

Chem. J. Chinese Universities ›› 2016, Vol. 37 ›› Issue (5): 946.doi: 10.7503/cjcu20150779
• Physical Chemistry • Previous Articles Next Articles
YAN Shan1, ZHANG Shengjian1,*( ), ZHAO Yingxian1, LI Xianming2, ZHANG Yongming2, ZHANG Hong1, WANG Jian2, FU Jianqiong2
), ZHAO Yingxian1, LI Xianming2, ZHANG Yongming2, ZHANG Hong1, WANG Jian2, FU Jianqiong2
Received:2015-10-08
Online:2016-05-10
Published:2016-04-01
Contact:
ZHANG Shengjian
E-mail:zsj@nit.zju.edu.cn
Supported by:CLC Number:
TrendMD:
YAN Shan, ZHANG Shengjian, ZHAO Yingxian, LI Xianming, ZHANG Yongming, ZHANG Hong, WANG Jian, FU Jianqiong. Adsorption Mechanisms of TMPDO and 4-NOH-TMPD in HTS/H2O2 System and Effects on the Stabilization of Ti—OOH†[J]. Chem. J. Chinese Universities, 2016, 37(5): 946.
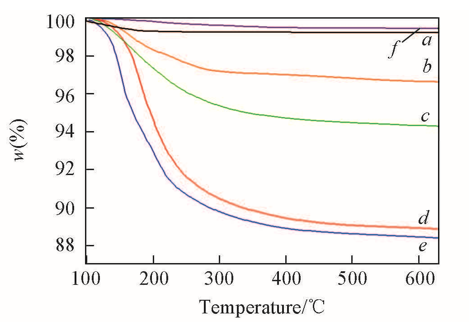
Fig.1 TG curves of different systemsa. HTS/H2O2, 0.1 g HTS and 10 g H2O2; b. HTS/H2O/TMPDO, 0.1 g HTS, 10 g H2O and 0.1 g TMPDO; c. HTS/H2O/4-NOH-TMPD, 0.1 g HTS, 10 g H2O and 0.1 g 4-NOH-TMPD; d. HTS/H2O2/TMPDO, 0.1 g HTS, 10 g H2O2, and 0.1 g TMPDO; e. HTS/H2O2/4-NOH-TMPD, 0.1 g HTS, 10 g H2O2, and 0.1 g 4-NOH-TMPD; f. HTS/H2O, 0.1 g HTS and 10 g H2O.
| System | Mass loss, w(%) | |||
|---|---|---|---|---|
| 100—200 ℃ | 200—400 ℃ | 400—700 ℃ | Total | |
| HTS/H2O | 0.1 | 0.1 | 0 | 0.2 |
| HTS/H2O2 | 0.2 | 0.3 | 0 | 0.5 |
| HTS/H2O/TMPDO | 1.7 | 1.3 | 0.4 | 3.4 |
| HTS /H2O/4-NOH-TMPD | 2.8 | 2.6 | 0.4 | 5.8 |
| HTS/H2O2/TMPDO | 5.4 | 5.1 | 0.8 | 11.3 |
| HTS /H2O2/4-NOH-TMPD | 7.0 | 4.0 | 0.6 | 11.6 |
Table 1 Mass loss of different systems
| System | Mass loss, w(%) | |||
|---|---|---|---|---|
| 100—200 ℃ | 200—400 ℃ | 400—700 ℃ | Total | |
| HTS/H2O | 0.1 | 0.1 | 0 | 0.2 |
| HTS/H2O2 | 0.2 | 0.3 | 0 | 0.5 |
