

Chem. J. Chinese Universities ›› 2016, Vol. 37 ›› Issue (5): 920.doi: 10.7503/cjcu20160028
• Physical Chemistry • Previous Articles Next Articles
LI Tan, ZHANG Xiaochao*( ), WANG Kai, LI Rui, FAN Caimei*
), WANG Kai, LI Rui, FAN Caimei*
Received:2016-01-13
Online:2016-05-10
Published:2016-04-12
Contact:
ZHANG Xiaochao,FAN Caimei
E-mail:zhangxiaochao@tyut.edu.cn
Supported by:CLC Number:
TrendMD:
LI Tan, ZHANG Xiaochao, WANG Kai, LI Rui, FAN Caimei. First-principle Calculations on Electronic Structures and Optical Properties of α, β, γ, δ, ε, η-Bi2
| Species | Crystal system | Space group | Unit cell parameter | V0/nm3 |
|---|---|---|---|---|
| α-Bi2O3 | Monoclinic | P21/c | a=0.58496(3) nm, b=0.81648(4) nm, c=0.75101(4) nm, | 0.330235 |
| α=γ=90°, β=112.977(3)° | ||||
| β-Bi2O3 | Tetragonal | P | a=b=0.5738(3) nm, c=0.5731(8) nm, α=β=γ=90° | 0.343153 |
| γ-Bi2O3 | Cubic | I23 | a=b=c=1.025 nm, α=γ=β=90o | 0.343153 |
| δ-Bi2O3 | Cubic | Fm | a=b=c=0.56595(4) nm, α=β=γ=90° | 0.181277 |
| ε-Bi2O3 | Orthorhombic | Pbnb | a=0.49555(1) nm, b=0.55854(2) nm, c=1.27299(3) nm, | 0.352347 |
| α=β=γ=90° | ||||
| η-Bi2O3 | Hexagonal | P | a=b=0.3878(1) nm, c=0.6303(1) nm, α=β=90°, γ=120° | 0.082096 |
Table 1 Crystal lattice parameters of α, β, γ, δ, ε, η-Bi2 O3[5-7,9]
| Species | Crystal system | Space group | Unit cell parameter | V0/nm3 |
|---|---|---|---|---|
| α-Bi2O3 | Monoclinic | P21/c | a=0.58496(3) nm, b=0.81648(4) nm, c=0.75101(4) nm, | 0.330235 |
| α=γ=90°, β=112.977(3)° | ||||
| β-Bi2O3 | Tetragonal | P | a=b=0.5738(3) nm, c=0.5731(8) nm, α=β=γ=90° | 0.343153 |
| γ-Bi2O3 | Cubic | I23 | a=b=c=1.025 nm, α=γ=β=90o | 0.343153 |
| δ-Bi2O3 | Cubic | Fm | a=b=c=0.56595(4) nm, α=β=γ=90° | 0.181277 |
| ε-Bi2O3 | Orthorhombic | Pbnb | a=0.49555(1) nm, b=0.55854(2) nm, c=1.27299(3) nm, | 0.352347 |
| α=β=γ=90° | ||||
| η-Bi2O3 | Hexagonal | P | a=b=0.3878(1) nm, c=0.6303(1) nm, α=β=90°, γ=120° | 0.082096 |
| Species | Function | a/nm | b/nm | c/nm | α/(°) | β/(°) | γ/(°) | ETotal/eV |
|---|---|---|---|---|---|---|---|---|
| α-Bi2O3 | Experimental data | 0.58496 | 0.81648 | 0.75101 | 90 | 112.98 | 90 | |
| GGA(PBE) | 0.58688 | 0.81297 | 0.74127 | 90 | 112.13 | 90 | -6452.1483 | |
| GGA(RPBE) | 0.58964 | 0.82923 | 0.74865 | 90 | 112.35 | 90 | -6457.8904 | |
| GGA(PW91) | 0.58699 | 0.81178 | 0.74147 | 90 | 112.24 | 90 | -6458.9497 | |
| GGA(WC) | 0.58410 | 0.79654 | 0.73246 | 90 | 111.90 | 90 | -6442.5043 | |
