

Chem. J. Chinese Universities ›› 2016, Vol. 37 ›› Issue (12): 2191.doi: 10.7503/cjcu20160487
• Physical Chemistry • Previous Articles Next Articles
WANG Zhicheng1, XI Hailing1,2,*( ), KONG Lingce1, ZHAO Sanping2, ZUO Yanjun1
), KONG Lingce1, ZHAO Sanping2, ZUO Yanjun1
Received:2016-07-08
Online:2016-12-10
Published:2016-11-18
Contact:
XI Hailing
E-mail:fhxihl@163.com
CLC Number:
TrendMD:
WANG Zhicheng, XI Hailing, KONG Lingce, ZHAO Sanping, ZUO Yanjun. Kinetics and Mechanism of Decontamination Reaction of CEES in H2O2/[CnMIm]HCO3†[J]. Chem. J. Chinese Universities, 2016, 37(12): 2191.
| IL | 1H NMR (600 MHz, CDCl3), δ | 13C NMR (600 MHz, CDCl3), δ | |
|---|---|---|---|
| [EMIm]HCO3 | 1.61(t, 3H), 3.74(s, 3H), 4.43—4.44(m, 2H), 7.73—7.74(d, 2H), 10.13(s, 1H) | 16.18, 37.12, 45.65, 122.61, 124.26, 137.08 | |
| [BMIm]HCO3 | 0.86(t, 3H), 1.38(m, 2H), 1.89—1.91(m, 2H), 3.91(s, 3H), 4.22(t, 2H), 7.59(s, 1H), 7.70(s, 1H), 10.05(s, 1H) | 13.99, 19.95, 32.66, 37.28, 50.30, 122.78, 124.38, 137.48 | |
| [HMIm]HCO3 | 0.87(t, 3H), 1.30—1.31(m, 6H), 1.91(m, 2H), 3.84(s, 3H), 4.32 (t, 2H), 7.54 (s, 1H), 7.69 (s, 1H), 9.88 (s, 1H) | 14.42, 22.84, 26.33, 30.69, 31.55, 37.22, 50.54, 122.69, 124.41, 137.29 | |
| [OMIm]HCO3 | 0.87(t, 3H), 1.25—1.33(m, 8H), 1.90—1.91(m, 2H), 3.85(s, 3H), 4.32(t, 2H), 7.50(s, 1H), 7.67(s, 1H), 10.03(s, 1H) | 14.61, 23.11, 28.79, 29.50, 29.58, 30.85, 32.21, 37.35, 50.67, 122.62, 124.42, 137.58 | |
Table 1 1H NMR and 13C NMR data of compounds [CnMIm]HCO3(n=2, 4, 6, 8)
| IL | 1H NMR (600 MHz, CDCl3), δ | 13C NMR (600 MHz, CDCl3), δ | |
