

Chem. J. Chinese Universities ›› 2018, Vol. 39 ›› Issue (9): 1859.doi: 10.7503/cjcu20180114
• Articles:Inorganic Chemistry • Previous Articles Next Articles
WANG Shuang1, YU Yue1, BAI Pu1, LIU Jiancong1, LI Lin1, SONG Xiaowei1,2,*( )
)
Received:2018-02-07
Online:2018-09-07
Published:2018-08-03
Contact:
SONG Xiaowei
E-mail:xiaoweisong@jlu.edu.cn
Supported by:CLC Number:
TrendMD:
WANG Shuang,YU Yue,BAI Pu,LIU Jiancong,LI Lin,SONG Xiaowei. Synthesis of Aluminophosphate Molecular Sieves by Ball Milling and Recrystallization†[J]. Chem. J. Chinese Universities, 2018, 39(9): 1859.
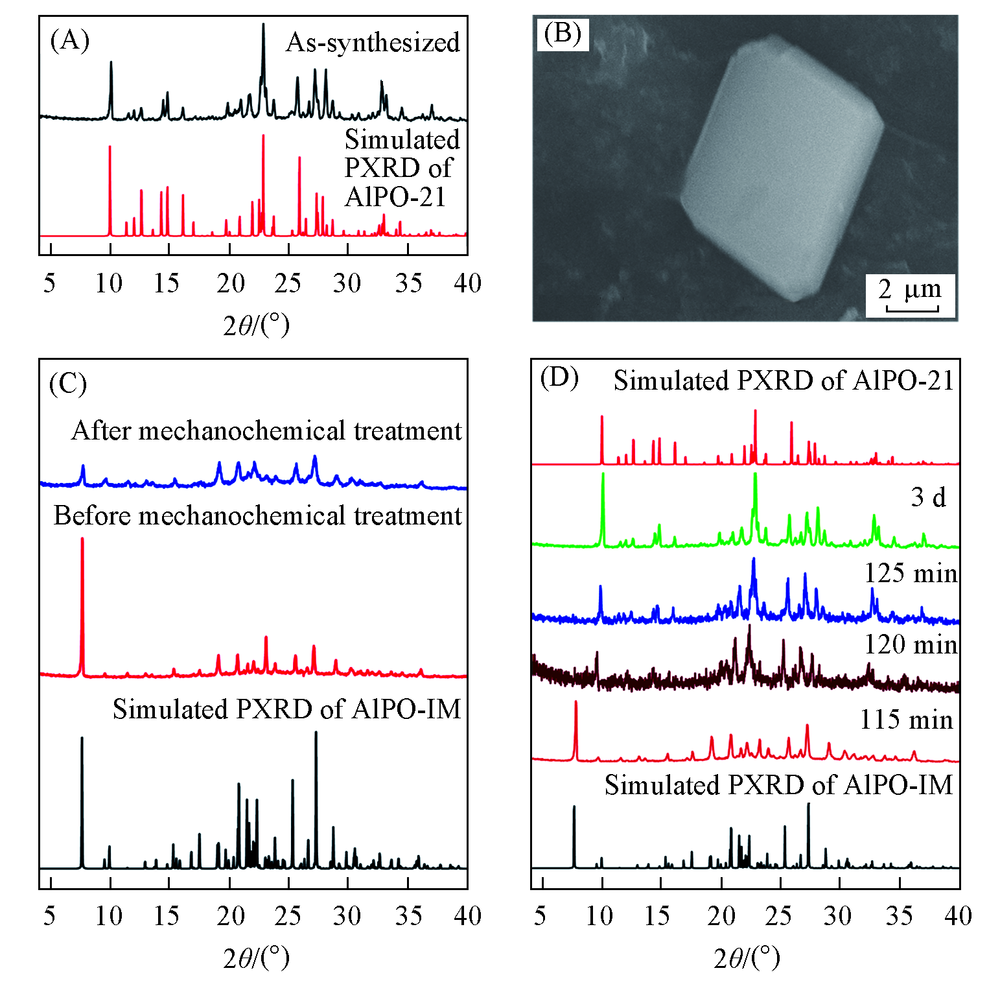
Fig.1 XRD patterns(A) and SEM image(B) of AlPO-CJ73a, XRD patterns of AlPO-IM before and after the mechanochemical treatment(C) and XRD patterns of AlPO-CJ73a after different reaction time(D)
| Sample | Content, w(%) | |||||
|---|---|---|---|---|---|---|
| Al | M | P | C | N | H | |
| MgAPO-CJ73b | 12.0 | 3.16 | 17.8 | 8.15 | 6.72 | 1.24 |
| ZnAPO-CJ73c | 10.5 | 11.2 | 16.9 | 7.29 | 5.76 | 0.98 |
Table 1 Elemental analysis of the MAPO-CJ73(M=Mg, Zn)
| Sample | Content, w(%) | |||||
|---|---|---|---|---|---|---|
| Al | M | P | C | N | H | |
| MgAPO-CJ73b | 12.0 | 3.16 | 17.8 | 8.15 | 6.72 | 1.24 |
| ZnAPO-CJ73c | 10.5 | 11.2 | 16.9 | 7.29 | 5.76 | 0.98 |
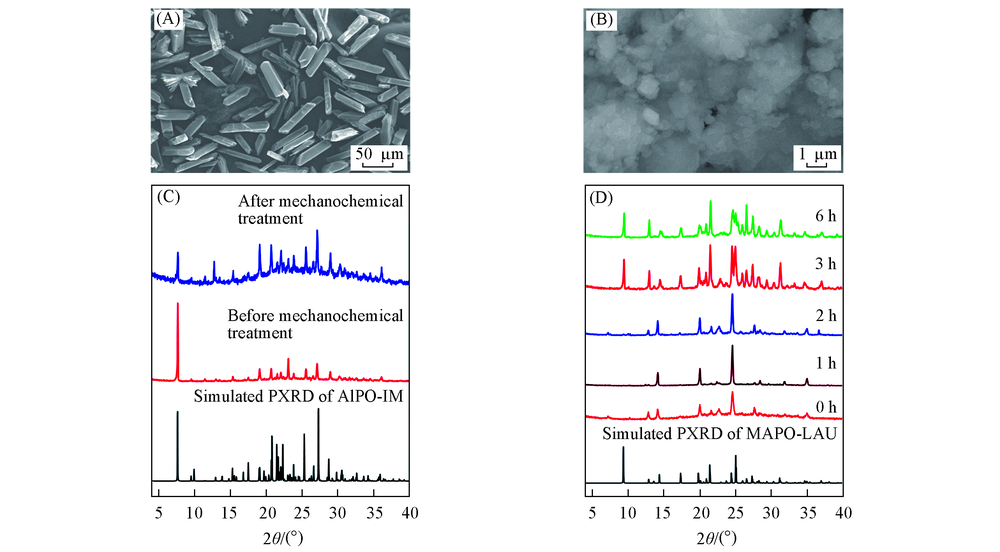
Fig.5 SEM images of AlPO-IM before(A) and after(B) the mechanochemical treatment, XRD patterns of AlPO-IM before and after the mechanochemical treatment(C) and the products under different ball-milling time(D)
| [1] | van Speybroeck V., Hemelsoet K., Joos L., Waroquier M., Bell R. G., Catlow C. R., Chem. Soc. Rev., 2015, 44(20), 7044—7111 |
| [2] | Zhu R., Chen Y. X., Ke Q. F., Zhang C. Q., Guo Y. P., Mater. Design, 2017, 122, 118—127 |
| [3] | Li J., Corma A., Yu J., Chem. Soc. Rev., 2015, 44(20), 7112—7127 |
| [4] | Parnham E. R., Morris R. E., Accounts Chem. Res., 2007, 40(10), 1005—1013 |
