

Chem. J. Chinese Universities ›› 2016, Vol. 37 ›› Issue (11): 2085.doi: 10.7503/cjcu20160496
• Polymer Chemistry • Previous Articles Next Articles
LI Weiwei, SHI Yan*( ), YANG Wantai, FU Zhifeng
), YANG Wantai, FU Zhifeng
Received:2016-07-13
Online:2016-11-10
Published:2016-10-14
Contact:
SHI Yan
E-mail:shiyan@mail.buct.edu.cn
Supported by:CLC Number:
TrendMD:
LI Weiwei, SHI Yan, YANG Wantai, FU Zhifeng. Cobalt-mediated Radical Polymerization(CMRP) of Chloroprene by CoⅡ(salen*)†[J]. Chem. J. Chinese Universities, 2016, 37(11): 2085.
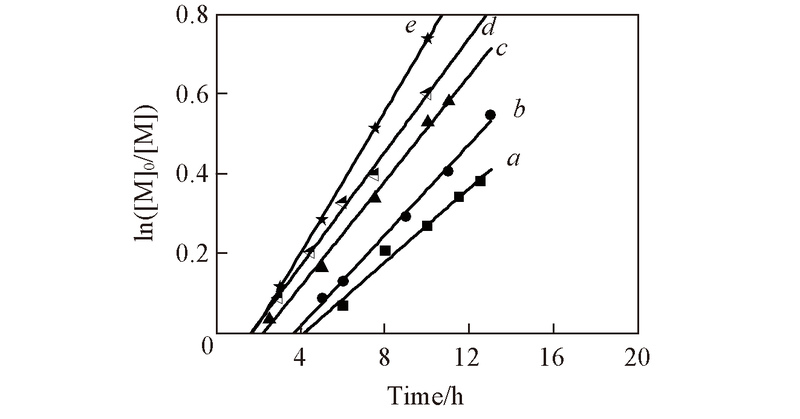
Fig.2 First order kinetic plots for polymerization of CP in benzene[V(CP)/V(benzene)=1/2, 50 ℃] with different ABVN concentrationsConditions: [CoⅡ(salen*)]0=9.0×10-3 mol/L, [CP]0=3.6 mol/L, [ABVN]0/[CoⅡ(salen*)]0: a. 2.0; b. 2.5; c. 3.0; d. 3.5; e. 4.0.
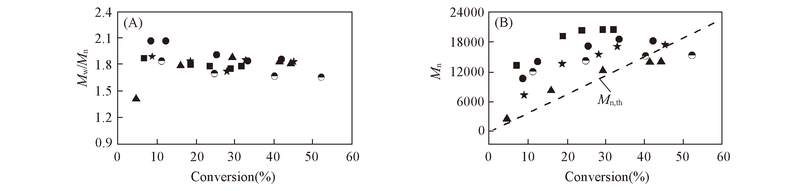
Fig.3 Molecular weight distribution(A) and number-average molecular weight(B) for CoⅡ(salen*) mediated CP polymerization in benzene(CP/benzene=1/2, 50 ℃) with different ABVN concentrationsMn,th=([M]0/[CoⅡ(salen*)]0)×MCP×monomer conversion+MCoⅡ(salen*; [M]0=initial monomer concentration; [CoⅡ(salen*)]0=initial CoⅡ(salen*) concentration; MCP=molecular weight of CP=88.54; MCoⅡ(salen*=604. Conditions are the same as those in Fig.2. [CP]∶[CoⅡ(salen*)]∶[ABVN]: 400∶1∶2; 400∶1∶2.5; 400∶1∶3; 400∶1∶3.5; 400∶1∶4.
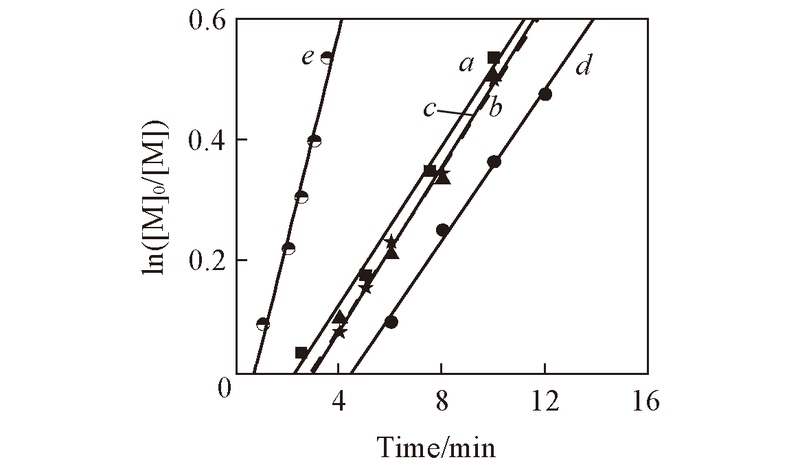
Fig.4 First order kinetic plots for polymerization of CP in different solutions at 50 ℃ Conditions: [CP]0∶[ABVN]0∶[CoⅡ(salen*)]0= 400∶3∶1; a. benzene; b. THF; c. EA; d. toluene; e. bulk.
