

Chem. J. Chinese Universities ›› 2019, Vol. 40 ›› Issue (2): 350.doi: 10.7503/cjcu20180241
• Physical Chemistry • Previous Articles Next Articles
ZHU Hongtai2, SONG Liyun1,2, HE Hong1,2,*( ), YIN Mengqi2, CHENG Jie2, SUN Yanming2, LI Shining2, QIU Wenge1,2
), YIN Mengqi2, CHENG Jie2, SUN Yanming2, LI Shining2, QIU Wenge1,2
Received:2018-03-29
Online:2019-02-10
Published:2018-09-10
Contact:
HE Hong
E-mail:hehong@bjut.edu.cn
Supported by:CLC Number:
TrendMD:
ZHU Hongtai,SONG Liyun,HE Hong,YIN Mengqi,CHENG Jie,SUN Yanming,LI Shining,QIU Wenge. Sulfur Tolerance of the CeTiOx Catalysts for Selective Catalytic Reduction of NO with NH3†[J]. Chem. J. Chinese Universities, 2019, 40(2): 350.
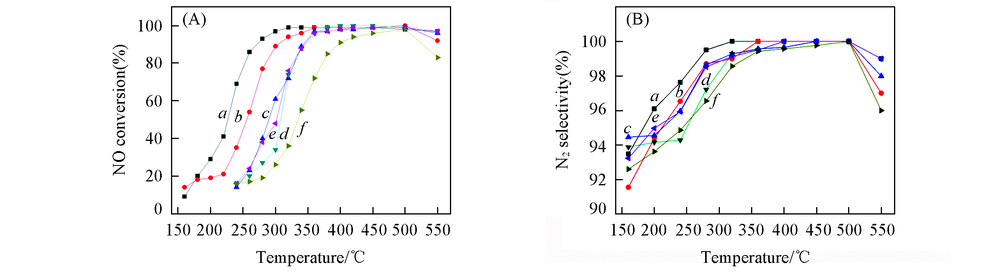
Fig.1 NH3-SCR activity(A) and N2 selectivity(B) over the catalystsReaction conditions: 0.1%NO+0.1%NH3+6%O2+0.0175%SO2+6%H2O, He balance, GHSV: 3×104 h-1. a. CeTiOx-A; b. CeTiOx-B; c. 40CeTiOx-A; d. 40CeTiOx-B; e. 60CeTiOx-A; f. 60CeTiOx-B.
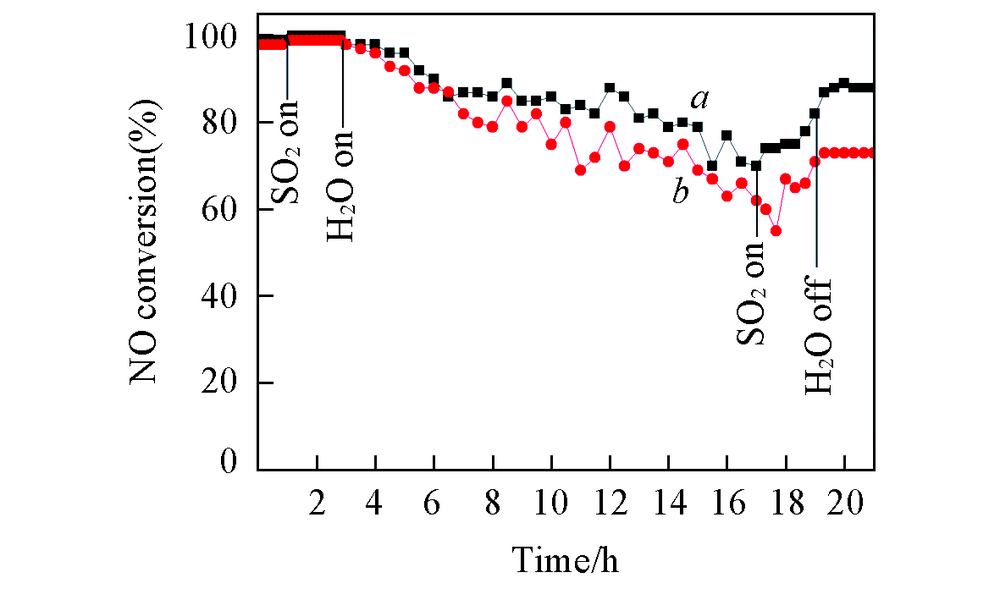
Fig.2 NH3-SCR activity over CeTiOx-A(a) and CeTiOx-B(b) in the presence of SO2/H2O at 300 ℃Reaction condition: 0.1%NO+0.1%NH3+6%O2+0.0175%SO2+6%H2O, N2 balance, GHSV: 3×104 h-1.
