

Chem. J. Chinese Universities ›› 2019, Vol. 40 ›› Issue (1): 55.doi: 10.7503/cjcu20180369
• Analytical Chemistry • Previous Articles Next Articles
LIU Qianrong1,2, CAI Huiwu1,*( ), GUAN Ming2,3, QIAO Juan2,3, QI Li2,3,*(
), GUAN Ming2,3, QIAO Juan2,3, QI Li2,3,*( )
)
Received:2018-05-18
Online:2019-01-10
Published:2018-11-09
Contact:
CAI Huiwu,QI Li
E-mail:caihuiwu@126.com;qili@iccas.ac.cn
Supported by:CLC Number:
TrendMD:
LIU Qianrong,CAI Huiwu,GUAN Ming,QIAO Juan,QI Li. Turn-off Fluorescence of Gold Nanoclusters for Sensing of Ferric Ion†[J]. Chem. J. Chinese Universities, 2019, 40(1): 55.
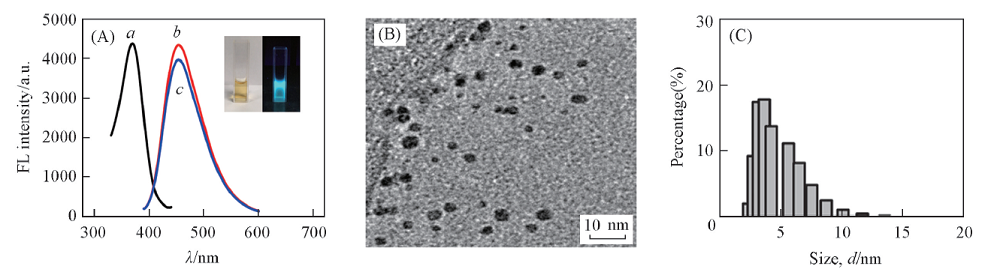
Fig.2 Fluorescence excitation(a), emission spectra of fresh D-Trp@AuNCs(b) and stored for a month(c)(A), TEM image(B) and DLS analysis(C) of D-Trp@AuNCsInset of (A): photographs of D-Trp@AuNCs under irradiation of daylight(left) and UV light(right).
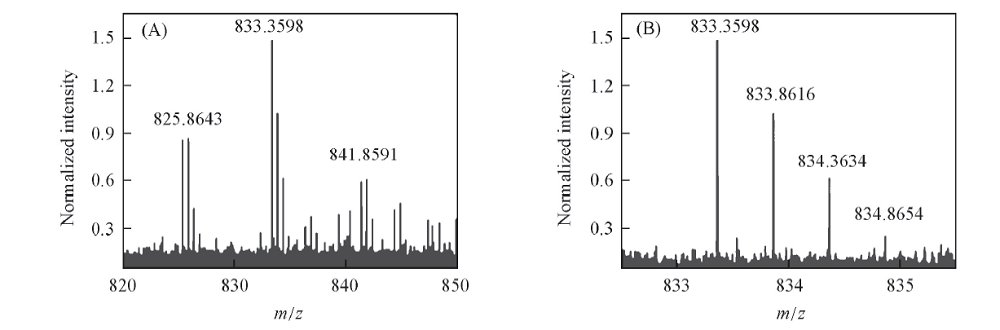
Fig.3 Enlarged ESI mass spectra of D-Trp@AuNCs from m/z 820—850(A) and the expanded views of the peaks originated from [Au3Y4+Na10+K-H9]2+(m/z 833.5245)(B)
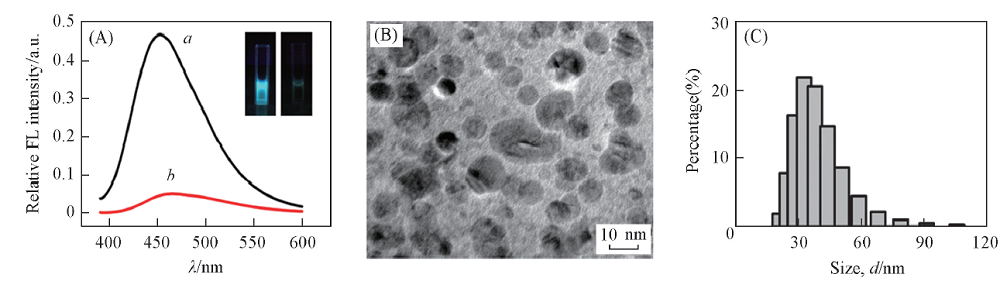
Fig.5 Fluorescence spectra of D-Trp@AuNCs(a) and D-Trp@AuNCs+Fe3+(b)(A), TEM image of D-Trp@AuNCs+Fe3+(B) and DLS analysis of D-Trp@AuNCs+Fe3+(C)Inset of (A): under irradiation of UV light, photographs of D-Trp@AuNCs(left) and D-Trp@AuNCs + Fe3+(right).