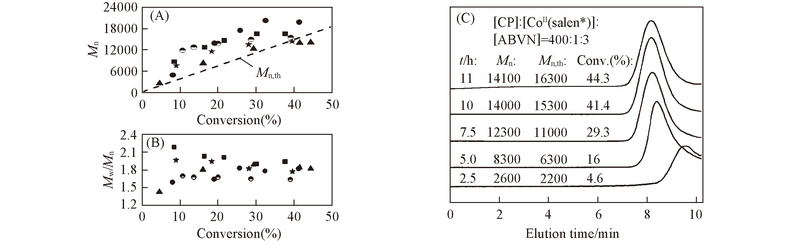
Fig.5 Plots of conversion versus molecular weight(Mn)(A) and PDI(B) for polymerization of CP in different solutions at 50 ℃ and typical GPC traces from the synthesis of PCP in benzene at 50 ℃(C)Conditions are the same as those in Fig.4. Toluene; benzene; bulk; THF; EA.
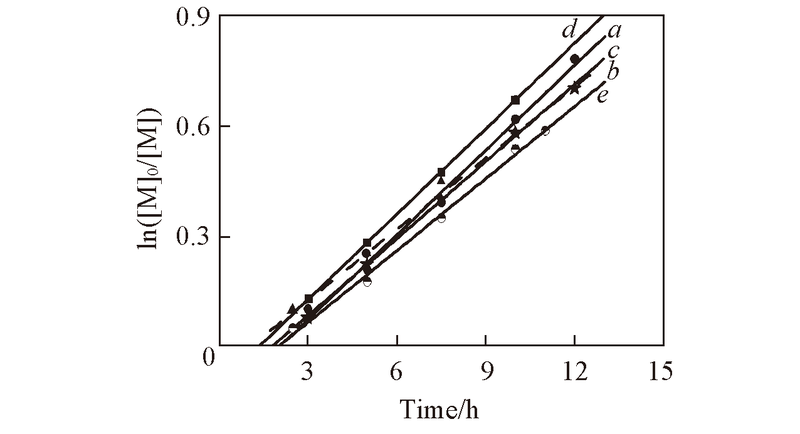
Fig.6 First order kinetic plots for polymerization of CP in benzene at 50 ℃Conditions: [CP]0∶[ABVN]0∶[CoⅡ(salen*)]0=400∶3∶1 and [Co]∶[ED]=1∶25; a. DMSO; b. THF; c. NEt3; d. Py; e. no addition.
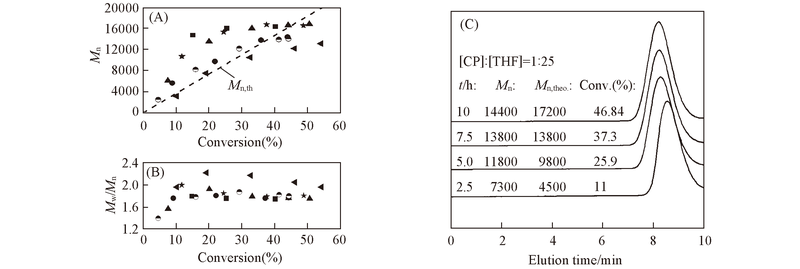
Fig.7 Plots of molecular weight(A) and PDI(B) versus conversion for the addition of electron donors to polymerization of CP in benzene at 50 ℃ and typical GPC traces from the synthesis of PCP with addition of THF(C)Conditions are the same as those in Fig.6. DMSO; THF; water; Py; NEt3; no addition.
