

Chem. J. Chinese Universities ›› 2016, Vol. 37 ›› Issue (5): 886.doi: 10.7503/cjcu20160124
• Organic Chemistry • Previous Articles Next Articles
LIU Zhiqing, XUE Fei, LEI Zhenkai, LIU Chenjiang*( )
)
Received:2016-03-01
Online:2016-05-10
Published:2016-04-20
Contact:
LIU Chenjiang
E-mail:pxylcj@126.com
Supported by:CLC Number:
TrendMD:
LIU Zhiqing, XUE Fei, LEI Zhenkai, LIU Chenjiang. Synthesis of ILs 1-Alkyl-3-carboxymethyl Benzimidazole Double Trifluoromethanesulfonimide and Application in Desulfurization of Fuels†[J]. Chem. J. Chinese Universities, 2016, 37(5): 886.
| ILs | Appearance | m. p. /℃ | ESI-MS, m/z | IR(KBr), νmax/cm-1 |
|---|---|---|---|---|
| 2a | White solid | 66—69 | 205.1[M]+, 279.9[M]- | 3159, 3098, 2999, 2956, 1747, 1575, 1428, 1354, 1193, 1135, 1054, 757, 617, 570 |
| 2b | White solid | 76—79 | 233.1[M]+, 279.8[M]- | 3540, 3150, 3084, 2967, 2880, 1729, 1569, 1463, 1355, 1195, 1135, 1053, 754, 614, 571 |
| 2c | White solid | 67—69 | 247.1[M]+, 279.9[M]- | 3149, 3084, 2963, 2875, 1735, 1570, 1463, 1355, 1196, 1135, 1054, 754, 614, 570 |
| 2d | Red liquid | 289.2[M]+, 279.9[M]- | 3155, 3088, 2933, 2862, 1746, 1572, 1464, 1352, 1195, 1135, 1057, 750, 616, 571 |
Table 1 Appearance, melting points ESI-MS and IR data of ionic liquids 2a—2d
| ILs | Appearance | m. p. /℃ | ESI-MS, m/z | IR(KBr), νmax/cm-1 |
|---|---|---|---|---|
| 2a | White solid | 66—69 | 205.1[M]+, 279.9[M]- | 3159, 3098, 2999, 2956, 1747, 1575, 1428, 1354, 1193, 1135, 1054, 757, 617, 570 |
| 2b | White solid | 76—79 | 233.1[M]+, 279.8[M]- | 3540, 3150, 3084, 2967, 2880, 1729, 1569, 1463, 1355, 1195, 1135, 1053, 754, 614, 571 |
| 2c | White solid | 67—69 | 247.1[M]+, 279.9[M]- | 3149, 3084, 2963, 2875, 1735, 1570, 1463, 1355, 1196, 1135, 1054, 754, 614, 570 |
| 2d | Red liquid | 289.2[M]+, 279.9[M]- | 3155, 3088, 2933, 2862, 1746, 1572, 1464, 1352, 1195, 1135, 1057, 750, 616, 571 |
| ILs | 1H NMR(400 MHz, DMSO), δ | 13C NMR(100 MHz, DMSO), δ |
|---|---|---|
| 2a | 1.55(t, J=7.2 Hz, 3H, CH3), 4.58(q, 2H, CH2), 5.40(s, 2H, CH2), 7.68—8.11(m, 4H, ArH), 9.76(s, 1 H, CH) | 14.0, 42.1, 47.8, 113.4, 113.7, 119.4(q, 1JCF=320 Hz, 2C, CF3SO3), 126.4, 126.6, 130.4, 131.6, 142.7, 167.8 |
| 2b | 0.93(t, J=7.2 Hz, 3H, CH3), 1.29—1.38(m, 2H, CH2), 1.85—1.93(m, 2H, CH2), 4.56(t, J=7.2 Hz, 2H, CH2), 5.35(s, 2H, CH2), 7.67—8.13(m, 4H, ArH), 9.75(s, 1H, CH) | 13.1, 18.9, 30.4, 46.4, 47.9, 113.5, 113.7, 119.4(q, 1JCF=320 Hz, 2C, CF3SO3), 126.4, 126.6, 130.5, 131.5, 143.0, 167.7 |