| HTS/H2O/TMPDO | 1.7 | 1.3 | 0.4 | 3.4 |
| HTS /H2O/4-NOH-TMPD | 2.8 | 2.6 | 0.4 | 5.8 |
| HTS/H2O2/TMPDO | 5.4 | 5.1 | 0.8 | 11.3 |
| HTS /H2O2/4-NOH-TMPD | 7.0 | 4.0 | 0.6 | 11.6 |
| System | Adsorbate | Rwl(%) | cad/(μmol·g-1) | Adsorption rate*(%) |
|---|---|---|---|---|
| HTS/H2O | H2O | 0.2 | 111 | 12 |
| HTS/H2O2 | H2O+H2O2 | 0.5 | 147 | 16 |
| HTS/H2O/TMPDO | H2O+TMPDO | 3.4 | 203 | 22 |
| HTS/H2O/4-NOH-TMPD | H2O+4-NOH-TMPD | 5.8 | 328 | 36 |
| HTS/H2O2/TMPDO | H2O+H2O2+TMPDO | 11.3 | 674 | 74 |
| HTS/H2O2/4-NOH-TMPD | H2O+H2O2+4-NOH-TMPD | 11.6 | 643 | 71 |
Table 2 Molar adsorption quantity of vasious sytems
| System | Adsorbate | Rwl(%) | cad/(μmol·g-1) | Adsorption rate*(%) |
|---|---|---|---|---|
| HTS/H2O | H2O | 0.2 | 111 | 12 |
| HTS/H2O2 | H2O+H2O2 | 0.5 | 147 | 16 |
| HTS/H2O/TMPDO | H2O+TMPDO | 3.4 | 203 | 22 |
| HTS/H2O/4-NOH-TMPD | H2O+4-NOH-TMPD | 5.8 | 328 | 36 |
| HTS/H2O2/TMPDO | H2O+H2O2+TMPDO | 11.3 | 674 | 74 |
| HTS/H2O2/4-NOH-TMPD | H2O+H2O2+4-NOH-TMPD | 11.6 | 643 | 71 |
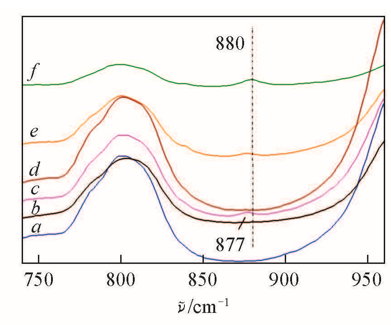
Fig.3 FTIR spectra of HTS(a), HTS/H2O2(b), HTS/H2O2/TMPDO(c) and HTS/H2O2/4-NOH-TMPD(d) with different mass ratios of HTS/4-NOH-TMPD(e, f)Sample compositions:a. 0.1 g HTS; b. 0.1 g HTS, 10 g H2O2; c. 0.1 g HTS, 10 g H2O and 0.1 g TMPDO; d. 0.1 g HTS, 10 g H2O and 0.01 g 4-NOH-TMPD; e. 0.1 g HTS, 10 g H2O2 and 0.05 g TMPDO; f. 0.1 g HTS, 10 g H2O2 and 0.1 g 4-NOH-TMPD.
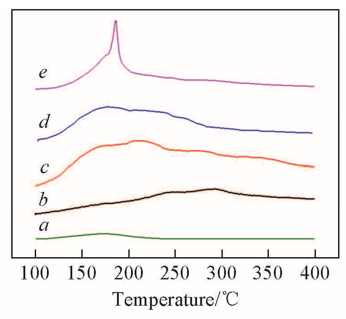
Fig.4 NH3-TPD profiles of HTS(a), HTS/H2O(b), HTS/H2O2(c), HTS/H2O2/TMPDO(d) and HTS/H2O2/4-NOH-TMPD(e)Sample compositions: a. 0.1 g HTS; b. 0.1 g HTS and 10 g H2O; c. 0.1 g HTS and 10 g H2O2; d. 0.1 g HTS, 10 g H2O2 and 0.1 g TMPDO; e. 0.1 g HTS, 10 g H2O2 and 0.1 g 4-NOH-TMPD.