|---|---|---|---|
| [EMIm]HCO3 | 1.61(t, 3H), 3.74(s, 3H), 4.43—4.44(m, 2H), 7.73—7.74(d, 2H), 10.13(s, 1H) | 16.18, 37.12, 45.65, 122.61, 124.26, 137.08 | |
| [BMIm]HCO3 | 0.86(t, 3H), 1.38(m, 2H), 1.89—1.91(m, 2H), 3.91(s, 3H), 4.22(t, 2H), 7.59(s, 1H), 7.70(s, 1H), 10.05(s, 1H) | 13.99, 19.95, 32.66, 37.28, 50.30, 122.78, 124.38, 137.48 | |
| [HMIm]HCO3 | 0.87(t, 3H), 1.30—1.31(m, 6H), 1.91(m, 2H), 3.84(s, 3H), 4.32 (t, 2H), 7.54 (s, 1H), 7.69 (s, 1H), 9.88 (s, 1H) | 14.42, 22.84, 26.33, 30.69, 31.55, 37.22, 50.54, 122.69, 124.41, 137.29 | |
| [OMIm]HCO3 | 0.87(t, 3H), 1.25—1.33(m, 8H), 1.90—1.91(m, 2H), 3.85(s, 3H), 4.32(t, 2H), 7.50(s, 1H), 7.67(s, 1H), 10.03(s, 1H) | 14.61, 23.11, 28.79, 29.50, 29.58, 30.85, 32.21, 37.35, 50.67, 122.62, 124.42, 137.58 | |
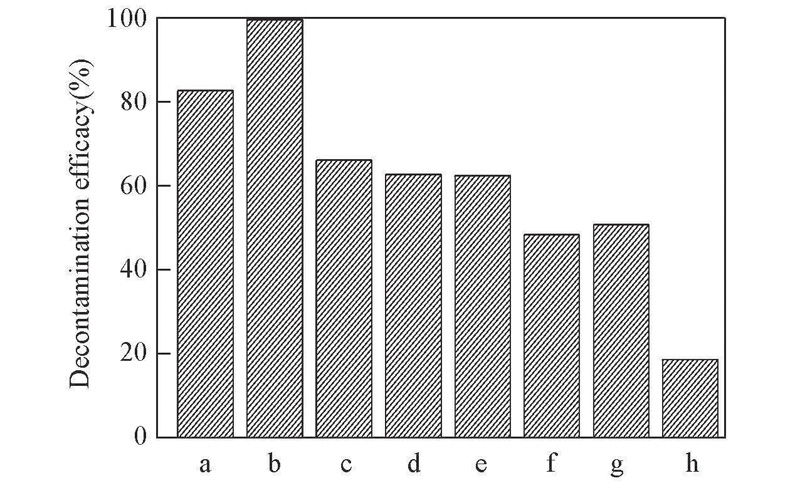
Fig.1 Decontamination efficacy of CEES by H2O2 in different solventsa. [EMIm]HCO3; b. [BMIm]HCO3; c. [HMIm]HCO3; d. [OMIm]HCO3; e. [BMIm]DCA; f. [BMIm]NTf2; g. C2H5OH; h. H2O. Experimental conditions: m(CEES)=10 mg, solvents 420 μL, 30% H2O2 aqueous solution 80 μL, n(oxidant)∶n(CEES)=10, 30 min.
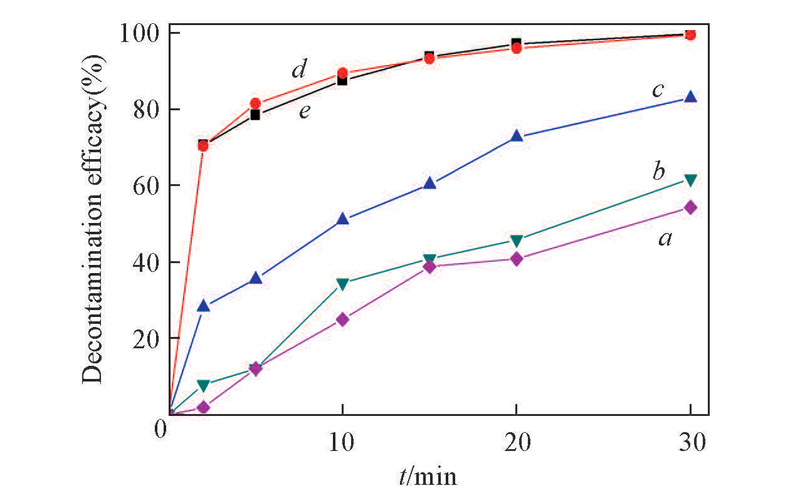
Fig.2 Decontamination efficacy of CEES using H2O2/[BMIm]HCO3 at different temperaturesExperimental conditions: c(CEES)=20 mg/mL,n(oxidant)∶n(CEES)=10, 30 min. Temperature/K:a. 243; b. 253; c. 273; d. 298; e. 313.
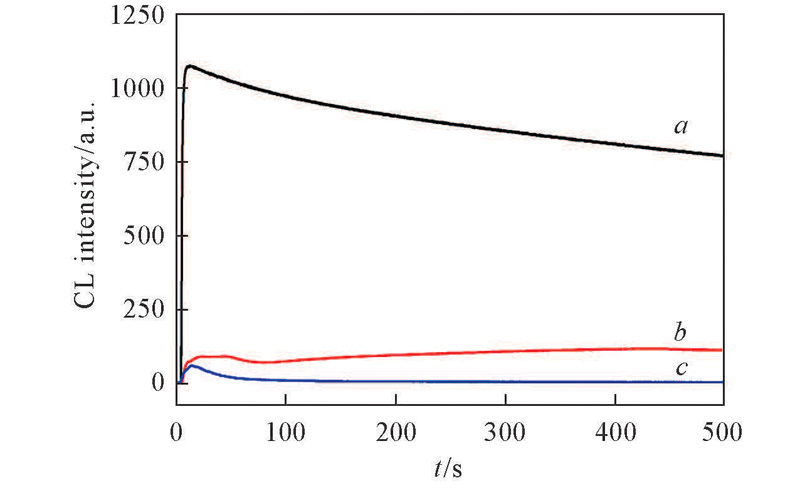
Fig.6 CL kinetics of H2O2 in lucigenin solution which containing basic ILsa. [BMIm]HCO3; b. [BMIm]DCA; c. [BMIm]NTf2.Experimental conditions: 2 mL lucigenin, 100 μL H2O2, 50 μL ILs.