| 2c | 0.86(t, J=6.4 Hz, 3H, CH3), 1.28—1.35(m, 4H, 2×CH2), 1.90—1.93(m, 2H, CH2), 4.56(t, J=7.2 Hz, 2H, CH2), 5.41(s, 2H, CH2), 7.68—8.12(m, 4H, ArH), 9.77(s, 1H, CH) | 13.5, 21.4, 27.7, 28.1, 46.6, 47.8, 113.5, 113.7, 119.4(q, 1JCF=320 Hz, 2C, CF3SO3), 126.4, 126.6, 130.5, 131.5, 143.0, 167.8 |
| 2d | 0.85(t, J=6.4 Hz, 3H, CH3), 1.18—1.31(m, 10H, 5×CH2), 1.91(t, J=6.8 Hz, 2H, CH2), 4.57(t, J=7.2 Hz, 2H, CH2), 5.45(s, 2H, CH2), 7.69—8.13(m, 4H, ArH), 9.76(s, 1H, CH) | 13.7, 21.9, 25.5, 28.2, 28.4, 31.0, 46.7, 47.6, 113.5, 113.7, 119.4(q, 1JCF=320 Hz, 2C, CF3SO3), 126.4, 126.6, 130.5, 131.1, 131.5, 143.0, 167.9 |
Table 2 1H NMR and 13C NMR data of ionic liquids 2a—2d
| ILs | 1H NMR(400 MHz, DMSO), δ | 13C NMR(100 MHz, DMSO), δ |
|---|---|---|
| 2a | 1.55(t, J=7.2 Hz, 3H, CH3), 4.58(q, 2H, CH2), 5.40(s, 2H, CH2), 7.68—8.11(m, 4H, ArH), 9.76(s, 1 H, CH) | 14.0, 42.1, 47.8, 113.4, 113.7, 119.4(q, 1JCF=320 Hz, 2C, CF3SO3), 126.4, 126.6, 130.4, 131.6, 142.7, 167.8 |
| 2b | 0.93(t, J=7.2 Hz, 3H, CH3), 1.29—1.38(m, 2H, CH2), 1.85—1.93(m, 2H, CH2), 4.56(t, J=7.2 Hz, 2H, CH2), 5.35(s, 2H, CH2), 7.67—8.13(m, 4H, ArH), 9.75(s, 1H, CH) | 13.1, 18.9, 30.4, 46.4, 47.9, 113.5, 113.7, 119.4(q, 1JCF=320 Hz, 2C, CF3SO3), 126.4, 126.6, 130.5, 131.5, 143.0, 167.7 |
| 2c | 0.86(t, J=6.4 Hz, 3H, CH3), 1.28—1.35(m, 4H, 2×CH2), 1.90—1.93(m, 2H, CH2), 4.56(t, J=7.2 Hz, 2H, CH2), 5.41(s, 2H, CH2), 7.68—8.12(m, 4H, ArH), 9.77(s, 1H, CH) | 13.5, 21.4, 27.7, 28.1, 46.6, 47.8, 113.5, 113.7, 119.4(q, 1JCF=320 Hz, 2C, CF3SO3), 126.4, 126.6, 130.5, 131.5, 143.0, 167.8 |
| 2d | 0.85(t, J=6.4 Hz, 3H, CH3), 1.18—1.31(m, 10H, 5×CH2), 1.91(t, J=6.8 Hz, 2H, CH2), 4.57(t, J=7.2 Hz, 2H, CH2), 5.45(s, 2H, CH2), 7.69—8.13(m, 4H, ArH), 9.76(s, 1H, CH) | 13.7, 21.9, 25.5, 28.2, 28.4, 31.0, 46.7, 47.6, 113.5, 113.7, 119.4(q, 1JCF=320 Hz, 2C, CF3SO3), 126.4, 126.6, 130.5, 131.1, 131.5, 143.0, 167.9 |
| ILs System | Sulfur-removal ratio(%) | |
|---|---|---|
| Extraction | Extraction and oxidationb | |
| [C2O2EBIM][Tf2N] | 10.9 | 96.4 |
| [C2O2BBIM][Tf2N] | 13.6 | 96.7 |
| [C2O2PBIM][Tf2N] | 14.8 | 96.8 |
| [C2O2OBIM][Tf2N] | 22.1 | 98.8/82.5c |
Table 3 Effect of different desulfurization systems on DBT removala
| ILs System | Sulfur-removal ratio(%) | |
|---|---|---|
| Extraction | Extraction and oxidationb | |
| [C2O2EBIM][Tf2N] | 10.9 | 96.4 |
| [C2O2BBIM][Tf2N] | 13.6 | 96.7 |
| [C2O2PBIM][Tf2N] | 14.8 | 96.8 |
| [C2O2OBIM][Tf2N] | 22.1 | 98.8/82.5c |
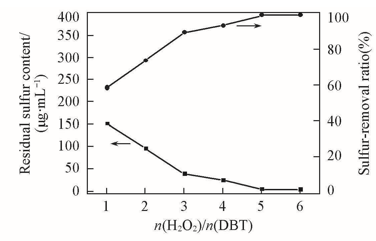
Fig.1 Effect of molar ratios of H2O2/DBT on DBT removalExperimental conditions: temperature=75 ℃, t=1 h, m(Model oil)∶m([C2O2OBIM][Tf2N])=5∶1, DBT(S: 367 mg/L) in n-octane.
