

Chem. J. Chinese Universities ›› 2018, Vol. 39 ›› Issue (10): 2178.doi: 10.7503/cjcu20180280
• Analytical Chemistry • Previous Articles Next Articles
MIAO Rui1, WU Dongxue1, WANG Qiuying1, ZHAO Huanxi1, LI Xue1, XIU Yang1,*( ), LIU Shuying1,2,*(
), LIU Shuying1,2,*( )
)
Received:2018-04-11
Online:2018-09-29
Published:2018-09-29
Contact:
XIU Yang,LIU Shuying
E-mail:ys830805@sina.com;syliu@ciac.ac.cn
Supported by:CLC Number:
TrendMD:
MIAO Rui,WU Dongxue,WANG Qiuying,ZHAO Huanxi,LI Xue,XIU Yang,LIU Shuying. Rapid Separation of Ginsenosides Based on Multi-walled Carbon Nanotubes†[J]. Chem. J. Chinese Universities, 2018, 39(10): 2178.
| Ginsenoside | Calibration curve | R2 | Linear range/ (μg·mL-1) | LOD/ (μg·mL-1) | LOQ/ (μg·mL-1) | Repeatability (RSD, %) | Recovery(%)/RSD(n=3, %) | ||
|---|---|---|---|---|---|---|---|---|---|
| 80% | 100% | 120% | |||||||
| Re | y=16733.09x+4922 | 0.995 | 0.5—200.0 | 0.09 | 0.31 | 2.86 | 103.12/1.40 | 98.24/3.65 | 96.38/3.61 |
| Rg1 | y=24013.13x+510.99 | 0.998 | 0.5—200.0 | 0.12 | 0.40 | 3.75 | 99.32/2.55 | 95.43/2.15 | 101.64/1.88 |
| 20(S)-Rf | y=3428.46x+930.66 | 0.998 | 0.5—200.0 | 0.10 | 0.34 | 2.23 | 93.54/2.77 | 108.75/1.23 | 89.45/2.43 |
| Rb1 | y=6777.37x+3176.51 | 0.997 | 0.5—200.0 | 0.14 | 0.45 | 2.61 | 108.71/4.27 | 95.01/2.53 | 92.17/3.40 |
| Rb2 | y=13982.11x+2949.74 | 0.994 | 0.5—200.0 | 0.13 | 0.44 | 1.08 | 101.55/2.43 | 98.34/3.54 | 95.35/1.88 |
| Rc | y=12188.45x-4503.28 | 0.997 | 0.5—200.0 | 0.14 | 0.45 | 3.47 | 89.16/3.58 | 93.15/3.16 | 103.34/4.88 |
| Rd | y=23267.82x-945.67 | 0.997 | 0.5—200.0 | 0.14 | 0.47 | 3.89 | 95.37/2.19 | 92.76/3.63 | 93.32/2.38 |