| LDA(CA-PZ) | 0.56575 | 0.77129 | 0.71422 | 90 | 112.57 | 90 | -6474.4689 | |
| β-Bi2O3 | Experimental data | 0.7738 | 0.7738 | 0.5731 | 90 | 90 | 90 | |
| GGA(PBE) | 0.79805 | 0.79805 | 0.56081 | 90 | 90 | 90 | -6451.4282 | |
| GGA(RPBE) | 0.81264 | 0.81264 | 0.57354 | 90 | 90 | 90 | -6457.4546 | |
| GGA(PW91) | 0.79418 | 0.79418 | 0.56212 | 90 | 90 | 90 | -6458.2178 | |
| GGA(WC) | 0.76856 | 0.76856 | 0.54938 | 90 | 90 | 90 | -6441.6260 | |
| LDA(CA-PZ) | 0.7471 | 0.7471 | 0.52843 | 90 | 90 | 90 | -6474.0463 | |
| γ-Bi2O3 | Experimental data | 1.025 | 1.025 | 1.025 | 90 | 90 | 90 | |
| GGA(PBE) | 1.02482 | 1.02482 | 1.02482 | 90 | 90 | 90 | -17905.0688 | |
| GGA(RPBE) | 1.04211 | 1.04211 | 1.04211 | 90 | 90 | 90 | -17921.4047 | |
| GGA(PW91) | 1.02284 | 1.02284 | 1.02284 | 90 | 90 | 90 | -17923.5416 | |
| GGA(WC) | 1.00512 | 1.00512 | 1.00512 | 90 | 90 | 90 | -17878.6071 | |
| LDA(CA-PZ) | 0.9768 | 0.9768 | 0.9768 | 90 | 90 | 90 | -17976.4001 | |
| δ-Bi2O3 | Experimental data | 0.56595 | 0.56595 | 0.56595 | 90 | 90 | 90 | |
| GGA(PBE) | 0.61886 | 0.61886 | 0.61886 | 90 | 90 | 90 | -14461.6655 | |
| GGA(RPBE) | 0.61992 | 0.61992 | 0.61992 | 90 | 90 | 90 | -14472.1577 | |
| GGA(PW91) | 0.61873 | 0.61873 | 0.61873 | 90 | 90 | 90 | -14477.5683 | |
| GGA(WC) | 0.61716 | 0.61716 | 0.61716 | 90 | 90 | 90 | -14441.4486 | |
| LDA(CA-PZ) | 0.61703 | 0.61703 | 0.61703 | 90 | 90 | 90 | -14469.0378 | |
| ε-Bi2O3 | Experimental data | 0.49555 | 0.55854 | 1.27299 | 90 | 90 | 90 | |
| GGA(PBE) | 0.52672 | 0.58311 | 1.32275 | 90 | 90 | 90 | -6442.5117 | |
| GGA(RPBE) | 0.52768 | 0.5792 | 1.31738 | 90 | 90 | 90 | -6454.1838 | |
| GGA(PW91) | 0.52626 | 0.59106 | 1.29263 | 90 | 90 | 90 | -6449.5727 | |
| GGA(WC) | 0.50199 | 0.58217 | 1.30291 | 90 | 90 | 90 | -6430.6840 | |
| LDA(CA-PZ) | 0.52219 | 0.5814 | 1.28529 | 90 | 90 | 90 | -6463.4572 | |
| η-Bi2O3 | Experimental data | 0.38781 | 0.38781 | 0.63031 | 90 | 90 | 120 | |
| GGA(PBE) | 0.38434 | 0.38434 | 0.59898 | 90 | 90 | 120 | -1612.6690 | |
| GGA(RPBE) | 0.38559 | 0.38559 | 0.60292 | 90 | 90 | 120 | -1613.9926 | |
| GGA(PW91) | 0.38414 | 0.38414 | 0.5994 | 90 | 90 | 120 | -1614.3683 | |
| GGA(WC) | 0.38261 | 0.38261 | 0.59165 | 90 | 90 | 120 | -1610.3816 | |
| LDA(CA-PZ) | 0.37171 | 0.37171 | 0.56963 | 90 | 90 | 120 | -1618.1124 |
Table 2 Comparison for the lattice parameters of α, β, γ, δ, ε and η-Bi2O3
| Species | Function | a/nm | b/nm | c/nm | α/(°) | β/(°) | γ/(°) | ETotal/eV |