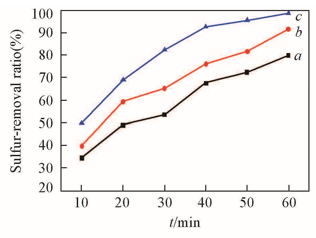
Fig.2 Effects of reaction temperatures and time on DBT removalExperimental conditions: m(Model oil)∶m([C2O2OBIM]·[Tf2N])=5∶1, n(H2O2)∶n(DBT)=5∶1, DBT(S: 367 mg/L) in n-octane. Temperature/℃: a. 55, b. 65, c. 75.
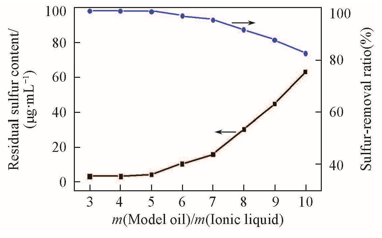
Fig.3 Effect of mass ratio of model oil/ionic liquid on DBT removalExperimental conditions: temperature=75 ℃, t=1 h, n(H2O2)∶n(DBT)=5∶1, DBT(S: 367 mg/L) in n-octane, IL=[C2O2OBIM][Tf2N].
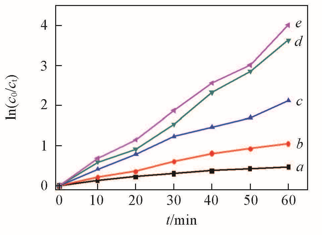
Fig.4 Oxidation of different sulfur-containing compoundsExperimental conditions: temperature=75 ℃, m(Model oil)∶m([C2O2OBIM][Tf2N])=5∶1, n(H2O2)∶n(DBT)=5∶1. a. T; b. 2.5-DMT; c. BT; d. 4,6-DMDBT; e. DBT.
| Substrate | DBT | 4,6-DMDBT | BT | 2,5-DMT | T |
|---|---|---|---|---|---|
| k1/min-1 | 0.065 | 0.060 | 0.034 | 0.019 | 0.009 |
| t1/2/min | 10.66 | 11.55 | 20.39 | 36.48 | 77.02 |
Table 4 First-order desulphurization rate and half-lives of different substrates
| Substrate | DBT | 4,6-DMDBT | BT | 2,5-DMT | T |
|---|---|---|---|---|---|
| k1/min-1 | 0.065 | 0.060 | 0.034 | 0.019 | 0.009 |
| t1/2/min | 10.66 | 11.55 | 20.39 | 36.48 | 77.02 |
| Temperature/℃ | k/min-1 | |
|---|---|---|
| DBT | BT | |
| 55 | 0.025 | 0.011 |
| 65 | 0.037 | 0.019 |
| 75 | 0.065 | 0.034 |
Table 5 First-order rate constant(k) of DBT and BT*
| Temperature/℃ | k/min-1 | |
|---|---|---|
| DBT | BT | |
| 55 | 0.025 | 0.011 |
| 65 | 0.037 | 0.019 |
| 75 | 0.065 | 0.034 |
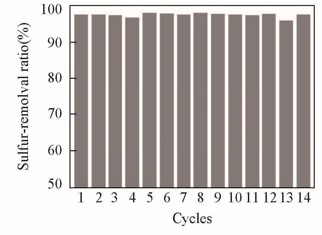
Fig.7 Recycling of IL on removal of DBT in model oilExperimental conditions: temperature=75 ℃, t=1 h,m(Model oil)∶m([C2O2OBIM][Tf2N])=5∶1,n(H2O2)∶n(DBT)=5∶1.