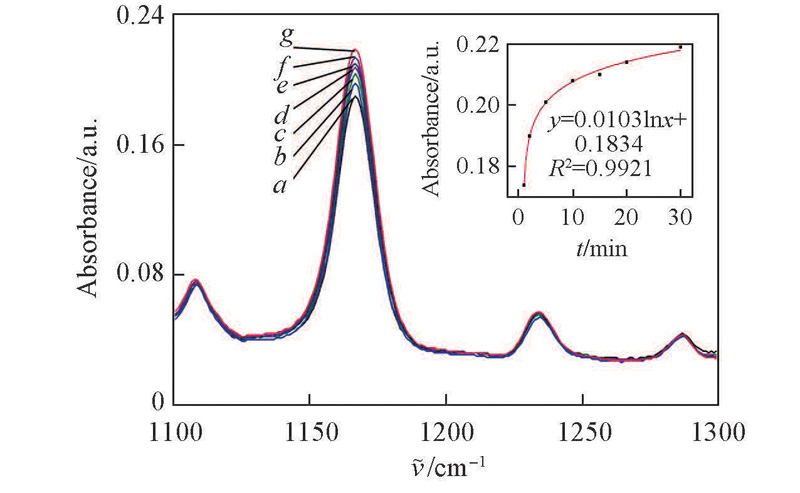
Fig.7 Formation dynamics of ·O2- in H2O2/[BMIm]HCO3Curves a—g were the FTIR spectrum of H2O2/[BMIm]HCO3 mixture at 1, 2, 5, 10, 15, 20, 30 min, respectively.Inset: the growth trend of ·O2- was fitted bo a logarithmic function.
| [1] | Talmage S. S., Watson A. P., Hauschild V., Munro N. B., King J., Curr. Org. Chem., 2007, 79(23), 285—298 |
| [2] | Wagner G. W., Yang Y., Ind. Eng. Chem. Res., 2002, 41(8), 1925—1928 |
| [3] | Fakhraian H., Valizadeh F., J. Mol. Catal. A: Chem., 2010, 333, 69—72 |
| [4] | Karunakaran C., Kamalam R., J. Chem. Soc., 2002, 2, 2011—2018 |
| [5] | Wagner G. W., Procell L. R., Yang Y., Bunton C. A., Langmuir, 2001, 17(16), 4809—4811 |
| [6] | Yan S., Zhang S. J., Zhao Y. X., Li X. M., Zhang Y. M., Zhang H., Wang J., Fu J. Q., Chem. J. Chinese Universities, 2016, 37(5), 946—955 |
| (严山, 张胜建, 赵迎宪, 李显明, 张永明, 张洪, 王健, 符建琼. 高等学校化学学报,2016, 37(5), 946—955) | |
| [7] | Wagner G. W., Sorrick D. C., Procell L. R., Brickhouse M. D., Mcvey I. F., Schwartz L. I., Langmuir, 2007, 23(3), 1178—1186 |
| [8] | Richardson D. E., Yao H., Frank K. M., Bennett D. A., J. Am. Chem. Soc., 2000, 122(8), 1729—1739 |
| [9] | Bokare A. D., Choi W., J. Hazard. Mater., 2016, 304, 313—319 |
| [10] | Jawad A., Chen Z., Yin G., Chinese J. Catal., 2016, 37(6), 810—825 |
| [11] | Peng J., Shi H., Li J., Wang L., Wang Z., Gao S., Chem. Eng. J., 2016, 306, 484—491 |
| [12] | Maurya M. R., Kumar N., J. Mol. Catal. A: Chem., 2015, 406, 204—212 |
| [13] | Zhang L. N., Cai K., Zhang F., Yue Q. F., Chem. Res. Chinese Universities, 2015, 31(1), 130—137 |
| [14] | Zhao Y. M., Cui H. M., Zheng C. Z., Chen X. G., Li C. Y., Chem. Res. Chinese Universities, 2016, 32(1), 112—117 |
| [15] | Wilkes J. S., Castle P. J., Levisky J. A., Hermosillo A., Cote P. J., Corley C. A., Montgomery E. A., Bird D. M., Hutchinson R. R., Ditson M. F., Ind. Eng. Chem. Res., 2009, 48(13), 6203—6211 |
| [16] | Voss B. A., Noble R. D., Gin D. L., Chem. Mater., 2012, 24(6), 1174—1180 |
| [17] | Gao D. L., Yu X. P., Guo Y. F., Wang S. Q., Liu M. M., Deng T. L., Chen Y. W., Belzile N., Chem. Res. Chinese Universities, 2015, 31(4), 621—626 |
| [18] | Li L. J., Li X. W., Ding J., Liu Y., Wu Q., Wang X. Z., Li M., Jin Y. R., Chem. J. Chinese Universities, 2016, 37(3), 454—459 |
| (李兰杰, 李绪文, 丁健, 刘迎, 吴谦, 王晓中, 李敏, 金永日. 高等学校化学学报,2016, 37(3), 454—459) | |
| [19] | Ma Y. Q., Wang R., Chem. J. Chinese Universities, 2014, 35(7), 1515—1522 |
| (马云倩, 王睿. 高等学校化学学报,2014, 35(7), 1515—1522) | |
| [20] | Wang X. D., Wu W. Y., Tu G. F., Jiang K. X., Chinese Science Bulletin, 2009, 54(1), 21—26 |