|---|---|---|---|---|---|---|---|---|
| α-Bi2O3 | Experimental data | 0.58496 | 0.81648 | 0.75101 | 90 | 112.98 | 90 | |
| GGA(PBE) | 0.58688 | 0.81297 | 0.74127 | 90 | 112.13 | 90 | -6452.1483 | |
| GGA(RPBE) | 0.58964 | 0.82923 | 0.74865 | 90 | 112.35 | 90 | -6457.8904 | |
| GGA(PW91) | 0.58699 | 0.81178 | 0.74147 | 90 | 112.24 | 90 | -6458.9497 | |
| GGA(WC) | 0.58410 | 0.79654 | 0.73246 | 90 | 111.90 | 90 | -6442.5043 | |
| LDA(CA-PZ) | 0.56575 | 0.77129 | 0.71422 | 90 | 112.57 | 90 | -6474.4689 | |
| β-Bi2O3 | Experimental data | 0.7738 | 0.7738 | 0.5731 | 90 | 90 | 90 | |
| GGA(PBE) | 0.79805 | 0.79805 | 0.56081 | 90 | 90 | 90 | -6451.4282 | |
| GGA(RPBE) | 0.81264 | 0.81264 | 0.57354 | 90 | 90 | 90 | -6457.4546 | |
| GGA(PW91) | 0.79418 | 0.79418 | 0.56212 | 90 | 90 | 90 | -6458.2178 | |
| GGA(WC) | 0.76856 | 0.76856 | 0.54938 | 90 | 90 | 90 | -6441.6260 | |
| LDA(CA-PZ) | 0.7471 | 0.7471 | 0.52843 | 90 | 90 | 90 | -6474.0463 | |
| γ-Bi2O3 | Experimental data | 1.025 | 1.025 | 1.025 | 90 | 90 | 90 | |
| GGA(PBE) | 1.02482 | 1.02482 | 1.02482 | 90 | 90 | 90 | -17905.0688 | |
| GGA(RPBE) | 1.04211 | 1.04211 | 1.04211 | 90 | 90 | 90 | -17921.4047 | |
| GGA(PW91) | 1.02284 | 1.02284 | 1.02284 | 90 | 90 | 90 | -17923.5416 | |
| GGA(WC) | 1.00512 | 1.00512 | 1.00512 | 90 | 90 | 90 | -17878.6071 | |
| LDA(CA-PZ) | 0.9768 | 0.9768 | 0.9768 | 90 | 90 | 90 | -17976.4001 | |
| δ-Bi2O3 | Experimental data | 0.56595 | 0.56595 | 0.56595 | 90 | 90 | 90 | |
| GGA(PBE) | 0.61886 | 0.61886 | 0.61886 | 90 | 90 | 90 | -14461.6655 | |
| GGA(RPBE) | 0.61992 | 0.61992 | 0.61992 | 90 | 90 | 90 | -14472.1577 | |
| GGA(PW91) | 0.61873 | 0.61873 | 0.61873 | 90 | 90 | 90 | -14477.5683 | |
| GGA(WC) | 0.61716 | 0.61716 | 0.61716 | 90 | 90 | 90 | -14441.4486 | |
| LDA(CA-PZ) | 0.61703 | 0.61703 | 0.61703 | 90 | 90 | 90 | -14469.0378 | |
| ε-Bi2O3 | Experimental data | 0.49555 | 0.55854 | 1.27299 | 90 | 90 | 90 | |
| GGA(PBE) | 0.52672 | 0.58311 | 1.32275 | 90 | 90 | 90 | -6442.5117 | |
| GGA(RPBE) | 0.52768 | 0.5792 | 1.31738 | 90 | 90 | 90 | -6454.1838 | |
| GGA(PW91) | 0.52626 | 0.59106 | 1.29263 | 90 | 90 | 90 | -6449.5727 | |
| GGA(WC) | 0.50199 | 0.58217 | 1.30291 | 90 | 90 | 90 | -6430.6840 | |
| LDA(CA-PZ) | 0.52219 | 0.5814 | 1.28529 | 90 | 90 | 90 | -6463.4572 | |
| η-Bi2O3 | Experimental data | 0.38781 | 0.38781 | 0.63031 | 90 | 90 | 120 | |
| GGA(PBE) | 0.38434 | 0.38434 | 0.59898 | 90 | 90 | 120 | -1612.6690 | |
