65 |
| 10 | 2-ClC6H4 | 75 | 46 |
Table 7 Results of asymmetric Aldol reaction of acetophenones and aromatic aldehydes catalyzed by chiral ZABDPa
| Entry | R | Yieldb(%) | e.e.c(%) | Entry | R | Yieldb(%) | e.e.c(%) |
|---|---|---|---|---|---|---|---|
| 1 | Ph | 74 | 82 | 11 | 3-MeOC6H4 | 99 | 85 |
| 2 | 4-MeC6H4 | 23 | 71 | 12 | 2-MeOC6H4 | 48 | 56 |
| 3 | 3-MeC6H4 | 77 | 80 | 13 | 1-Napth | 57 | 80 |
| 4 | 2-MeC6H4 | 60 | 76 | 14 | 2-Napth | 80 | 80 |
| 5 | 4-NO2C6H4 | 90 | 80 | 15 | 2-Furyl | 63 | 90 |
| 6 | 3-NO2C6H4 | 35 | 75 | 16 | 2-Thiophene | 85 | 77 |
| 7 | 2-NO2C6H4 | 94 | 71 | 17 | 3-Thiophene | 33 | 73 |
| 8 | 4-ClC6H4 | 65 | 73 | 18 | Isovaleric | 64 | 83 |
| 9 | 3-ClC6H4 | 72 | 82 | 19 | n-Butyl | 68 | 65 |
| 10 | 2-ClC6H4 | 75 | 46 |
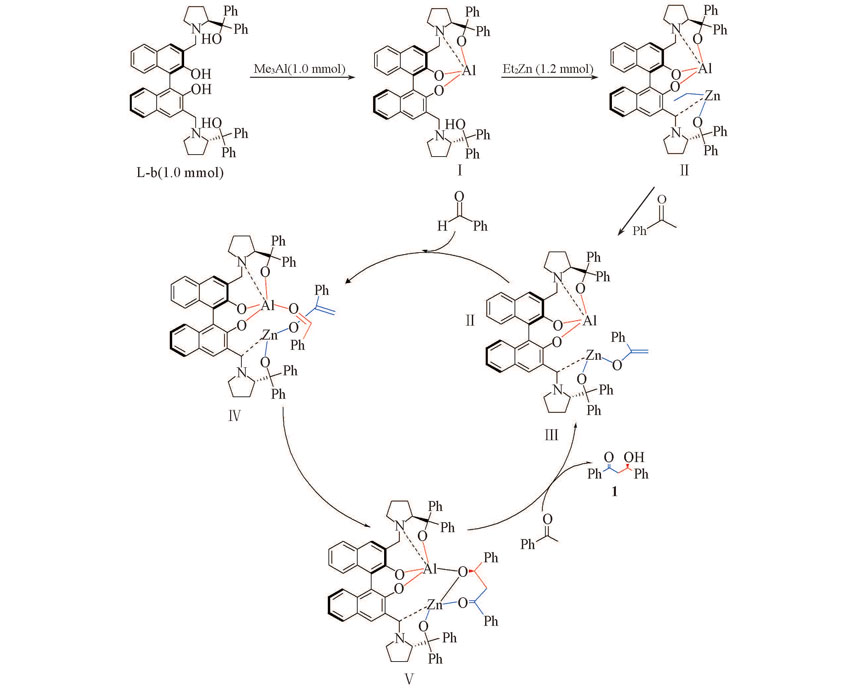
Scheme 2 Proposed reaction mechanism of direct asymmetic Aldol reaction of acetophenones and aromatic aldehydes catalyzed by chiral Al/Zn heterobimetallic compounds ZABP
| [1] | Alcaide B., Almendros P., Eur. J. Org. Chem., 2002, 2002(10), 1595—1601 |
| [2] | Trost B. M., Brindle C. S., Chem. Soc. Rev., 2010, 39(5), 1600—1632 |
| [3] | Palomo C., Oiarbide M., García J. M., Chem. Soc. Rev., 2004, 33(2), 65—75 |
| [4] | Xu D., Xie X. T., Zheng P., Wang Y. C., Yunnan Chemical Technology, 2016, 43(3), 52—61 |
| (徐丹, 谢笑天, 郑萍, 王永超.云南化工, 2016,43(3), 52—61) | |
| [5] | Li H., Xu D. Z., Wu L. L., Wang Y. M., Chem. Res. Chinese Universities, 2012, 28(6), 1003—1010 |
| [6] | Xiao J., Li G. W., Zhang W. Q., Chem. Res. Chinese Universities, 2013, 29(2), 256—262 |
| [7] | Li X., Han Y., Tan B. X., Wang B., Cheng J. P., Chem. J. Chinese Universities, 2014, 35(9), 1908—1911 |
| (李鑫, 韩玉, 谈柏轩, 王斌, 程津培.高等学校化学学报, 2014,35(9), 1908—1911) | |
| [8] | Yu X. X., Wang Q., Zhou Y., Gao R. J., Wang Y. W., Chem. J. Chinese Universities, 2015, 36(12), 2454—2460 |
| (于潇潇, 王琦, 周烨, 高仁钧, 王英武.高等学校化学学报, 2015,36(12), 2454—2460) | |
| [9] | Mahrwald R., Modern Aldol Reactions, Vol.2: Metal Catalysis, Wiley-VCH, Weinheim, 2004, 1—342 |
| [10] | Casiraghi G., Zanardi F., Appendino G., Rassu G., Chem. Rev., 2000, 100(6), 1929—1972 |
| [11] | Singh P., Bhardwaj A., J. Med. Chem., 2010, 53(9), 3707—3717 |
| [12] | Johnson J. S., Evans D. A., Acc. Chem. Res., 2000, 33(6), 325—335 |
| [13] | Li H. J., Tian H. Y., Wu Y. C., Chen Y. J., Liu L., Wang D., Li C. J., Adv. Synth. Catal., 2005, 347(9), 1247—1256 |
| [14] | Mlynarski J., Jankowska J., Adv. Synth. Catal., 2005, 347(4), 521—525 |