| [5] | Tompsett G. A., Conner W. C., Yngvesson K. S., Chem. Phys. Chem., 2006, 7(2), 296—319 |
| [6] | Cundy C. S., Cox P. A., Chem. Rev., 2003, 103(3), 663—702 |
| [7] | Guo J. H., Yan W. F., Shi W., Xu R. R., Chinese J. Inorg. Chem., 2018, 39(5), 841—848 |
| (郭俊辉, 闫文付, 师唯, 徐如人. 无机化学学报, 2018, 39(5), 841—848) | |
| [8] | Bértolo R., Silva J. M., Ribeiro M. F., Martins A., Fernandes A., Appl. Catal. A: Gen., 2017, 542, 28—37 |
| [9] | Ma Z., Yu J., Dai S., Adv. Mater., 2010, 22(2), 261—285 |
| [10] | Ralphs K., Hardacre C., James S. L., Chem. Soc. Rev., 2013, 42(18), 7701—7718 |
| [11] | Suryanarayana C., Prog. Mater. Sci., 2001, 46(1), 1—184 |
| [12] | Zhang P., Li H., Veith G. M., Dai S., Adv. Mater., 2015, 27(2), 234—239 |
| [13] | Xu C., De S., Balu A. M., Ojeda M., Luque R., Chem. Commun., 2015, 51(31), 6698—6713 |
| [14] | Majano G., Borchardt L., Mitchell S., Valtchev V., Pérez-Ramírez J., Micropor. Mesopor. Mat., 2014, 194, 106—114 |
| [15] | Mukhtar N. Z. F., Borhan M. Z., Rusop M., Abdullah S., 2nd International Conference on Sustainable Materials, 2013, 795, 711—715 |
| [16] | Iida T., Sato M., Numako C., Nakahira A., Kohara S., Okubo T., Wakihara T., J. Mater. Chem. A, 2015, 3(11), 6215—6222 |
| [17] | Kurniawan T., Muraza O., Miyake K., Hakeem A. S., Hirota Y., Al-Amer A. M., Nishiyama N., Ind. Eng. Chem. Res., 2017, 56(15), 4258—4266 |
| [18] | Chen Y., Zhang Y., Zhang C., Jiang J., Gu X., J. CO2 Util., 2017, 18, 30—40 |
| [19] | Chlubná P., Roth W. J., Zukal A., Kubu M., Pavlatová J., Catal. Today, 2012, 179(1), 35—42 |
| [20] | Roth W. J., Nachtigall P., Morris R. E., Wheatley P. S., Seymour V. R., Ashbrook S. E., Chlubna P., Grajciar L., Polozij M., Zukal A., Shvets O., Cejka J., Nat. Chem., 2013, 5(7), 628—633 |
| [21] | Wilson S. T., Lok B. M., Messina C. A., Cannan T. R., Flanigen E. M., J. Am. Chem. Soc., 1982, 104(4), 1146—1147 |
| [22] | Yu J. H., Xu R. R., Chem. Soc. Rev., 2006, 35(7), 593—604 |