| GGA(RPBE) | 0.38559 | 0.38559 | 0.60292 | 90 | 90 | 120 | -1613.9926 | |
| GGA(PW91) | 0.38414 | 0.38414 | 0.5994 | 90 | 90 | 120 | -1614.3683 | |
| GGA(WC) | 0.38261 | 0.38261 | 0.59165 | 90 | 90 | 120 | -1610.3816 | |
| LDA(CA-PZ) | 0.37171 | 0.37171 | 0.56963 | 90 | 90 | 120 | -1618.1124 |
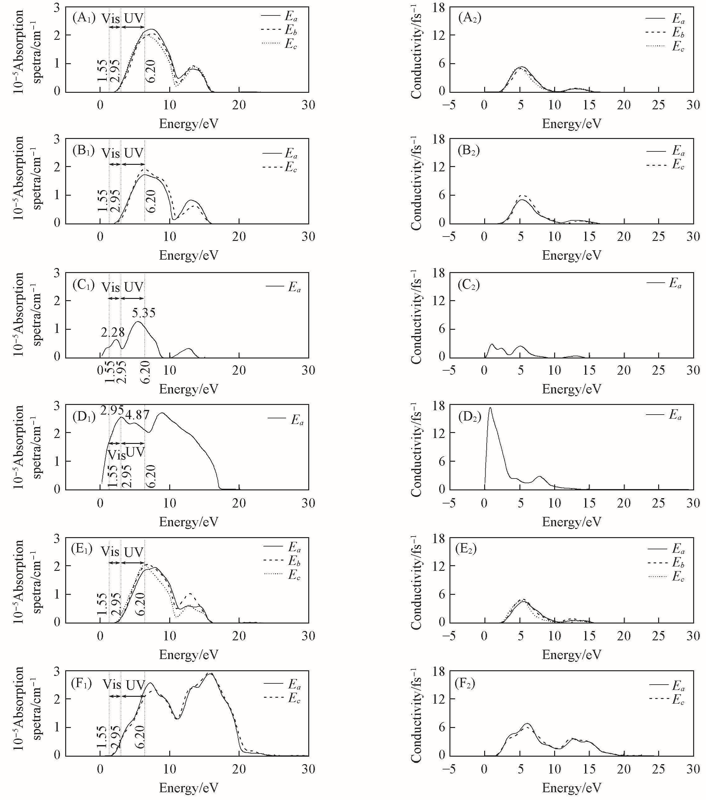
Fig.9 Calculated absorption spectra(A1—F1) and conductivity(A2—F2) of α-Bi2O3(A), β-Bi2O3(B),γ-Bi2O3(C), δ-Bi2O3(D), ε-Bi2O3(E), η-Bi2O3(F) along respective direction of polarization
| [1] | Dumbaugh W. H., Lapp J. C., J. Am. Ceram.Soc., 1992, 75(9), 2315—2326 |
| [2] | Bandoli G., Barreca D., Brescacin E., Rizzi G. A., Tondello E., Chem. Vap.Deposition, 1996, 2(6), 238—242 |
| [3] | Du R. G., Cui X. H., Wang B. H., Ma Y. D., Chem. J. Chinese Universities,1991, 12(3), 394—396 |
| (杜尧国, 崔湘浩, 王宝辉, 马英德. 高等学校化学学报, 1991, 12(3), 394—396) | |
| [4] | Leontie L., Caraman M., Visinoiu A., Rusu G. I., Thin SolidFilms, 2005, 473(2), 230—235 |
| [5] | Harwig H. A., Z. Anorg. Allg.Chem., 1978, 444(1), 151—166 |
| [6] | Sillén L. G., Naturwissenschaften, 1940, 28(13), 206—207 |
| [7] | Cornei N., Tancret N., Abraham F., Mentre O., Inorg.Chem., 2006, 45(13), 4886—4888 |
| [8] | Gualtieria A. F., Immovilli S., Prudenziatia M., PowderDiffr., 1997, 12(2), 90—92 |
| [9] | Atou T., Faqir H., Kikuchi M., Chiba H., Syono Y., Mater. Res.Bull., 1998, 33(2), 289—292 |
| [10] | Klinkova L. A., Nikolaichik V. I., Barkovskii N. V., Fedotov V. K., Russ. J. Inorg.Chem., 2007, 52(12), 1822—1829 |
| [11] | Levin E. M., McDaniel C. L., J. Res. Nat. Bur. Stand. A, 1965, 69A(3), 237—243 |
| [12] | Levin E. M., Roth R. S., J. Res. Nat. Bur. Stand. A, 1964, 68A(2), 197—206 |
| [13] | Harwig H. A., Gerards A. G., Thermochim.Acta, 1979, 28(1), 121—131 |
| [14] | Zou W., Hao W. C., Xin X., Wang T. M., Chinese J. Inorg.Chem., 2009, 25(11), 1971—1976 |
| (邹文, 郝维昌, 信心, 王天民. 无机化学学报, 2009, 25(11), 1971—1976) | |
| [15] | Cheng H., Huang B., Lu J., Wang Z., Xu B., Qin X., Zhang X. Y., Dai Y., PCCP, 2010, 12(47), 15468—15475 |
| [16] | Qiu Y., Liu D., Yang J., Yang S., Adv. Mater., 2006, 18(19), 2604—2608 |
| [17] | Gou X., Li R., Wang G., Chen Z., Wexler D., Nanotechnology, 2009, 20(49), 495501 |
| [18] | George J., Pradeep B., Joseph K. S., Phys. Status. Solidi.C, 1987, 103(2), 607—612 |
| [19] | Schlesinger M., Weber M., Schulze S., Hietschold M., Mehring M., ChemistryOpen, 2013, 2(4), 146—155 |