| [15] | Denmark S. E., Heemstra J. R. Jr., Org. Lett., 2003, 5(13), 2303—2306 |
| [16] | Kiyooka S., Takeshita Y., Tanaka Y., Higaki T., Wada Y., Tetrahedron Lett., 2006, 47(26), 4453—4456 |
| [17] | Li H.J., Tian H. Y., Chen Y. J., Wang D., Li C. J.,Chem. Commun., 2002, (24), 2994—2995 |
| [18] | Zhao J. F., Tan B. H., Loh T. P., Chem. Sci., 2011, 2(2), 349—352 |
| [19] | Yu J., Zhao X., Miao Z., Chen R., Org. Biomol. Chem., 2011, 9(19), 6721—6726 |
| [20] | Yoshikawa N., Kumagai N., Mutsunaga S., Moll G., Ohshima T., Suzuki T., Shibasaki M., J. Am. Chem. Soc., 2001, 123(10), 2466—2467 |
| [21] | Kumagai N., Matsunaga S., Yoshikawa N., Ohshima T., Shibasaki M., Org. Lett., 2001, 3(10), 1539—1542 |
| [22] | Trost B. M., Ito H., J. Am. Chem. Soc., 2000, 122(48), 12003—12004 |
| [23] | Trost B. M., Ito H., Silcoff E. R., J. Am. Chem. Soc., 2001, 123(14), 3367—3368 |
| [24] | Trost B. M., Yeh V. S. C., Angew. Chem. Int. Ed., 2002, 41(5), 861—863 |
| [25] | Trost B. M., Mino T., J. Am. Chem. Soc., 2003, 125(9), 2410—2411 |
| [26] | Trost B. M., Fettes A., Shireman B. T., J. Am. Chem. Soc., 2004, 126(9), 2660—2661 |
| [27] | Trost B. M., Shin S., Sclafani J. A., J. Am. Chem. Soc., 2005, 127(24), 8602—8603 |
| [28] | Li G. W., Wang X. J., Zhao W. X., Lu L. J., Liu G. J., Wang M. C., Progress in Chemistry, 2012, 24(2/3), 348—360 |
| (李高伟, 王晓娟, 赵文献, 鲁刘杰, 刘冠军, 王敏灿.化学进展, 2012,24(2/3), 348—360) | |
| [29] | Li H., Da C. S., Xiao Y. H., Li X., Su Y. N., J. Org. Chem., 2008, 73(18), 7398—7401 |
| [30] | Shibasaki M., Yoshikawa N., Chem. Rev., 2002, 102(6), 2187—2210 |
| [31] | Notz W., Tanaka F., Barbas C. F., Acc. Chem. Res., 2004, 37(8), 580—591 |
| [32] | Matsunaga S., Shibasaki M., Bull. Chem. Soc. Jpn., 2008, 81(1), 60—75 |
| [33] | Mihara H., Xu Y., Sheperd N. E., Matsunaga S., Shibasaki M., J. Am. Chem. Soc., 2009, 131(24), 8384—8385 |
| [34] | Handa S., Gnanadesikan V., Matsunaga S., Shibasaki M., J. Am. Chem. Soc., 2010, 132(13), 4925—4934 |
| [35] | Iwata M., Yazaki R., Chen I. H., Sureshkumar D., Kumagai N., Shibasaki M., J. Am. Chem. Soc., 2011, 133(14), 5554—5560 |
| [36] | Li K., Yang G. Q., Liu Y. Y., Zhang W. B., Chinese Journal of Organic Chemistry, 2013, 33(4), 749—759 |
| (李昆, 杨国强, 刘媛媛, 张万斌.有机化学, 2013,33(4), 749—759) | |
| [37] | Morohashi N., Hattori T., Yokomakura K., Kabuto C., Miyano S., Tetrahedron Letters, 2002, 43(43), 7769—7772 |
| [38] | Downey C. W., Johnson M. W., Tetrahedron Letters, 2007, 48(20), 3559—3562 |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||