| [1] | Zhang C., Pan X. Y., Wang F., Liu X. Q., Fuel,2012, 102, 580—584 |
| [2] | Zhang H. X., Gao J. J., Meng H., Li C. X., Ind. Eng. Chem.Res., 2012, 51, 6658—6665 |
| [3] | Wan M. W., Yen T. F., Appl. Catal.A-Gen., 2007, 319, 237—245 |
| [4] | Dharaskar S. A., Wasewar K. L., Varma M. N., Shende D. Z., Yoo C. K., Ind. Eng. Chem.Res., 2014, 53, 19845—19854 |
| [5] | Carnaroglio D., Gaudino E. C., Mantegna S., Moreira E. M., Castro A. V., Flores E. M. M., Cravotto G., EnergyFuels, 2014, 28, 1854—1859 |
| [6] | Subhan F., Aslam S., Yan Z. F., Ikram M., Rehman S., Microporous MesoporousMater., 2014, 199, 108—116 |
| [7] | Jiang W., Zhu W. S., Li H. P., Wang X., Yin S., Chang Y. H., Li H. M., Fuel,2015, 140, 590—596 |
| [8] | Wang A. J., Ruan L. F., Teng Y., Li X., Lu M. H., Ren J., Wang Y., Hu Y. K., J. Catal., 2005, 229, 314—321 |
| [9] | Yu F. L., Wang Y. Y., Liu C. Y., Xie C. X., Yu S. T., Chem. Eng.J., 2014, 255, 372—376 |
| [10] | Bhatia S., Sharma D. K., Pet. Sci.Technol., 2006, 24, 1125—1159 |
| [11] | Dinamarca M. A., Rojas A., Baeza P., Espinoza G., Ibacache-Quiroga C., Ojeda J., Fuel,2014, 116, 237—241 |
| [12] | Kulkarni P. S., Afonso C. A. M., GreenChem., 2010, 12, 1139—1149 |
| [13] | Rodríguez-Cabo B., Arce A., Soto A., Fluid PhaseEquilib., 2013, 356, 126—135 |
| [14] | Dharaskar S. A., Wasewar K. L., Varma M. N., Shende D. Z., Yoo C. K., Ind. Eng. Chem.Res., 2014, 53, 19845—19854 |
| [15] | Wang F., Zhang Z. Q., Yang J., Wang L. P., Lin Y., Wei Y., Fuel,2013, 107, 394—399 |
| [16] | Khan N. A., Hasan Z., Jhung S. H., Chem. Eur.J., 2014, 20, 376—380 |
| [17] | Ma C. H., Dai B., Liu P., Zhou N., Shi A. J., Ban L. L., Chen H. W., J. Ind. Eng.Chem., 2014, 20, 2769—2774 |
| [18] | Xiao J., Wu L. M., Wu Y., Liu B., Dai L., Li Z., Xia Q. B., Xi H. X., Appl.Energ., 2014, 113, 78—85 |
| [19] | Xu P. P., Wang C. F., Sun D., Chen Y. J., Zhun K. L., Chem. Res. ChineseUniversities, 2015, 31, 730—735 |
| [20] | Ma Y. Q., Wang R., Chem. J. Chinese Universities,2014, 35(7), 1515—1522 |
| (马云倩, 王睿. 高等学校化学学报, 2014, 35(7), 1515—1522) | |
| [21] | Xue F., Ma R., Sun Y. D., Abdukadera A., Zhang Y. H., Liu C. J., Chem. J. Chinese Universities,2015, 36(7), 1298—1303 |
| (薛飞, 麻荣, 孙亚栋, 阿布力米提·阿布都卡德, 张永红, 刘晨江. 高等学校化学学报, 2015, 36(7), 1298—1303) | |
| [22] | Otsuki S., Nonaka T., Takashima N., Qian W. H., Ishihara A., Imai T., Kabe T., EnergyFuels, 2000, 14, 1232—1239 |
| [1] | CUI Wei, ZHAO Deyin, BAI Wenxuan, ZHANG Xiaodong, YU Jiang. CO2 Absorption in Composite of Aprotic Solvent and Iron-based Ionic Liquid [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220120. |
| [2] | PENG Kuilin, LI Guilin, JIANG Chongyang, ZENG Shaojuan, ZHANG Xiangping. Research Progress for the Role of Electrolytes in the CO2 Electrochemical Reduction [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220238. |