| [23] | Thomas J. M., Raja R., Lewis D. W., Angew. Chem. Int. Ed., 2005, 44(40), 6456—6482 |
| [24] | Guo G., Sun Q., Wang N., Bai R., Yu J., Chem. Commun., 2018, 54(30), 3697—3700 |
| [25] | Gianotti E., Manzoli M., Potter M. E., Shetti V. N., Sun D., Paterson J., Mezza T. M., Levy A., Raja R., Chem. Sci., 2014, 5(5), 1810—1819 |
| [26] | Moliner M., Martínez C., Corma A., Chem. Mater., 2013, 26(1), 246—258 |
| [27] | Song X., Li Y., Gan L., Wang Z., Yu J., Xu R., Angew. Chem. Int. Ed., 2009, 48(2), 314—317 |
| [28] | Yu J., Terasaki O., Williams I. D., Quiv S., Xu R., Supramol. Sci., 1998, 5(3), 297—302 |
| [29] | Cirera J., Ruiz E., Alvarez S., Organometallics, 2005, 24(7), 1556—1562 |
| [30] | Zabrodsky H., Peleg S., Avnir D., J. Am. Chem. Soc., 1992, 114(20), 7843—7851 |
| [31] | |
| [32] | Song X., Li J., Guo Y., Pan Q., Gan L., Yu J., Xu R., Inorg. Chem., 2009, 48(1), 198—203 |
| [33] | Schröder K. P., Sauer J., Leslie M., Richard C., Catlow A., Thomas J. M., Chem. Phys. Lett., 1992, 188(3), 320—325 |
| [34] | Gale J. D., Henson N. J., J. Chem. Soc. Faraday Trans., 1994, 90(20), 3175—3179 |
| [35] | Gale J. D., J. Chem. Soc. Faraday Trans., 1997, 93(4), 629—637 |
| [36] | Song Y., Li J., Yu J., Wang K., Xu R., Top. Catal., 2005, 35(1), 3—8 |
| [37] | Bennett J. M., Cohen J. M., Artioli G., Pluth J. J., Smith J. V., Inorg. Chem., 1985, 24(2), 188—193 |
| [38] | Xiao L., Li J., Shen X., Yu J., Pang W., Xu R., Micropor. Mesopor. Mat., 2005, 84(1), 21—26 |
| [39] | Thomas J. M., Raja R., Top. Catal., 2006, 40(1—4), 3—17 |
| [40] | Akolekar D. B., Bhargava S., Bronswijk W. V., Appl. Spectrosc., 1999, 53(8), 931—937 |
| [41] | Meng X., Xiao F.S., Chem. Rev., 2014, 114(2), 1521—1543 |
| [42] | Guo Y., Shao L., Song X., Li J., Micropor. Mesopor. Mat., 2013, 165, 14—19 |
| [43] | Navrotsky A., Petrovic I., Hu Y., Chen C. Y., Davis M. E., Micropors. Mater., 1995, 4(1), 95—98 |
| [44] | Cundy C. S., Cox P. A., Micropor. Mesopor. Mat., 2005, 82(1/2), 1—78 |