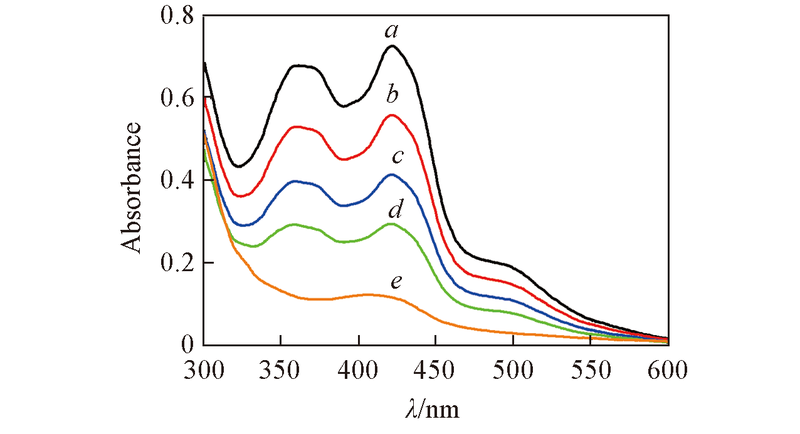
Fig.8 Time dependent UV-Vis spectra illustrating the transformation of CoⅡ(salen*) to CoⅢ(salen*)-R during the induction period of CP polymerizationConditions: [CP]0∶[ABVN]0∶[CoⅡ(salen*)]0=400∶3∶1 in benzene at 50 ℃. Time/min: a. 0; b. 30; c. 60; d. 90; e. 120.
| [1] | Georges M., K. , Veregin R. P., N. , Kazmaier P., M. , Hamer G., K. , Macromolecules, 1993, 26( 11), 2987- 2988 |
| [2] | Matyjaszewski, K. , Tsarevsky N., V. , J. Am. Chem. Soc., 2014, 136( 18), 6513- 6533 |
| [3] | Goto, A. , Sato, K. , Tsujii, Y. , Fukuda, T. , Moad, G. , Rizzardo, E. , Thang S., H. , Macromolecules, 2001, 34( 3), 402- 408 |
| [4] | Lligadas, G. , Ladislaw J., S. , Guliashvili, T. , Percec, V. , Journal of Polymer Science Part A: Polymer Chemistry, 2008, 46( 1), 278- 288 |
| [5] | Iovu M., C. , Matyjaszewski, K. , Macromolecules, 2003, 36( 25), 9346- 9354 |
| [6] | Peng C., H. , Yang T., Y. , Zhao, Y. , Fu X., F. , Org. Biomol. Chem., 2014, 12( 43), 8580- 8587 |
| [7] | Peng C., H. , Li, S. , Wayland B., B. , Journal of the Chinese Chemical Society, 2009, 56( 2), 219- 233 |
| [8] | Peng C. H., Li S., Wayland B. B., ACS Symposium Series, 2009, 1024, 115- 129 |
| [9] | 赵亚光, 禹蒙蒙, 刘禹初, 付雪峰. 中国科学: 化学, 2014, 44( 2), 236- 253 |
| Zhao Y., G. , Yu M., M. , Liu Y., C. , Fu X., F. , Scientia Sinics Chimica, 2014, 44( 2), 236- 253 ( | |
| [10] | Wayland B., B. , Poszmik, G. , Mukerjee S., L. , Journal of the American Chemical Society, 1994, 116( 17), 7943- 7944 |
| [11] | Arvanitopoulos L., D. , Greuel M., P. , Harwood H., J. , Polym. Prepr. Am. Chem. Soc., 1994, 35, 549- 550 |
| [12] | Debuigne, A. , Caille, J. , Jé, rôme R. , Angewandte Chemie International Edition, 2005, 44( 7), 1101- 1104 |
| [13] | Buchmeiser M., R. , Marino M., G. , Macromolecular Materials and Engineering, 2012, 297( 9), 894- 901 |
| [14] | Peng C., H. , Liao, C. , Hsu, C. , Wang, F. , Wayland B., B. , Polymer Chemistry, 2013, 4( 10), 3098- 3104 |
| [15] | Benoit, D. , Harth, E. , Fox, P. , Waymouth R., M. , Hawker C., J. , Macromolecules, 2000, 33( 2), 363- 370 |
| [16] | Georges M., K. , Hamer G., K. , Listigovers N., A. , Macromolecules, 1998, 31( 25), 9087- 9089 |
| [17] | Hua, J. , Li, X. , Li, Y.S. , Xu, L. , Li Y., X. , Journal of Applied Polymer Science, 2007, 104( 6), 3517- 3522 |
| [18] | 杨海强, 华静, 徐玲, 黄宝琛. 合成橡胶与工业, 2006, 29( 3), 228) |
| Yang H., Q. , Hua, J. , Xu, L. , Huang B., C. , China Synthetic Rubber Industry, 2006, 29( 3), 228 ( | |
| [19] | Jitchum, V. , Perrier, S. , Macromolecules, 2007, 40( 5), 1408- 1412 |
| [20] | Germack D., S. , Wooley K., L. , Journal of Polymer Science Part A: Polymer Chemistry, 2007, 45( 17), 4100- 4108 |
| [21] | Ajellal, N. , Thomas C., M. , Carpentier, J. , Polymer, 2008, 49( 20), 4344- 4349 |
| [22] | Hui, J. , Dong Z., J. , Shi, Y. , Fu Z., F. , Yang W., T. , RSC Adv., 2014, 4( 98), 55529- 55538 |
| [23] | Hui, J. , Shi, Y. , Li, T. , Fu Z., F. , Yang W., T. , RSC Adv., 2015, 5( 55), 44326- 44335 |
| [24] | Gu J., M. , Fu Z., F. , Yang W., T. , Shi, Y. , Journal of Applied Polymer Science, 2013, 128( 4), 2291- 2296 |