| (王晓丹, 吴文远, 涂赣峰, 蒋开喜. 科学通报,2009, 54(1), 21—26) | |
| [21] | Zhou T., Chen L., Ye Y., Chen L., Qi Z., Freund H., Sundmacher K., Ind. Eng. Chem. Res., 2012, 51(17), 6256—6264 |
| [22] | Freire M. G., Neves C. M. S. S., Shimizu K., Bernardes C. E. S., Marrucho I. M., Coutinho J. A. P., Lopes J. N. C., Rebelo L. P. N., J. Phys. Chem. B, 2010, 114(48), 15925—15934 |
| [23] | Fallis I. A., Griffiths P. C., Cosgrove T., Dreiss C. A., Govan N., Heenan R. K., Holden I., Jenkins R. L., Mitchell S. J., Notman S., Platts J. A., Riches J., Tatchell T., J. Am. Chem. Soc., 2009, 131(28), 9746—9755 |
| [24] | Liu Q. Z., Xue F., Lei Z. K., Liu C. J., Chem. J. Chinese Universities, 2016, 37(5), 886—891 |
| (刘庆治, 薛飞, 雷振凯, 刘晨江. 高等学校化学学报,2016, 37(5), 886—891) | |
| [25] | Zhang L., Xi H. L., Environmental Chimistry, 2011, 30(10), 1695—1699 |
| (张磊, 习海玲. 环境化学.,2011, 30(10), 1695—1699) | |
| [26] | AlNashef I. M., Hashim M. A., Mjalli F. S., Ali M. Q. A., Hayyan M., Tetrahedron Letters, 2010, 51(15), 1976—1978 |
| [27] | Khajvand T., Alijanpour O., Chaichi M. J., Hashemi M. V., Anal. Bioanal. Chem., 2015, 407, 6127—6136 |
| [28] | Islam M. M., Imase T., Okajima T., Takahashi M., Niikura Y., Kawashima N., Nakamura Y., Ohsaka T., J. Phys. Chem. A, 2009, 113(5), 912—916 |
| [29] | Xu A., Li X., Xiong H., Yin G., Chemosphere, 2011, 82(8), 1190—1195 |
| [30] | Hayyan M., Hashim M. A., AlNashef I. M., Ionics, 2015, 21, 719—728 |
| [31] | Yao H., Richardson D. E., J. Am. Chem. Soc., 2003, 125(20), 6211—6221 |
| [32] | Zhao S.P., Study on the Reactive Oxygen Species in the Activated H2O2 Solution and Their Decontamination Mechanisms on Chemical Warfare Agents(CWAs), Research Institute of Chemical Defence,Beijing, 2015 |
| (赵三平. 典型活化H2O2消毒体系的活性氧及其对化学毒剂的消毒机制研究, 北京: 防化研究院, 2015) |
| [1] | CUI Wei, ZHAO Deyin, BAI Wenxuan, ZHANG Xiaodong, YU Jiang. CO2 Absorption in Composite of Aprotic Solvent and Iron-based Ionic Liquid [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220120. |
| [2] | PENG Kuilin, LI Guilin, JIANG Chongyang, ZENG Shaojuan, ZHANG Xiangping. Research Progress for the Role of Electrolytes in the CO2 Electrochemical Reduction [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220238. |
| [3] | JI Shuangqi, JIN Zhao, GUAN Wenna, PAN Xiangyu, GUAN Tong. Preparation and Chromatographic Performance of Mixed-mode Silica Stationary Phase Modified by Double Cationic Ionic Liquid and Octadecyl Group [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220008. |
| [4] | FENG Li, SHAO Lanxing, LI Sijun, QUAN Wenxuan, ZHUANG Jinliang. Synthesis of Ultrathin Sm-MOF Nanosheets and Their Visible-light Induced Photodegradation of Mustard Simulant [J]. Chem. J. Chinese Universities, 2022, 43(4): 20210867. |