| [20] | Deng H., Hao W., Xu H., Wang C., J. Phys. Chem.C, 2012, 116(1), 1251—1255 |
| [21] | Music D., Konstantinidis S., Schneider J. M., J. Phys.: Condens.Matter, 2009, 21(17), 175403 |
| [22] | Walsh A., Watson G. W., Payne D. J., Edgell R. G., Guo J., Glans P. A., Learmonth T., Smith K. E., Phys. Rev.B, 2006, 73(23), 235104 |
| [23] | Milman V., Winkler B., White J. A., Pickard C. J., Payne M. C., Akhmatskaya E. V., Nobes R. H., Int. J. QuantumChem., 2000, 77(5), 895—910 |
| [24] | Perdew J. P., Burke K., Ernzerhof M., Phys. Rev.Lett., 1996, 77(18), 3865—3868 |
| [25] | Hammer B., Hansen L. B., Norskov J. K., Phys. Rev.B, 1999, 59(11), 7413—7421 |
| [26] | Perdew J. P., Chevary J. A., Vosko S. H., Jackson K. A., Pederson M. R., Singh D. J., Fiolhais C., Phys. Rev.B, 1992, 46(11), 6671—6687 |
| [27] | Wu Z., Cohen R. E., Phys. Rev.B, 2006, 73(23), 235116 |
| [28] | Ceperley D. M., Alder B. J., Phys. Rev.Lett., 1980, 45(7), 566—569 |
| [29] | Perdew J. P., Zunger A., Phys. Rev.B, 1981, 23(10), 5048—5079 |
| [30] | Monkhorst H. J., Pack J. D., Phys. Rev.B, 1976, 13(12), 5188—5192 |
| [31] | Wang Y., Zhang X. C., Zhao L. J., Zhao X. X., Shi B. P., Fan C. M., Chem. J. ChineseUniversites, 2014, 35(12), 2624—2631 |
| (王艳, 张小超, 赵丽君, 赵晓霞, 史宝萍, 樊彩梅. 高等学校化学学报, 2014, 35(12), 2624—2631) | |
| [32] | Pack J. D., Monkhorst H. J., Phys. Rev.B, 1977, 16(4), 1748—1749 |
| [33] | Shanno D. F., Phua K. H., Math.Program., 1978, 14(1), 149—160 |
| [34] | Kroeze J. E., Savenije T. J., Vermeulen M. J., Warman J. M., J. Phys. Chem.B, 2003, 107(31), 7696—7705 |
| [35] | Xu Y., Schoonen M. A. A., Am.Mineral., 2000, 85(4), 543—556 |
| [1] | XIA Tian, WAN Jiawei, YU Ranbo. Progress of the Structure-property Correlation of Heteroatomic Coordination Structured Carbon-based Single-atom Electrocatalysts [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220162. |
| [2] | ZHENG Xuelian, YANG Cuicui, TIAN Weiquan. The Second Order Nonlinear Optical Properties of Azulene-defect Graphene Nanosheets with Full Armchair Edge [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210806. |
| [3] | WANG Zumin, MENG Cheng, YU Ranbo. Doping Regulation in Transition Metal Phosphides for Hydrogen Evolution Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220544. |
| [4] | ZHUO Zengqing, PAN Feng. Progress of Key Electronic States in Lithium Ion Battery Materials Probed Through Soft X-ray Spectroscopy [J]. Chem. J. Chinese Universities, 2021, 42(8): 2332. |