| Sample | Component content(mass fraction, %) | BET surface area/(m2·g-1) | Pore volume/(cm3·g-1) | ||
|---|---|---|---|---|---|
| TiO2 | CeO2 | SO3 | |||
| CeTiOx-A | 67.5 | 31.8 | 0.41 | 112 | 0.34 |
| CeTiOx-B | 64.6 | 34.7 | 0.36 | 105 | 0.30 |
| 40CeTiOx-A | 61.5 | 31.8 | 6.32 | 66 | 0.26 |
| 40CeTiOx-B | 61.2 | 33.9 | 4.51 | 52 | 0.19 |
| 60CeTiOx-A | 62.1 | 31.7 | 5.83 | 60 | 0.25 |
| 60CeTiOx-B | 60.4 | 33.2 | 6.06 | 52 | 0.19 |
Table 1 Component content, BET surface area and pore volume of the catalysts
| Sample | Component content(mass fraction, %) | BET surface area/(m2·g-1) | Pore volume/(cm3·g-1) | ||
|---|---|---|---|---|---|
| TiO2 | CeO2 | SO3 | |||
| CeTiOx-A | 67.5 | 31.8 | 0.41 | 112 | 0.34 |
| CeTiOx-B | 64.6 | 34.7 | 0.36 | 105 | 0.30 |
| 40CeTiOx-A | 61.5 | 31.8 | 6.32 | 66 | 0.26 |
| 40CeTiOx-B | 61.2 | 33.9 | 4.51 | 52 | 0.19 |
| 60CeTiOx-A | 62.1 | 31.7 | 5.83 | 60 | 0.25 |
| 60CeTiOx-B | 60.4 | 33.2 | 6.06 | 52 | 0.19 |
| Sample | H2 consumption/(μmol·g-1) | Sample | H2 consumption/(μmol·g-1) | ||||
|---|---|---|---|---|---|---|---|
| Peak 1 | Peak 2 | Total | Peak 1 | Peak 2 | Total | ||
| CeTiOx-A | 36 | - | 36 | 40CeTiOx-B | 270 | 163 | 432 |
| CeTiOx-B | 30 | - | 30 | 60CeTiOx-A | 117 | 69 | 186 |
| 40CeTiOx-A | 106 | 53 | 159 | 60CeTiOx-B | 319 | 187 | 506 |
Table 2 H2 consumption of the catalysts
| Sample | H2 consumption/(μmol·g-1) | Sample | H2 consumption/(μmol·g-1) | ||||
|---|---|---|---|---|---|---|---|
| Peak 1 | Peak 2 | Total | Peak 1 | Peak 2 | Total | ||
| CeTiOx-A | 36 | - | 36 | 40CeTiOx-B | 270 | 163 | 432 |
| CeTiOx-B | 30 | - | 30 | 60CeTiOx-A | 117 | 69 | 186 |
| 40CeTiOx-A | 106 | 53 | 159 | 60CeTiOx-B | 319 | 187 | 506 |
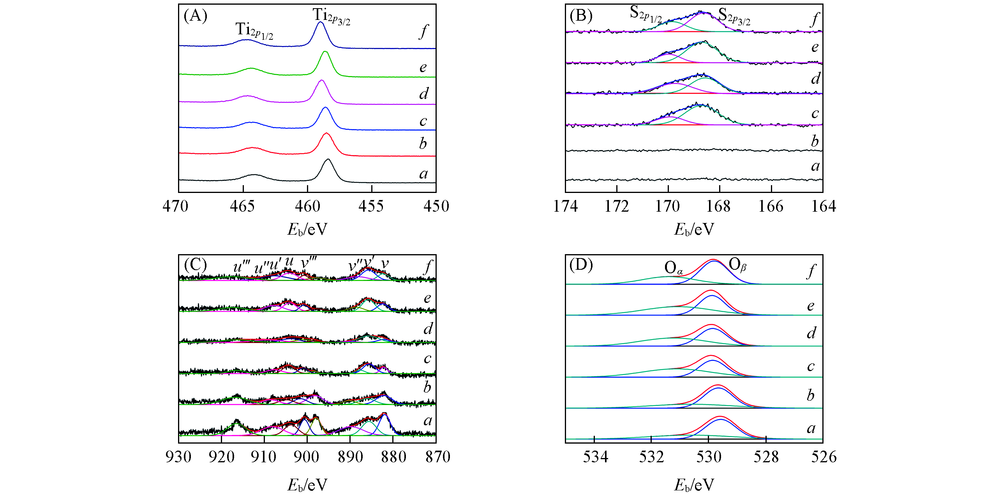
Fig.5 XPS results of Ti2p(A), S2p(B), Ce3d(C) and O1s(D) of the catalystsa. CeTiOx-A; b. CeTiOx-B; c. 40CeTiOx-A; d. 40CeTiOx-B; e. 60CeTiOx-A; f. 60CeTiOx-B.