| [5] | CHANG Sihui, CHEN Tao, ZHAO Liming, QIU Yongjun. Thermal Degradation Mechanism of Bio-based Polybutylactam Plasticized by Ionic Liquids [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220353. |
| [6] | WANG Man, WANG Xin, ZHOU Jing, GAO Guohua. Efficient Synthesis of Dimethyl Carbonate via Transesterification of Methanol and Ethylene Carbonate Catalyzed by Poly(ionic liquid)s [J]. Chem. J. Chinese Universities, 2021, 42(12): 3701. |
| [7] | WAN Ren, SONG Fan, PENG Changjun, LIU Honglai. Group Contribution Method for Infinite Dilution Molar Conductivity of Unconventional Ions in Water [J]. Chem. J. Chinese Universities, 2021, 42(12): 3672. |
| [8] | ZHOU Molin, JIANG Xin, YI Ting, YANG Xiangguang, ZHANG Yibo. Improvement of Interface Stability Between Sulfide Solid Electrolyte Li10GeP2S12 and Lithium Metal [J]. Chem. J. Chinese Universities, 2020, 41(8): 1810. |
| [9] | GAO Chong,YU Fengli,XIE Congxia,YU Shitao. Baeyer-Villiger Oxidation of Cyclic Ketones Catalyzed by Amino Alcohol Heteropoly Acid Ionic Liquid † [J]. Chem. J. Chinese Universities, 2020, 41(5): 1101. |
| [10] | GAO Naiwei, MA Qiang, HE Yonglin, WANG Yapei. Green Electronic Devices Based on Ionic Liquids † [J]. Chem. J. Chinese Universities, 2020, 41(5): 901. |
| [11] | CHENG Shifu,HU Hao,CHEN Bihua,WU Haihong,GAO Guohua,HE Mingyuan. Preparation and Electrochemical Performance of Porous Carbons Prepared from Binary Ionic Liquids † [J]. Chem. J. Chinese Universities, 2020, 41(5): 1048. |
| [12] | PIAO Huilan,MA Pinyi,QIN Zucheng,JIANG Yanxiao,SUN Ying,WANG Xinghua,SONG Daqian. Determination of Triazine Herbicides from Fruit Juice Samples Using Effervescence Assisted Microextraction Method Based on Acidic Ionic Liquid Packed Syringe [J]. Chem. J. Chinese Universities, 2020, 41(2): 228. |
| [13] | ZHANG Li,QIAN Mingchao,LIU Xueke,Gao Shuaitao,YU Jiang,XIE Haishen,WANG Hongbin,SUN Fengjiang,SU Xianghong. Dynamic Study of Oxidative Desulfurization by Iron-based Ionic Liquids/NHD † [J]. Chem. J. Chinese Universities, 2020, 41(2): 317. |
| [14] | WANG Nan,YAO Kaisheng,ZHAO Chenchen,LI Tianjin,LU Weiwei. Ionic Liquid-assisted Synthesis of AuPd Nanosponges and Their Catalytic Performance † [J]. Chem. J. Chinese Universities, 2020, 41(1): 62. |
| [15] | LIU Xiaozhou, GUAN Xinyu, FANG Qianrong, JIN Yongri. Three-dimensional Covalent Organic Frameworks Synthesized by Room Temperature Ionic Liquid Method† [J]. Chem. J. Chinese Universities, 2019, 40(7): 1341. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||