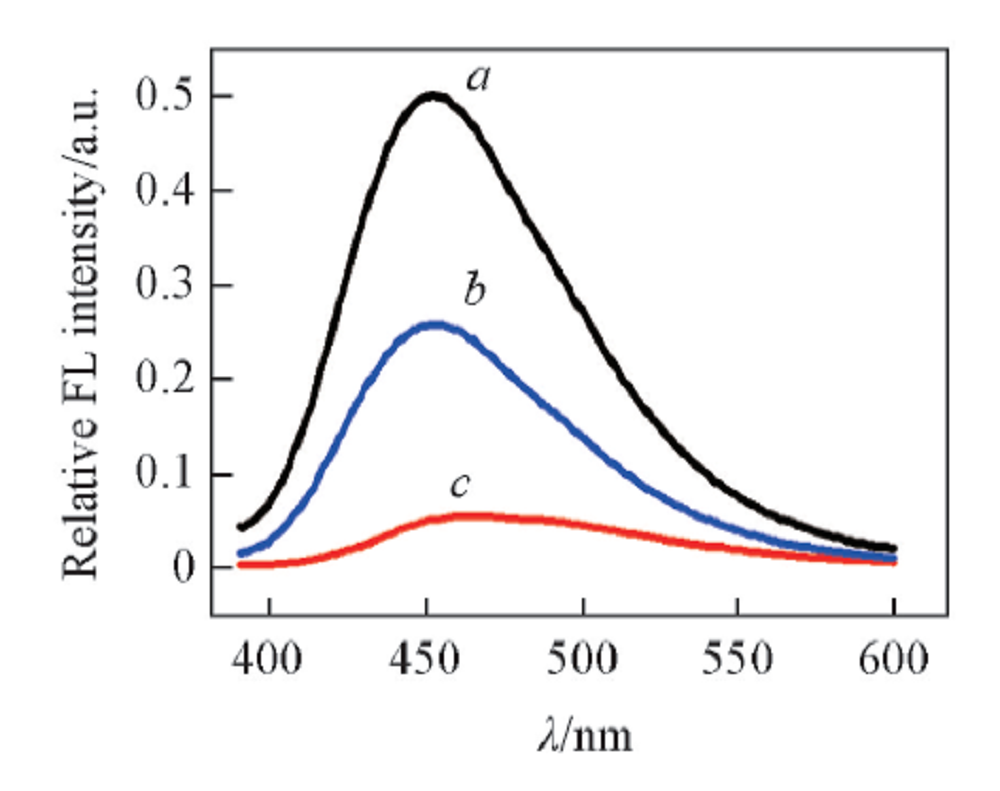
Fig.6 Fluorescence spectra of D-Trp@AuNCs(a), D-Trp@AuNCs+Fe3+(10.0 mmol/L) in the presence of 10.0 mmol/L EDTA(b) and D-Trp@AuNCs in the presence of 10.0 mmol/L Fe3+ (c)
| Sample | Detected/ (μmol·L-1) | Spiked/ (μmol·L-1) | Found/(μmol·L-1) | Recovery(%) | RSD(%) |
|---|---|---|---|---|---|
| Lake water | —— | 50.0 | 48.8 | 97.6 | 5.4 |
| —— | 70.0 | 68.9 | 98.4 | 2.3 | |
| —— | 120.0 | 112.6 | 93.8 | 3.5 | |
| Pond water | —— | 50.0 | 43.3 | 86.6 | 4.5 |
| —— | 70.0 | 63.8 | 91.1 | 5.6 | |
| —— | 120.0 | 105.4 | 87.8 | 2.6 | |
| Tap water | —— | 50.0 | 49.3 | 98.6 | 3.8 |
| —— | 70.0 | 69.9 | 99.8 | 4.5 | |
| —— | 120.0 | 127.8 | 106.5 | 5.6 |
Table 1 Determination of Fe3+ in real water samples(n=6)*
| Sample | Detected/ (μmol·L-1) | Spiked/ (μmol·L-1) | Found/(μmol·L-1) | Recovery(%) | RSD(%) |
|---|---|---|---|---|---|
| Lake water | —— | 50.0 | 48.8 | 97.6 | 5.4 |
| —— | 70.0 | 68.9 | 98.4 | 2.3 | |
| —— | 120.0 | 112.6 | 93.8 | 3.5 | |
| Pond water | —— | 50.0 | 43.3 | 86.6 | 4.5 |
| —— | 70.0 | 63.8 | 91.1 | 5.6 | |
| —— | 120.0 | 105.4 | 87.8 | 2.6 | |
| Tap water | —— | 50.0 | 49.3 | 98.6 | 3.8 |
| —— | 70.0 | 69.9 | 99.8 | 4.5 | |
| —— | 120.0 | 127.8 | 106.5 | 5.6 |
| [1] | Bindhu M. R., Umadevi M., J. Cluster Sci.,2014, 25, 969—978 |
| [2] | Lin S. M., Geng S., Li N., Liu S. G., Li N. B., Luo H. Q., Sens. Actuator B: Chem.,2017, 252, 912—918 |
| [3] | Ministry of Health and National Committee for Standardization, GB5749-2006, Standards for Drinking Water Quality, Standards Press of China, Beijing, 2006-12-29 |
| (卫生部和国家标准化委员会, GB5749-2006, 生活饮用水卫生标准, 北京: 中国标准出版社, 2006-12-29) | |
| [4] | Kang J. O., Clin. Lab. Sci., 2001, 14, 208—219 |
| [5] | Dungier Y.,Alcohol., 2003, 145—150 |
| [6] | Knobel Y., Glei M., Osswald K., Pool-Zobel B. L., Toxicol. Vitro.,2006, 20, 793—800 |
| [7] | Fargion S., Mattioli M., Fracanzani A.L., Fiorelli G.,Can. J. Gastroenterol., 2000, 89D—92D |
| [8] | Walker E., Walker S.,Clin. Lab. Sci., 2000, 330—354 |
| [9] | Pomazal K., Prohaska C., Stean I., Reich G., Huber J. F. K., Analyst,1999, 124, 657—663 |
| [10] | Andersen J. E. T., Analyst>, 2005, 130, 385—390 |
| [11] | Cao H. Y., Chen Z. H., Zheng H. Z., Huang Y. M., Biosens. Bioelectron.,2014, 62, 189—195 |
| [12] | Wang R., Yu F. B., Liu P., Chen L. X., Chem. Commun.,2012, 48, 5310—5312 |
| [13] | Xiang Y., Tong A. J., Org. Lett.,2006, 8, 1549—1552 |
| [14] | Lee D. Y., Singh N., Jang D. O., Tetrahedron Lett., 2011, 52, 1368—1371 |