| Temperature/℃ | Acidity/(μmol·g-1) | ||||
|---|---|---|---|---|---|
| HTS | HTS/H2O | HTS/H2O2 | HTS/H2O2/TMPDO | HTS/H2O2/4-NOH-TMPD | |
| 150—200 | 78 | 23 | 157 | 389 | 432 |
| 200—250 | 10 | 63 | 165 | 120 | 64 |
| 250—300 | 0 | 82 | 124 | 47 | 28 |
| 300—350 | 0 | 0 | 75 | 0 | 0 |
| Total | 88 | 168 | 521 | 556 | 524 |
Table 3 Acid strength distribution of HTS in different mediums measured by NH3-TPD
| Temperature/℃ | Acidity/(μmol·g-1) | ||||
|---|---|---|---|---|---|
| HTS | HTS/H2O | HTS/H2O2 | HTS/H2O2/TMPDO | HTS/H2O2/4-NOH-TMPD | |
| 150—200 | 78 | 23 | 157 | 389 | 432 |
| 200—250 | 10 | 63 | 165 | 120 | 64 |
| 250—300 | 0 | 82 | 124 | 47 | 28 |
| 300—350 | 0 | 0 | 75 | 0 | 0 |
| Total | 88 | 168 | 521 | 556 | 524 |
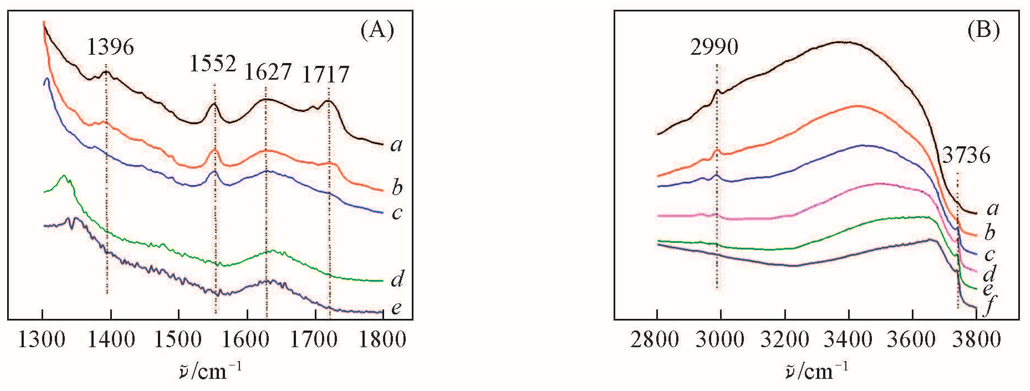
Fig.5 IR spectra of HTS/H2O2 sample after TMPDO adsorption at 70 ℃ followed by desorption in vacuum(<1 Pa) at different temperatures(A) 1300—1800 cm-1. a. 80 ℃; b. 150 ℃; c. 200 ℃; d. 300 ℃; e. 350 ℃.(B) 2800—3800 cm-1. a. 80 ℃; b. 150 ℃; c. 200 ℃; d. 250 ℃; e. 300 ℃; f. 350 ℃.
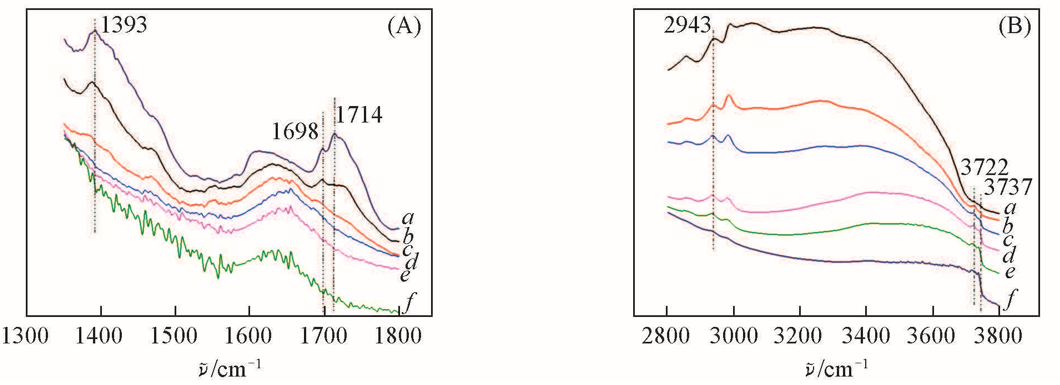
Fig.6 IR spectra of HTS/H2O2 sample after 4-NOH-TMPD adsorption at 70 ℃ followed by desorption in vacuum(<1 Pa) at different temperatures(A) 1300—1800 cm-1; (B) 2800—3800 cm-1. a. 70 ℃; b. 150 ℃; c. 200 ℃; d. 250 ℃; e. 300 ℃; f. 350 ℃.