| Ro | y=15753.62x+983.03 | 0.994 | 0.5—200.0 | 0.10 | 0.35 | 3.15 | 106.24/2.75 | 88.67/5.34 | 97.52/4.36 |
Table 1 Validation of the developed HPLC-QqQ/MS method used for the quantification of the 8 ginsenosides
| Ginsenoside | Calibration curve | R2 | Linear range/ (μg·mL-1) | LOD/ (μg·mL-1) | LOQ/ (μg·mL-1) | Repeatability (RSD, %) | Recovery(%)/RSD(n=3, %) | ||
|---|---|---|---|---|---|---|---|---|---|
| 80% | 100% | 120% | |||||||
| Re | y=16733.09x+4922 | 0.995 | 0.5—200.0 | 0.09 | 0.31 | 2.86 | 103.12/1.40 | 98.24/3.65 | 96.38/3.61 |
| Rg1 | y=24013.13x+510.99 | 0.998 | 0.5—200.0 | 0.12 | 0.40 | 3.75 | 99.32/2.55 | 95.43/2.15 | 101.64/1.88 |
| 20(S)-Rf | y=3428.46x+930.66 | 0.998 | 0.5—200.0 | 0.10 | 0.34 | 2.23 | 93.54/2.77 | 108.75/1.23 | 89.45/2.43 |
| Rb1 | y=6777.37x+3176.51 | 0.997 | 0.5—200.0 | 0.14 | 0.45 | 2.61 | 108.71/4.27 | 95.01/2.53 | 92.17/3.40 |
| Rb2 | y=13982.11x+2949.74 | 0.994 | 0.5—200.0 | 0.13 | 0.44 | 1.08 | 101.55/2.43 | 98.34/3.54 | 95.35/1.88 |
| Rc | y=12188.45x-4503.28 | 0.997 | 0.5—200.0 | 0.14 | 0.45 | 3.47 | 89.16/3.58 | 93.15/3.16 | 103.34/4.88 |
| Rd | y=23267.82x-945.67 | 0.997 | 0.5—200.0 | 0.14 | 0.47 | 3.89 | 95.37/2.19 | 92.76/3.63 | 93.32/2.38 |
| Ro | y=15753.62x+983.03 | 0.994 | 0.5—200.0 | 0.10 | 0.35 | 3.15 | 106.24/2.75 | 88.67/5.34 | 97.52/4.36 |
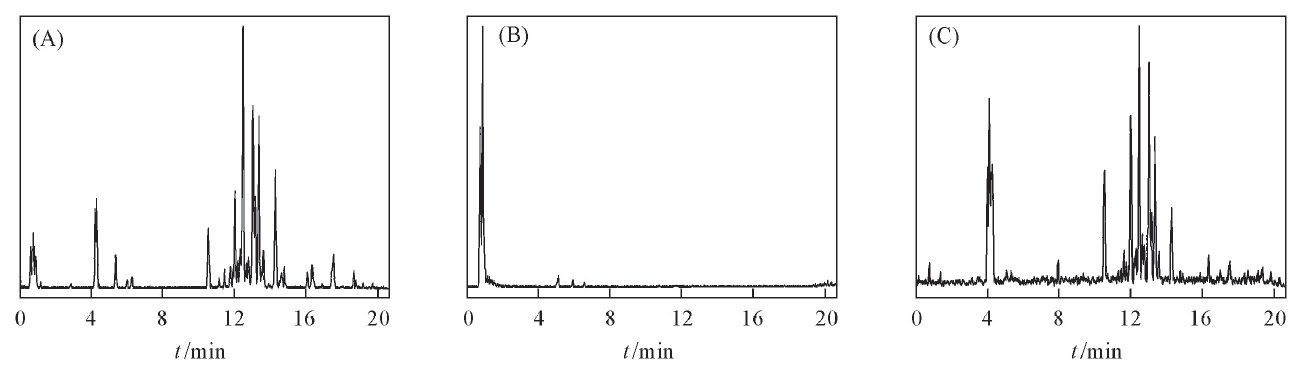
Fig.1 Base peak intensity chromatograms of ginseng extract(A), ginseng extract after being absorbed by MWCNTs(B) and ginsenosides desorbed from MWCNTs(C)
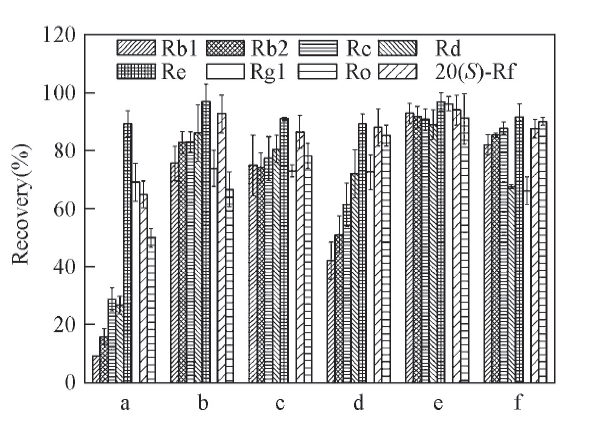
Fig.4 Recovery of the 8 ginsenosides desorbed by different kinds of solventa. 40% Ethanol; b. 70% ethanol; c. acetonitrile;d. acetone; e. n-BuOH; f. 95% ethanol.