| Sample | Surface atomic concentration(%) | Surface atomic ratio(%) | ||||
|---|---|---|---|---|---|---|
| Ce | Ti | O | S | Ce3+/(Ce3++Ce4+) | Oα/(Oα+Oβ) | |
| CeTiOx-A | 2.6 | 32.6 | 63.6 | 1.2 | 22.8 | 35.9 |
| CeTiOx-B | 1.6 | 34.2 | 63.2 | 1.0 | 19.8 | 35.1 |
| 40CeTiOx-A | 1.4 | 29.1 | 65.3 | 4.2 | 45.2 | 55.9 |
| 40CeTiOx-B | 1.3 | 31.4 | 64.3 | 3.0 | 42.0 | 52.1 |
| 60CeTiOx-A | 1.4 | 29.9 | 64.9 | 3.9 | 36.1 | 53.9 |
| 60CeTiOx-B | 1.0 | 30.8 | 64.5 | 3.7 | 35.1 | 37.5 |
Table 3 XPS results of the catalysts
| Sample | Surface atomic concentration(%) | Surface atomic ratio(%) | ||||
|---|---|---|---|---|---|---|
| Ce | Ti | O | S | Ce3+/(Ce3++Ce4+) | Oα/(Oα+Oβ) | |
| CeTiOx-A | 2.6 | 32.6 | 63.6 | 1.2 | 22.8 | 35.9 |
| CeTiOx-B | 1.6 | 34.2 | 63.2 | 1.0 | 19.8 | 35.1 |
| 40CeTiOx-A | 1.4 | 29.1 | 65.3 | 4.2 | 45.2 | 55.9 |
| 40CeTiOx-B | 1.3 | 31.4 | 64.3 | 3.0 | 42.0 | 52.1 |
| 60CeTiOx-A | 1.4 | 29.9 | 64.9 | 3.9 | 36.1 | 53.9 |
| 60CeTiOx-B | 1.0 | 30.8 | 64.5 | 3.7 | 35.1 | 37.5 |
| [1] | Dunn J. P., Koppula P. R., Stenger H. G., Wachs I. E., Appl. Catal. B: Environ., 1998, 19, 103-117 |
| [2] | Dunn J. P., Stenger H. G. Jr., Wachs I. E., J. Catal., 1999, 181, 233-243 |
| [3] | Yates M., Martin J. A., Martin-Luengo M. A., Suarez S., Suarez J., Catal. Today,2005, 107/108, 120-125 |
| [4] | Koebel M., Elsener M., Kleemann M., Catal. Today,2000, 59, 335-345 |
| [5] | Qu R. Y., Gao X., Cen K. F., Li J. H., Appl. Catal. B: Environ., 2013, 142/143, 290-297 |
| [6] | Ma Z., Weng D., Wu X. D., Si Z. C., Wang B., Catal. Commun., 2012, 27, 97-100 |
| [7] | Li X. L., Li Y. H., Deng S. S., Rong T. A., Catal. Commun., 2013, 40, 47-50 |
| [8] | Sun X. L., He H., Su Y. C., Yan J. F., Song L. Y., Qiu W. G., Chem. J. Chinese Universities,2017, 38(5), 814-822 |
| (孙向丽, 何洪, 苏垚超, 闫京芳, 宋丽云, 邱文革. 高等学校化学学报, 2017, 38(5), 814-822) | |
| [9] | Gao X., Jiang Y., Fu Y. C., Zhong Y., Luo Z. Y., Cen K. F., Catal. Commun., 2010, 11, 465-469 |
| [10] | Zhang Q. L., Song Z. X., Ning P., Liu X., Li H., Gu J. J., Catal. Commun., 2015, 59, 170-174 |
| [11] | Gao S., Wang P. L., Chen X. B., Wang H. Q., Wu Z. B., Liu Y., Weng X. L., Catal. Commun., 2014, 43, 223-226 |
| [12] | Zhang L., Zou W. X., Ma K. L., Cao Y., Xiong Y., Wu S. G., Tang C. J., Gao F., Dong L., J. Phys. Chem. C., 2015, 119, 1155-1163 |
| [13] | Yan J. F., Qiu W. G., Song L. Y., Chen Y., Su Y. C., Bai G. M., Zhang G. Z., He H., Chem. Commun.,2017, 53, 1321-1324 |
| [14] | Song L. Y., Zhan Z. C., Liu X. J., He H., Qiu W. G., Zi X. H., Chinese J. Catal.,2014, 35(7), 1030-1035 |
| (宋丽云, 展宗城, 刘晓军, 何洪, 邱文革, 訾学红. 催化学报, 2014, 35(7), 1030-1035) | |
| [15] | Chao J. D., He H., Song L. Y., Fang Y. J., Liang Q. M., Zhang G. G., Qiu W. G., Zhang R., Chem. J. Chinese Universities,2015, 36(3), 523-530 |
| (晁晶迪, 何洪, 宋丽云, 房玉娇, 梁全明, 张桂臻, 邱文革, 张然. 高等学校化学学报, 2015, 36(3), 523-530) | |