| [3] | JI Shuangqi, JIN Zhao, GUAN Wenna, PAN Xiangyu, GUAN Tong. Preparation and Chromatographic Performance of Mixed-mode Silica Stationary Phase Modified by Double Cationic Ionic Liquid and Octadecyl Group [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220008. |
| [4] | SUN Cuihong, LYU Liqiang, LIU Ying, WANG Yan, YANG Jing, ZHANG Shaowen. Mechanism and Kinetics on the Reaction of Isopropyl Nitrate with Cl, OH and NO3 Radicals [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210591. |
| [5] | CHANG Sihui, CHEN Tao, ZHAO Liming, QIU Yongjun. Thermal Degradation Mechanism of Bio-based Polybutylactam Plasticized by Ionic Liquids [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220353. |
| [6] | CAO Kaiyue, PENG JinWu, LI Hongbin, SHI Chengying, WANG Peng, LIU Baijun. High-temperature Proton Exchange Membranes Based on Cross-linked Polybenzimidazole/hyperbranched-polymer Blends [J]. Chem. J. Chinese Universities, 2021, 42(6): 2049. |
| [7] | MA Zihui, WANG Mengyan, CAO Hongyu, TANG Qian, WANG Lihao, ZHENG Xuefang. Transient Absorption and Decay Kinetic Properties of Photo-excited Metal Coordinated Tetraphenylporphyrin [J]. Chem. J. Chinese Universities, 2021, 42(3): 767. |
| [8] | WANG Man, WANG Xin, ZHOU Jing, GAO Guohua. Efficient Synthesis of Dimethyl Carbonate via Transesterification of Methanol and Ethylene Carbonate Catalyzed by Poly(ionic liquid)s [J]. Chem. J. Chinese Universities, 2021, 42(12): 3701. |
| [9] | SHUAI Die, ZHAO Meijuan, CHEN Bingnian, WANG Li. Inhibitory Effect of Four Kinds of Keegin-type Phosphomolybdate on Tyrosinase and Melanin Formation and Its Antioxidant Activities [J]. Chem. J. Chinese Universities, 2021, 42(12): 3579. |
| [10] | WAN Ren, SONG Fan, PENG Changjun, LIU Honglai. Group Contribution Method for Infinite Dilution Molar Conductivity of Unconventional Ions in Water [J]. Chem. J. Chinese Universities, 2021, 42(12): 3672. |
| [11] | ZHANG Aiqin, WANG Man, SHEN Gangyi, JIN Jun. Interactions Between Polybrominated Diphenyl Ethers and Human Serum Albumin Using SPR and Molecular Docking [J]. Chem. J. Chinese Universities, 2020, 41(9): 2054. |
| [12] | ZHOU Molin, JIANG Xin, YI Ting, YANG Xiangguang, ZHANG Yibo. Improvement of Interface Stability Between Sulfide Solid Electrolyte Li10GeP2S12 and Lithium Metal [J]. Chem. J. Chinese Universities, 2020, 41(8): 1810. |
| [13] | GAO Chong,YU Fengli,XIE Congxia,YU Shitao. Baeyer-Villiger Oxidation of Cyclic Ketones Catalyzed by Amino Alcohol Heteropoly Acid Ionic Liquid † [J]. Chem. J. Chinese Universities, 2020, 41(5): 1101. |
| [14] | GAO Naiwei, MA Qiang, HE Yonglin, WANG Yapei. Green Electronic Devices Based on Ionic Liquids † [J]. Chem. J. Chinese Universities, 2020, 41(5): 901. |
| [15] | CHENG Shifu,HU Hao,CHEN Bihua,WU Haihong,GAO Guohua,HE Mingyuan. Preparation and Electrochemical Performance of Porous Carbons Prepared from Binary Ionic Liquids † [J]. Chem. J. Chinese Universities, 2020, 41(5): 1048. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||