| [1] | Baek S.H., Bae O. N., Park J. H., J. Ginseng Res., 2012, 36(2), 119—134 |
| [2] | Corbit M.R., Ferreira J. F. S., Ebbs S. D., Murphy L. L., J. Agric. Food Chem., 2005, 53, 9867—9873 |
| [3] | Wang H.P., Zhang Y. B., Yang X. W., Zhao D. Q., Wang Y. P., J. Ginseng Res., 2016, 40(4), 382—394 |
| [4] | Zhu G.Y., Li Y. W., Hau D. K. P., Jing Z. H., Yu Z. L., Fong W. F., J. Agric. Food Chem., 2011, 59(1), 200—205 |
| [5] | Xu X.F., Cheng X. L. Lin Q. H., Li S. S., Jia Z., Han T., Lin R. C., Wang D., Wei F., Li X. R., J. Ginseng Res., 2016, 40, 344—350 |
| [6] | Liu Z.Q., Chem. Rev., 2012, 112(6), 3329—3355 |
| [7] | Zhao Y., Chen B., Yao S.Z., Sep. Purif. Technol., 2007, 52(3), 533—538 |
| [8] | Kuang P.Q., Wang G., Yuan Q. P., Liang H., Nat. Prod. Res., 2012, 26(3), 286—290 |
| [9] | Lin H.M., Zhang Y. G., Han M., Yang L. M., Ultrason. Sonochem., 2013, 20(2), 680—684 |
| [10] | Kwon J.H., Belanger J. M., Pare J. R., J. Agric. Food Chem., 2003, 51(7), 1807—1810 |
| [11] | Lee J.W., Mo E. J., Choi J. E., Jo Y. H., Jang H., Jeong J. Y., Jin Q., Chung H. N., Hwang B. Y., Lee M. K., J. Ginseng Res., 2016, 40(3), 229—236 |
| [12] | Wong J.W., Zhang K., Tech K., Hayward D. G., Krynitsky A. J., Cassias I., Schenck F. J., Banerjee K., Dasgupta S., Brown D., J. Agric. Food Chem., 2010, 58(10), 5884—5896 |
| [13] | Han Y.H., Ren L. M., Xu K., Yang F., Li Y. F., Cheng T. T., Kang X. M., Xu C. M., Shi Q., J. Chromatogr.A, 2015, 1395, 1—6 |
| [14] | Wepasnick K.A., Smith B. A., Schrote K. E., Wilson H. K., Diegelmann S. R., Fairbrother D. H., Carbon, 2011, 49(1), 24—36 |
| [15] | Lou Z., Chen C., Chen Q., J. Phys. Chem. B, 2005, 109(21), 10557—10560 |
| [16] | Brus L., Nano Lett., 2010, 10(2), 363—365 |
| [17] | Liang X.J., Liu S. J., Wang S., Guo Y., Jiang S. X., J. Chromatogr.A, 2014, 1357, 53—67 |
| [18] | Tasis D., Tagmatarchis N., Bianco A., Prato M., Chem. Rev., 2006, 106, 1105—1136 |
| [19] | Xu J.J., An M., Yang R., Tan Z., Hao J., Cao J., Peng L. Q., Cao W., J. Agric. Food Chem., 2016, 64(12), 2647—2654 |
| [20] | Liu J.L., Zhang C., Wang X. J., Wang T., Li Y., Chem. J. Chinese Universities, 2012, 33(1), 37—43 |
| (刘建林, 张琛, 王夏娇, 王婷, 李鱼. 高等学校化学学报, 2012, 33(1), 37—43) | |
| [21] | Ma J., Jiang L., Jiang L., Wu G., Xia Y., Lu W., Li J., Chen L., J. Chromatogr. A, 2016, 1466, 12—20 |
| [22] | Liu S., Li J.Y., Luo M. B., Hua R., Lin H. L., Ma J. G., Chem. J. Chinese Universities , 2013, 47(1), 7—13 |
| (刘淑娟, 李金英, 罗明标, 花榕, 林海禄, 马建国. 高等学校化学学报, 2013, 47(1), 7—13) | |
| [23] | Wu H., Wang X.C., Liu B., Lu J., Du B. X., Zhang L. X., Ji J. J., Yue Q. Y., Han B. P., J. Chromatogr.A, 2010, 1217, 2911—2917 |
| [24] | Masuko T., Minami A., Iwasaki N., Majima T., Nishimura S., Lee Y.C., Anal. Biochem., 2005, 339(1), 69—72 |
| [25] | Xiu Y., Li X., Sun X., Xiao D., Miao R., Zhao H., Liu S., J. Ginseng Res., 2017, DOI: 10.1016/j.jgr.2017.12.001 |
| [26] | Zhao Y., Chen B., Yao S., Sep. Purif. Technol., 2007, 52(3), 533—538 |
| [1] | LIU Jie, LI Jinsheng, BAI Jingsen, JIN Zhao, GE Junjie, LIU Changpeng, XING Wei. Constructing a Water-blocking Interlayer Containing Sulfonated Carbon Tubes to Reduce Concentration Polarization in Direct Methanol Fuel Cells [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220420. |
| [2] | ZHU Ling,WANG Yuchen,ZHAO Jiangyuan,WEN Mengliang,LI Minggang,HAN Xiulin. Transformation of Ginsenoside Rb3 and C-Mx by Recombinant β-Xylosidase † [J]. Chem. J. Chinese Universities, 2020, 41(5): 1010. |
| [3] | GUAN Fanglan,LI Xin,ZHANG Qun,GONG Yan,LIN Ziyu,CHEN Yao,WANG Lejun. Fabrication and Capacitance Performance of Laser-machined RGO/MWCNT/CF In-plane Flexible Micro-supercapacitor † [J]. Chem. J. Chinese Universities, 2020, 41(2): 300. |
| [4] | WANG Tianqi,YU Qiongwei,FENG Yuqi. Analysis of Imidazole Propionic Acid in Serum of Patients with Type 2 Diabetes Based on NiO@SiO2 Solid-phase Extraction Coupled with Liquid Chromatography-Mass Spectrometry † [J]. Chem. J. Chinese Universities, 2020, 41(2): 262. |
| [5] |
XIAO Yongkun,LIU Chunying,YU Hongshan,YI Tea-Hoo,XU Longquan,SONG Jianguo,IM Wan-Teak,SUN Changkai,JIN Fengxie.