| [1] | Taramasso M., Perego G., Notari B., Preparation of Porous Crystalline Synthetic Material Comprised of Silicon and Titanium Oxides, US Patent 4410501, 1983-10-18 |
| [2] | Bhaumik A., Mukherjee P., Kumar R., J. Catal., 1998, 178(1), 101—107 |
| [3] | Bengoa J. F., Gallegos N. G., Marchetti S. G., Alvarez A. M., Cagnoli M. V., Yeramian A. A., Microporous MesoporousMater., 1998, 24(4—6), 163—172 |
| [4] | Le Bars J., Dakka J., Sheldon R. A., Appl. Catal. A:Gen., 1996, 136(1), 69—80 |
| [5] | Tatsumi T., Jappar N., J. Catal., 1996, 161(2), 570—576 |
| [6] | Shi C. F., Zhu B., Lin M., Long J., Wang R. W., Catal.Today, 2011, 175(1), 398—403 |
| [7] | Chen R. Z., Bu Z., Li Z. H., Zhong Z. X., Jin W. Q., Xing W. H., Chem. Eng.J., 2010, 156(14), 418—422 |
| [8] | Zheng A. G., Xia C. J., Xiang Y. J., Xin M. D., Zhu B., Lin M., Xu G. T., Shu X. T., Catal.Commun., 2014, 45, 34—38 |
| [9] | Bordiga S., Bonino F., Damin A., Lamberti C., Phys. Chem. Chem.Phys., 2007, 9(35), 4854—4878 |
| [10] | Spanó E., Tabacchi G., Gamba A., Fois E., J. Phys. Chem.B, 2006, 110(43), 21651—21661. |
| [11] | Panyaburapa W., Nanok T., Limtrakul J., J. Phys. Chem.C, 2007, 111(8), 3433—3441 |
| [12] | Wang L. L., Xiong G., Su J., Li P., Guo H. C., J. Phys. Chem.C, 2012, 116(16), 9122—9131 |
| [13] | Yoon C. W., Hirsekorn K. F., Neidig M. L.,Yang X. Z., Tilley T. D., ACSCatal., 2011, 1(12), 1665—1678 |
| [14] | Cordeiro P. J., Tilley T. D., ACSCatal., 2011, 1(5), 455—467 |
| [15] | Wang Y., Zhang S. J., Zhao Y. X., Lin M., J. Mol. Catal. A: Chem., 2014, 385, 1—6 |
| [16] | Tozzola G., Mantegazza M. A., Ranghino G., Petrini G., Bordiga S., Ricchiardi G., Lamberti C., Zulian R., Zecchina A., J. Catal., 1998, 179(1), 64—71 |
| [17] | Zhuang J. Q., Yan Z. M., Liu X. M., Liu X. C., Han X. W., Bao X. H., Mueller U., Catal.Lett., 2002, 83(1/2), 87—91 |
| [18] | Zhu Y. X., Lin W., Tian H. P., Long J., PetrochemicalTechnol., 2006, 35(7), 607—614 |
| (朱玉霞, 林伟, 田辉平, 龙军. 石油化工, 2006, 35(7), 607—614) | |
| [19] | Li H., Lei Q., Zhang X. M., Suo J. S., Chinese J.Catal., 2013, 34(7), 1363—1372 |
| (李颢,雷骞,张小明,索继栓.催化学报, 2013, 34(7), 1363—1372) | |
| [20] | Li H., Lei Q., Zhang X. M., Suo J. S., Microporous MesoporousMater., 2012, 147(1), 110—116 |
| [21] | Katada N., Niwa M., Catal. Surv.Asia, 2004, 8, 161—170 |
| [22] | Prakash A. M., Sung-Suh H. M., Kevan L., J. Phys. Chem.B, 1998, 102(5), 857—864 |
| [23] | Yang G., Zhou L. J., Han X. W., J. Mol. Catal. A: Chem., 2012, 363/364, 371—379 |