| [5] | CHEN Mingsu, ZHANG Huiru, ZHANG Qi, LIU Jiaqin, WU Yucheng. First-principles Study on the Catalytic Effect of Co,P co-Doped MoS2 in Lithium-sulfur Batteries [J]. Chem. J. Chinese Universities, 2021, 42(8): 2540. |
| [6] | SHI Haihan,WU Xiangping,PENG Xinzhe,YU Guojing,DONG Chaoyang,JI Yaoyao,YANG Siwen,CHEN Junlin,WANG Jin,RAN Xueqin,YANG Lei,XIE Linghai,HUANG Wei. An Effective Method of Reducing the Internal Reorganization Energy Based on Windmill-like Grid Composed of Four Carbazoles† [J]. Chem. J. Chinese Universities, 2020, 41(7): 1670. |
| [7] | KANG Huimin,WANG Hongqiang,WANG Huiying,WU Lixin,QIU Yongqing. Theoretical Study on the Second-order Nonlinear Optical Properties of a Porphyrin-o-Carborane-Boron-dipyrromethene Triad Compound† [J]. Chem. J. Chinese Universities, 2019, 40(5): 965. |
| [8] | SUN Guodong,WANG Xue,JIANG Guoliang,XU Zhiyong,LIU Hongmei. Effects of Gas Adsorption on Two Dimensional Metal-hexaiminobenzene Frameworks† [J]. Chem. J. Chinese Universities, 2019, 40(5): 995. |
| [9] | ZHOU Hegen,JIN Hua,GUO Huirui,LIN Jing,ZHANG Yongfan. Electronic Structures and Optical Properties of Cu-based Semiconductors with Chalcopyrite-type Structure† [J]. Chem. J. Chinese Universities, 2019, 40(3): 518. |
| [10] | JI Yuchun,MAO Wenhui,LIAO Hejie,WANG Jilin,LONG Fei,GU Yunle. Boron Nitride Nanotube-nanosheet Hierarchical Structures andIts Optical/adsorption Properties† [J]. Chem. J. Chinese Universities, 2019, 40(2): 216. |
| [11] | ZHANG Zhaoyan,CHEN Hongshan. Theoretical Studies on the Zintl Crystals Assembled by Al6ONa2 Clusters † [J]. Chem. J. Chinese Universities, 2019, 40(11): 2354. |
| [12] | WU Dan, LI Man, ZHONG Yao, AN Kuisheng, CHEN Yanwei. Property of Au Nanoshuttles with Arrow-head Prepared via Wet Chemical Synthetic Method† [J]. Chem. J. Chinese Universities, 2018, 39(8): 1617. |
| [13] | JI Tianhao, TIAN Yanqing. Optical Properties and Applications of Halide-perovskite Nanocrystals† [J]. Chem. J. Chinese Universities, 2018, 39(6): 1113. |
| [14] | LIU Jingwei,YU Guangtao,SHEN Xiaopeng,HUANG Xuri,CHEN Wei. Theoretical Studies on the Structures, Electronic and Magnetic Properties of Fully and Partically Fluorizated Germanene Nanoribbons† [J]. Chem. J. Chinese Universities, 2018, 39(5): 977. |
| [15] | LI Kaifeng,WU Dan,CHEN Yanwei. Effect of Copper Doping on the Growth and Optical Properties of Au Nanorods† [J]. Chem. J. Chinese Universities, 2018, 39(5): 855. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||