| [45] | Fan F., Feng Z., Sun K., Guo M., Guo Q., Song Y., Li W., Li C., Angew. Chem. Int. Ed., 2009, 48(46), 8743—8747 |
| [46] | Li C., Li M.J., J. Mol. Catal.(China), 2003, 17(3), 213—239 |
| (李灿, 李美俊. 分子催化, 2003, 17(3), 213—239) | |
| [47] | Li S. B., Dou Z. Y., He X. Q., Cui L. L., Zhang Y. T., Chinese J. Inorg. Chem., 2013, 34(2), 319—323 |
| (李双宝, 窦志宇, 何兴权, 崔丽莉, 张誉腾. 无机化学学报, 2013, 34(2), 319—323) |
| [1] | ZHU Hongtai,SONG Liyun,HE Hong,YIN Mengqi,CHENG Jie,SUN Yanming,LI Shining,QIU Wenge. Sulfur Tolerance of the CeTiOx Catalysts for Selective Catalytic Reduction of NO with NH3† [J]. Chem. J. Chinese Universities, 2019, 40(2): 350. |
| [2] | SHAO Guoquan,ZHU Hui,MA Wei,YAN Pan,MA Jilong. Synthesis of High-performance AlPO4-14 Zeolite Membranes for Gas Separation † [J]. Chem. J. Chinese Universities, 2019, 40(11): 2265. |
| [3] | GUO Junhui,YAN Wenfu,SHI Wei,XU Ruren. Rapid Synthesis of Aluminophosphate Molecular Sieve AlPO4-5 Under Ambient Pressure and In situ Thermogravimetric-Mass Spectroscopy Investigation of the Crystallization† [J]. Chem. J. Chinese Universities, 2018, 39(5): 841. |
| [4] | CHANG Xiaowen, YAN Wenfu, SHI Wei, XU Ruren. Influence of Environment Change Around N-atom on the Structure-directing Effect of Methylamine in the Synthesis of Open-framework Aluminophosphates† [J]. Chem. J. Chinese Universities, 2018, 39(1): 12. |
| [5] | WANG Aitian, SUN Yangyang, XU Ruren, YAN Wenfu. Synthesis of a New Open-framework Aluminophosphate and the Co-templating Effect in the Crystallization† [J]. Chem. J. Chinese Universities, 2017, 38(5): 701. |
| [6] | TIAN Ye, WANG Shurong, YAN Wenfu, XU Ruren, WANG Yongrui, MU Xuhong. Influence of F- on the Structure-directing Effect of Organic Amines in the Synthesis of Open-framework Aluminophosphates† [J]. Chem. J. Chinese Universities, 2015, 36(3): 428. |