Dynamic Biotransformation of Protopanaxadiol-ginsenosides and Preparation of Minor Ginsenosides C-K or |
| [6] | ZHAO Huanxi,WANG Qiuying,SUN Xiuli,LI Xue,MIAO Rui,WU Dongxue,LIU Shuying,XIU Yang. Discrimination of Ginseng Origins and Identification of Ginsenoside Markers Based on HPLC-MS Combined with Multivariate Statistical Analysis† [J]. Chem. J. Chinese Universities, 2019, 40(2): 246. |
| [7] | SUN Mengmeng,CHANG Chunrui,ZHANG Zhiming,AN Libao. Preparation and Electrical Contact Properties of Palladium-doped Multi-walled Carbon Nanotubes† [J]. Chem. J. Chinese Universities, 2019, 40(1): 11. |
| [8] | ZHAO Lefeng, JIAO Chuanxin, LI Hui, JIAO Lili, MA Yue, WU Wei, LIU Shuying. Chemical Transformation of Protopanaxadiol Type Ginsenoside Rb1, Rb2 and Rc Analyzed by RRLC-Q-TOF-MS† [J]. Chem. J. Chinese Universities, 2018, 39(4): 667. |
| [9] | QIAO Mengdan, LIU Shang, ZHANG Yan, LI Jing, ZHENG Fei, DAI Yulin, YUE Hao. Biotransformation of Ginsenosides in Fermented Ginseng Using UPLC-Q-Orbitrap MS/MS† [J]. Chem. J. Chinese Universities, 2018, 39(2): 219. |
| [10] | LIU Ying, CHEN Yanxin, WU Qian, LI Peng, LI Xuwen, SHI Xiaolei, JIN Yongri. Preparation of 20(S/R)-Ginsenoside Rg3 and Their Effects on Regulation of Th1/Th2 Imbalance† [J]. Chem. J. Chinese Universities, 2018, 39(11): 2419. |
| [11] | LIU Xinru, LIU Chunying, XU Longquan, SONG Jianguo, YU Hongshan. Construction of Ginsenoside-β-glucosidase Gene Vector and Biotransformation in Pichia Pastoris† [J]. Chem. J. Chinese Universities, 2018, 39(11): 2451. |
| [12] | LI Ruigang,ZHU Na,ZHAO Huanxi,WANG Nan,SUN Hongmei,YUE Hao,LI Jing. Effects of Ginseng Polysaccharides on the Metabolism of Ginsenoside Re in vivo and Transformation of Ginsenoside Re in vitro† [J]. Chem. J. Chinese Universities, 2018, 39(10): 2192. |
| [13] | LI Nan, ZHAO Huanxi, LI Jing, WANG Nan, YU Bohao, YUE Hao, YU Shanshan. Transformation of Total Notoginsenosides by Recombinant Endocellulase Fpendo5A† [J]. Chem. J. Chinese Universities, 2017, 38(12): 2185. |
| [14] | XU Kaige, ZHANG Di, LEI Jie, PENG Yage, PENG Juan, JIN Xiaoyong. Au Nanowires-MWCNTs Modified Electrode for Catalyzing the Oxidization of Glucose† [J]. Chem. J. Chinese Universities, 2017, 38(10): 1864. |
| [15] | LI Xue, ZHAO Huanxi, MIAO Rui, LI Wenying, XIU Yang, LIU Shuying. Structure and Pathway Research on Chemical Transformation of Ginsenoside Rb1 via HPLC-HRMS/MSn/QqQ Technique† [J]. Chem. J. Chinese Universities, 2017, 38(10): 1730. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||