| [16] | Casanova M., Rocchini E., Trovarelli A., Schermanz K., Begsteiger I., J. Alloys. Compd., 2006, 408/412, 1108-1112 |
| [17] | Guo X. Y., Bartholomew C., Hecker W., Baxter L. L., Appl. Catal. B: Environ., 2009, 92, 30-40 |
| [18] | Sheng Z. Y., Hu Y. F., Xue J. M., Wang X. M., Liao W. P., J. Rare. Earth., 2012, 30, 676-682 |
| [19] | Arakawa K., Matsuda S., Kinoshita H., Appl. Surf. Sci.., 1997, 121(1), 382-386 |
| [20] | Liu F. D., Asakura K., He H., Shan W. P., Shi X. Y., Zhang C. B., Appl. Catal. B: Environ., 2011, 103, 369-377 |
| [21] | Waqif M., Bazin P., Saur O., Lavalley J. C., Blanchard G., Touret O., Appl. Catal. B: Environ., 1997, 11, 193-205 |
| [22] | Yang S. J., Guo Y. F., Chang H. Z., Ma L., Peng Y., Qu Z., Yan N. Q., Wang C. Z., Li J. H., Appl. Catal. B: Environ., 2013, 136/137, 19-28 |
| [23] | Zhang L., Li L. L., Cao Y., Yao X. J., Ge C. Y., Gao F., Deng Y., Tang C. J., Dong L., Appl. Catal. B: Environ., 2015, 165, 589-598 |
| [24] | Chen Y. X., Jiang Y., Li W. Z., Jin R. C., Tang S. Z., Hu W. B., Catal. Today,1999, 50, 39-47 |
| [25] | Wu Z. B., Jin R. B., Wang H. Q., Liu Y., Catal. Commun., 2009, 10, 935-939 |
| [26] | Karami A., Salehi V., J. Catal., 2012, 292, 32-43 |
| [27] | Ferrizz R. M., Gorte R. J., Vohs J. M., Catal. Lett., 2002, 82, 123-129 |
| [28] | Hino M., Kurashige M., Matsuhashi H., Arata K., Thermochim. Acta,2006, 441, 35-41 |
| [29] | Shinde V. M., Madras G., Appl. Catal. B: Environ., 2013, 138/139, 51-61 |
| [30] | Rodriguez J. A., Jirsak T., Freitag A., Hanson J. C., Larese J. Z., Chaturvedi S., Catal. Lett., 1999, 62, 113-119 |
| [31] | Boaro M., Leitenburg C. D., Dolcetti G., Trovarelli A., Graziani M., Top. Catal., 2001, 16/17, 229-306 |
| [32] | Chen L., Li J. H., Ge M. F., Zhu R. H., Catal. Today,2010, 153, 77-83 |
| [33] | Yao X. J., Zhang L., Li L. L., Liu L. C., Cao Y., Dong X., Gao F., Deng Y., Tang C. J., Chen Z., Dong L., Chen Y., Appl. Catal. B: Environ., 2014, 150/151, 315-329 |
| [34] | Zhang R., Zhong Q., Zhao W., Yu L. M., Qu H. X., Appl. Surf. Sci., 2014, 289, 237-244 |
| [35] | Guo R.T., Zhou Y., Pan W. G., Hong J. N., Zhen W. L., Jin Q., Ding C. G., Guo S. Y., J. Ind. Eng. Chem., 2013, 19, 2022-2025 |
| [36] | Xu T. F., Wu X. D., Liu X. S., Cao L., Lin Q. W., Weng D., J. Environ. Sci., 2016, 57, 110-117 |
| [37] | Zhu M. H., Lai J. K., Tumuluri U., Wu Z. L., Wachs I. E., J. Am. Chem. Soc., 2017, 139, 44, 15624-15627 |
| [1] | YU Xia, SONG Chenhai, GUO Xiangke, XUE Nianhua, DING Weiping. Cooperative Catalysis of Adjacent Acid Sites in Zeolite HZSM-5 [J]. Chem. J. Chinese Universities, 2021, 42(1): 239. |
| [2] | ZHANG Ling,DUAN Hongchang,TAN Zhengguo,WU Qinming,MENG Xiangju,XIAO Fengshou. Recent Advances in the Preparation of 8MR Zeolites for the Selective Catalytic Reduction of NOx(NH3-SCR) in Diesel Engines † [J]. Chem. J. Chinese Universities, 2020, 41(1): 19. |