| [7] | WANG Ning, SUN Qiming, YAN Yan, LIU Jiancong, YU Jihong, XU Ruren. Organotemplate-free Synthesis and Proton Conduction Properties of a Layered Aluminophosphate Na4[Al4P4O18]·H2O† [J]. Chem. J. Chinese Universities, 2015, 36(11): 2311. |
| [8] |
ZHU Jingran, LI Ke, YAN Yan, LI Xu, SONG Xiaowei.
Co-templates Synthesis and Characterization of a New Two-dimensional Co-containing Aluminophosphate [C4 |
| [9] | GAO Na, GUO Yuting, LI Jiyang, WANG Jianzhong. Rational Synthesis of Microporous Aluminophosphates Based on the Genetic Programming Method† [J]. Chem. J. Chinese Universities, 2014, 35(10): 2067. |
| [10] | LIU Hao, XU Fen, SUN Li-Xian, CAO Zhong, ZHOU Huai-Ying. Preparation and Hydrogen Generation for Al-LiBH4 Composite Materials [J]. Chem. J. Chinese Universities, 2013, 34(8): 1953. |
| [11] | SHAO Lang, LI Yi, WANG Xiao-Fang, YU Ji-Hong. Synthesis of Divalent-metal-containing Open-framework Aluminophosphates M(Ⅱ)-CJ50 Using Ether Amine as the Organic Template [J]. Chem. J. Chinese Universities, 2013, 34(8): 1806. |
| [12] | LU Hui-Ying, TONG Xiao-Qiang, YAN Yan, YAN Wen-Fu, YU Ji-Hong, XU Ru-Ren. Synthesis, Structure and Phase Transition of a New Microporous Aluminophosphate [C4N2H14]2+[H2Al3P3O14]2- [J]. Chem. J. Chinese Universities, 2013, 34(7): 1571. |
| [13] | LI Shuang-Bao, DOU Zhi-Yu, HE Xing-Quan, CUI Li-Li, ZHANG Yu-Teng. Relationship Between Framework Distortion and Heteroatom-substitution in Aluminophosphate Molecular Sieves [J]. Chem. J. Chinese Universities, 2013, 34(2): 319. |
| [14] | WANG Xiao-Ke, TIAN Mi*. Synthesis of Benzimidazole Derivatives in Water Under Imidazolium Salt Grafted Iodobenzene Diacetate Assisted [J]. Chem. J. Chinese Universities, 2010, 31(2): 296. |
| [15] | ZHANG Cheng-Liang1, LEI Ze1,2, LIU Fu-Chu1, ZHU Zheng-Hui1, JIANG Ming-Zhong1, ZHU Hong-You1,2*. Electrochemical Synthesis of 2-Alkylidene-5-hydroxymethyltetrahedrofunans [J]. Chem. J. Chinese Universities, 2009, 30(3): 513. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||