| [25] | 时永强. 席夫碱铜配合物的合成及催化性能的研究. 杭州: 浙江理工大学, 2013) |
| Shi Y., Q. , Synthesis and Catalytic Activity of Schiff Base Copper Complexes, Zhejiang Sci-Tech University, Hangzhou, 2013 ( | |
| [26] | Kermagoret, A. , Jé, rôme C. , Detrembleur, C. , Debuigne, A. , European Polymer Journal, 2015, 62, 312- 321 |
| [27] | Zhao, Y. , Yu, M. , Fu, X. , Chemical Communications, 2013, 49( 45), 5186- 5188 |
| [28] | Sherwood R., K. , Kent C., L. , Patrick B., O. , McNeil W., S. , Chemical Communications, 2010, 46( 14), 2456- 2458 |
| [29] | Hsu, C. , Yang, T. , Peng, C. , Polym. Chem., 2014, 5( 12), 3867- 3875 |
| [30] | Lin, Y. , Hsieh, Y. , Lin, Y. , Peng, C. , Macromolecules, 2014, 47( 21), 7362- 7369 |
| [31] | 张建国, 文强, 王永军, 张新军. 世界橡胶工业, 2013, 40( 07), 28- 35 |
| Zhang J., G. , Wen, Q. , Wang Y., J. , Zhang X., J. , World Rubber Industry, 2013, 40( 07), 28- 35 ( | |
| [32] | Maria, S. , Kaneyoshi, H. , Matyjaszewski, K. , Poli, R. , Chemistry: A European Journal, 2007, 13( 9), 2480- 2492 |
| [33] | Debuigne, A. , Champouret, Y. , Jé, rôme R. , Poli, R. , Detrembleur, C. , Chemistry: A European Journal, 2008, 14( 13), 4046- 4059 |
| [34] | Ren, W. , Wang, Y. , Zhang, R. , Jiang, J. , Lu, X. , Journal of Organic Chemistry, 2013, 78( 10), 4801- 4810 |
| [1] | ZHOU Chengsi, ZHAO Yuanjin, HAN Meichen, YANG Xia, LIU Chenguang, HE Aihua. Regulation of Silanes as External Electron Donors on Propylene/butene Sequential Polymerization [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220290. |
| [2] | ZHANG Xinping, ZHANG Jianping, CAI Lei, ZONG Xin, HE Aihua. Structure and Properties of Damping Materials with Super Fatigue Resistance Based on Chloroprene Rubber† [J]. Chem. J. Chinese Universities, 2019, 40(7): 1571. |
| [3] | SHAO Xiao-Na, ZHANG Xian-Fu. Self-assembly of Phthalocyanine-carbon Nanotubes Dispersed in Micelle and Light Induced Long-lived Charge Separation State [J]. Chem. J. Chinese Universities, 2012, 33(04): 806. |
| [4] | DENG Rui-Ping1,2, YU Jiang-Bo1, ZHANG Hong-Jie1*, LI Zhe-Feng1,2, ZHOU Liang1,2, PENG Ze-Ping1,2, GUO Zhi-Yong1,2. Investigation of Triboluminescence of Sm(TTA)3phen and the Relationship Between Triboluminescence Phenomena of Rare Earth Complexes and Properties of Ligands [J]. Chem. J. Chinese Universities, 2007, 28(6): 1005. |
| [5] | CAO Yun-Wei, CHAI Siang-Dong, CHEN Si-Guang, JIANG Yue-Shun, LI Tie-Jin. Synthesis and Characterization of Several Electron Donor-Acceptor Amphiphilic Compounds [J]. Chem. J. Chinese Universities, 1995, 16(2): 225. |
| [6] | CAO Xian-Yi, ZHANG Chuan-Bai, WU Guan-Ying . Carbocationic Polymerization of Isobutylene by the Initiator System of Diethyl Aluminium Chloride/Benzyl Chloride/Pyridine [J]. Chem. J. Chinese Universities, 1993, 14(10): 1476. |
| [7] | ZHANG Dong, JIANG Yue-Shun, WANG De-Jun, YANG Sai-Peng, LI Tie-Jin, ZHANG Bao-Wen. Electrochemical Behaviour of Cyanoethenyl Aniline Derivatives [J]. Chem. J. Chinese Universities, 1992, 13(11): 1420. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||