| [15] | Zhao Q., Chen S. N., Huang H. W., Zhang L. Y., Wang L. Q., Liu F. P., Chen J., Zeng Y. L., Chu P. K., Analyst,2014, 139, 1498—1503 |
| [16] | Zhang M. M., Qiao J., Zhang S. F., Qi L., Talanta,2018, 182, 595—599 |
| [17] | Cui K., Lu X. M., Cui W., Wu J., Chen X. M., Lu Q. H., Chem. Commun.,2011, 47, 920—922 |
| [18] | Selvaprakash K., Chen Y. C., Biosens. Bioelectron.,2014, 61, 88—94 |
| [19] | Tao Y., Li M. Q., Rena J. S., Qu X. G., Chem. Soc. Rev., 2015, 44, 8636—8663 |
| [20] | Devi J. S. A., Salini S., Anulekshmi A. H., Praveen G. L., Sony G., Sens. Actuator B: Chem.,2017, 246, 943—951 |
| [21] | Ho J. A., Chang H. C., Su W. T., Anal. Chem.,2012, 84, 3246—3253 |
| [22] | Liu X. F., Li C. H., Xun J. L., Lv J., Zhu M., Guo Y. B., Cui S., Liu H.B., Wang S., Li Y. L., J. Phys. Chem. C,2008, 112, 10778—10783 |
| [23] | Qu W. G., Wang S. M., Hu Z. J., Cheang T. Y., Xing Z. H., Zhang X. J., Xu A. W., J. Phys. Chem. C,2010, 114, 13010—13016 |
| [24] | Mu X. Y., Qi L., Dong P., Qiao J., Hou J., Nie Z. X., Ma H. M., Biosens. Bioelectron.,2013, 49, 249—255 |
| [25] | Li H. W., Yue Y., Liu T. Y., Li D., Wu Y. Q., J. Phys. Chem. C,2013, 117, 16159—16165 |
| [26] | Su Y., Qi L., Mu X. Y., Wang M. L., Anal. Methods,2015, 7, 684—689 |
| [27] | Cademartiri, Kitaev V., Nanoscale, 2011, 3, 3435—3446 |
| [1] | YAN Fanyong, SUN Zhonghui, PANG Jiping, JIANG Yingxia, CHEN Yuan. Functionalized Carbon Dots of Benzothiazine Derivatives for Detection of Quercetin in Ginkgo Biloba Tea [J]. Chem. J. Chinese Universities, 2020, 41(8): 1768. |
| [2] | GONG Minghui,YANG Shanshan,LI Shusheng,KUANG Rui,KONG Xiangzheng. Preparation of Fluorescence Polyurea Microspheres Through Precipitation Polymerization and Their Use for Fe3+ Determination† [J]. Chem. J. Chinese Universities, 2019, 40(6): 1317. |
| [3] | XU Yuan, CHEN Yanhua, DING Lan. One-pot Microwave-assisted Synthesis of Passivated Fluorescent Carbon Dots for Fe(Ⅲ) Detection† [J]. Chem. J. Chinese Universities, 2018, 39(7): 1420. |
| [4] | CHEN Junlei, ZHANG Wei, LI Xinxia, LI Jiutong, GUAN Ming. Detection of C-reactive Protein by Background Fluorescence Quenching-Immunochromatography† [J]. Chem. J. Chinese Universities, 2018, 39(1): 41. |
| [5] | XIA Meng, PENG Xiongwei, GAO Hongfei, YAN Chao, CHEN Huiru, CHENG Xiaohong. Synthesis and Properties of Barbituric Acid Based Taper Shaped Rodlike Liquid Crystal Compound and Hydrogen Bonded Complex with Triazine Derivative† [J]. Chem. J. Chinese Universities, 2017, 38(7): 1203. |