| [3] | LI Chunxiao, LI Jian, LIANG Wenjun, LIANG Quanming. Low Temperature NH3-SCR Activity of Cr Doped V2O5-WO3/TiO2 Catalyst† [J]. Chem. J. Chinese Universities, 2019, 40(7): 1447. |
| [4] | SUN Xiangli, HE Hong, SU Yaochao, YAN Jingfang, SONG Liyun, QIU Wenge. CeO2-TiO2 Mixed Oxides Catalysts for Selective Catalytic Reduction of NOx with NH3: Structure-properties Relationships† [J]. Chem. J. Chinese Universities, 2017, 38(5): 814. |
| [5] | CHAO Jingdi, HE Hong, SONG Liyun, FANG Yujiao, LIANG Quanming, ZHANG Guizhen, QIU Wenge, ZHANG Ran. Promotional Effect of Pr-Doping on the NH3-SCR Activity over the V2O5-MoO3/TiO2 Catalyst† [J]. Chem. J. Chinese Universities, 2015, 36(3): 523. |
| [6] | LIU Jiandong, HUANG Zhanggen, LI Zhe, GUO Qianqian, LI Qiaoyan. Ce Modification on Mn/TiO2/cordierite Monolithic Catalyst for Low-temperature NOx Reduction† [J]. Chem. J. Chinese Universities, 2014, 35(3): 589. |
| [7] | WANG Xue-Yan, HUA Wei-Ming, YUE Ying-Hong, GAO Zi. Porous Phosphate Heterostructure Materials as Green Catalysts in Prins Condensation [J]. Chem. J. Chinese Universities, 2013, 34(8): 1913. |
| [8] | HE Li-Fang, LIU Jian-Dong, HUANG Wei, LI Zhe. Effect of Preparation Methods on Performance of Mn-Ce/ZSM-5 Catalyst for Low-temperature Selective Catalytic Reduction of NO [J]. Chem. J. Chinese Universities, 2012, 33(11): 2532. |
| [9] |
LI Zhe, CHEN Bing, HUANG Wei, XIE Ke-Chang . Selective Catalytic Reduction of NO with Ammonia over Fe Promoted Mo/ZSM-5 Catalysts [J]. Chem. J. Chinese Universities, 2006, 27(10): 1907. |
| [10] |
LIN Qi-Chun, LIN Wei-Ming, HAO Ji-Ming, LI Jun-Hua, FU Li-Xin.
Effect of Support Acidity on Selective Catalytic Reduction of NO by C3H6 over Cu-based Pillared Clay Catalyst [J]. Chem. J. Chinese Universities, 2006, 27(1): 85. |
| [11] | ZHANG Chang-Bin, HE Hong, YU Yun-Bo, ZHANG Run-Duo . Research of Selective Catalystic Reduction of NO with Propene over Catalyst Cu/Al2O3 in Excess Oxygen [J]. Chem. J. Chinese Universities, 2004, 25(1): 136. |
| [12] | LI Jun-Hua, HAO Ji-Ming, FU Li-Xin, ZHU Tian-Le, CHEN Ling-Lin . Selective Catalytic Reduction of Nitrogen Oxide by Propene over Noble CatalystSIn the Pressence of Exess Oxygen [J]. Chem. J. Chinese Universities, 2003, 24(11): 2060. |
| [13] | JIA Ming-Jun, LI Xue-Mei, WANG Gui-Ying, ZHANG Wen-Xiang, WU Tong-Hao. Selective Catalytic Reduction of NO with C3H6 on CuCl/MCM-41 Prepared by Disperse Method [J]. Chem. J. Chinese Universities, 2002, 23(5): 923. |
| [14] | JIA Ming-Jun, ZHANG Wen-Xiang, XIAO Feng-Shou, WU Tong-Hao, SUN Tie . Selective Catalytic Reduction of NO with C3H6 over CuCl/ZSM-5 Zeolites Prepared by Disperse Method [J]. Chem. J. Chinese Universities, 1998, 19(4): 606. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||