| [24] | Liu W. H., Guo P., Su J., Hu J., Wang Y. M., Liu N., Guo H. C., Chinese J.Catal., 2009, 30(6), 482—484 |
| (刘文欢, 郭鹏, 苏际, 胡佳, 王艳梅, 刘娜, 郭洪臣. 催化学报, 2009, 30(6), 482—484) | |
| [25] | Zakrzewski J., Monatsh. Chem. ChemicalMonthly, 1990, 121(10), 803—808 |
| [26] | Bordiga S., Damin A., Bonino F., Zecchina A., Spanò G., Rivetti F., Bolis V., Prestipino C., Lamberti C., J. Phys. Chem.B, 2002, 106(38), 9892—9905 |
| [27] | Rozantsev E. G., Chudinov A. V., Sholle V. D., Bull. Acad. Sci. USSR Div. Chem. Sci., 1980, 29(9), 1510—1513 |
| [28] | Liu H. J., Liu P. L., You K. Y., Luo H. A., Catal.Commun., 2010, 11(10), 887—891 |
| [29] | Krinitskaya L. A., Volodarskii L. B., Bull. Acad. Sci. USSR Div. Chem. Sci., 1983, 32(2), 352—355 |
| [1] | LI Min, ZHAO Chun, FENG Qinzhong, FENG Jian, MENG Xiaojing. Experimental and DFT Studies on the Adsorption of Cd(II) Ions from Aqueous Solutions by Nanofiber Modified Thiourea Groups [J]. Chem. J. Chinese Universities, 2021, 42(12): 3680. |
| [2] | LI Bowen,WANG Ruoheng,LI Li,XIAO Yang. Adsorption of Toluene by Alkali Activated Porous Carbons and Activation/Adsorption Mechanism † [J]. Chem. J. Chinese Universities, 2020, 41(2): 284. |
| [3] | Ying MA,Tian WANG,Heng ZHANG. Molecular Dynamics Simulation of Adsorption of Methylene Blue by Graphene Oxide † [J]. Chem. J. Chinese Universities, 2019, 40(12): 2534. |
| [4] | LUAN Jingde, LIU Yawei, ZHANG Chengyu, KE Xin. Synchronization Capture of Zn2+ and p-Nitrophenyl Phenol on Montmorillonite Composites† [J]. Chem. J. Chinese Universities, 2018, 39(2): 270. |
| [5] | WANG Xiaobing, SHI Xiuyi, ZHOU Xingjun, QIU Xiuzhen, LU Wenguan. Adsorption Behavior of Metal-organic Framework NH2-MIL-53(Al) for Diclofenac Sodium in Aqueous Solution† [J]. Chem. J. Chinese Universities, 2018, 39(2): 206. |
| [6] | LI Ji-Hong1,2, ZHANG Yuan-Wei1, YANG Mei1, ZHANG Jing1, MA Yi1, YUAN Zhi1*. Preparation of Adsorbents for Oligopeptide and Adsorption Mechanism [J]. Chem. J. Chinese Universities, 2008, 29(6): 1159. |
| [7] | LI Ji-Hong1, YU Mei1, WANG Hui-Yan1, YUAN Zhi1*, MA Yi2. Studies on Adsorption Mechanisms of Adsorbents for Endotoxin [J]. Chem. J. Chinese Universities, 2006, 27(6): 1066. |
| [8] | CHEN Jian-Rong, LIN Jian-Jun, ZHONG Yi-Jun, DONG Xun-Jie, SHOU Kai-Sheng. A Study of Adsorption of Indium with N503 Levextrel Resin [J]. Chem. J. Chinese Universities, 1996, 17(8): 1169. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||