| [6] | LIU Jilin, MI Hongyu, GUAN Mingming, HUAN Yanfu, FEI Qiang, ZHANG Zhiquan, FENG Guodong. Preparation and Application of Novel Fluorescence Chemical Sensor for Determining Trace Levels of Picronitric Acid Based on Water Soluble Conjugated Polymer [J]. Chem. J. Chinese Universities, 2017, 38(12): 2163. |
| [7] | YU Xijuan, HAN Lulu, HUN Xu. Cu2+ Modified Gold Nanoclusters for Fluorescence Turn-on Detection of Dopamine† [J]. Chem. J. Chinese Universities, 2017, 38(12): 2169. |
| [8] | LI Bing, DONG Yongchun. Coordination Kinetics of Different Carboxylic Fiber with Fe3+ and Catalytic Degradation Performance of Their Fe3+ Complexes† [J]. Chem. J. Chinese Universities, 2014, 35(8): 1761. |
| [9] | LIN Mei-Na, ZHANG Wei-Ying, LI Xiao, YING Xiao-Guang, CHEN Shuang-Shuang. Non-covalent Interaction of Tryptophan with Functional Monomers in Self-assembly Systems [J]. Chem. J. Chinese Universities, 2013, 34(1): 198. |
| [10] | LIU Xue-Ping, OUYANG Xiang-Yuan, WU Hui-Wang, SHEN Guo-Li, YU Ru-Qin. Novel Fluorescent Technique for Detecting Adenosine Based on DNA Strand Displacement Induced by Target-aptamer Complex and Metal Deposition Catalyzed by Gold Nanoparticles [J]. Chem. J. Chinese Universities, 2012, 33(08): 1692. |
| [11] | YIN Peng-Fei, GONG Hui-Ping, LI Ping-Ping, LIU Zheng-Qing, HE You-Qiu. Fluorescence Spectrometry and UV-Vis Absorption Spectra Analysis of Interaction of Core-shell CdTe/CdS QDs with Palmatine Chloride and Its Applications [J]. Chem. J. Chinese Universities, 2012, 33(07): 1432. |
| [12] | YANG Mu, WANG Xiao-Na, XU Jin-Wu, WANG Ge. Preparation and Catalytic Properties of Fe-Ti-MCM-41 [J]. Chem. J. Chinese Universities, 2012, 33(07): 1559. |
| [13] | GAN Xiao-Juan, LIU Shao-Pu, LIU Zhong-Fang, HU Xiao-Li. Fluorescence Quenching Method for the Determination of Carbazochromum with Halide Fluorescein Dyes [J]. Chem. J. Chinese Universities, 2012, 33(04): 683. |
| [14] | TIAN Lun-Fu, LIU Zhong-Fang, HU Xiao-Li, KONG Ling, LIU Shao-Pu. Proteins Fluorescence Quenching by [Hg(SCN)4]2- and Its Analytical Application [J]. Chem. J. Chinese Universities, 2012, 33(01): 59. |
| [15] | XU Qian-Ying, LIU Zhong-Fang, HU Xiao-Li, KONG Ling, LIU Shao-Pu*. Fluorescence Spectroscopy Study on the Interaction Between Pd(Ⅱ) and Tryptophan, Tyrosine and Phenylalanine [J]. Chem. J. Chinese Universities, 2011, 32(7): 1492. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||