

Chem. J. Chinese Universities ›› 2015, Vol. 36 ›› Issue (5): 914.doi: 10.7503/cjcu20141076
• Organic Chemistry • Previous Articles Next Articles
YANG Fengzhi, ZHANG Man, XIE Jin, XIE Dongsheng*( ), FU Lei*(
), FU Lei*( )
)
Received:2014-12-05
Online:2015-05-10
Published:2015-04-15
Contact:
XIE Dongsheng,FU Lei
E-mail:dshxie@sjtu.edu.cn;leifu@sjtu.edu.cn
CLC Number:
TrendMD:
YANG Fengzhi, ZHANG Man, XIE Jin, XIE Dongsheng, FU Lei. Synthetic Method of Caffeic Acid Phenethyl Ester by Heck Reaction†[J]. Chem. J. Chinese Universities, 2015, 36(5): 914.
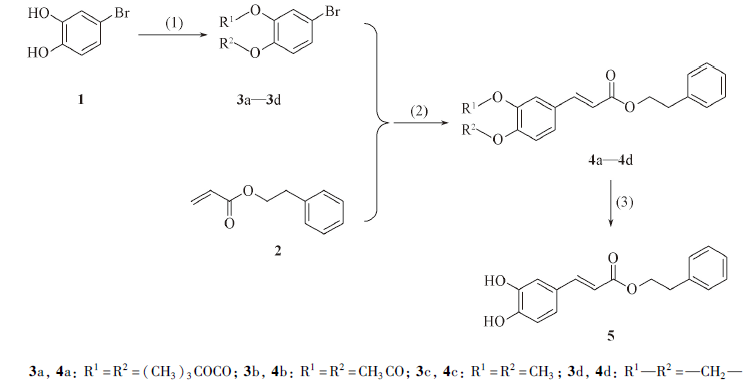
Synthesis of CAPE based on Heck reactionReaction conditions: (1) 3a: [(CH3)3COCO]2O, acetonitrile, pyridine, r. t., 30 min; 3b: (CH3CO)2O, dichloromethane, NEt3, r. t., 10 h; 3c: CH3I, N,N-dimethylformamide, K2CO3, r. t. -40 ℃, 3 h; 3d: CH2Cl2, N,N-dimethylformamide, KF, 110 ℃, 1.5 h; (2) solvent, Pd(OAc)2, Ph3P, base, nitrogen atmosphere, 70 ℃, reflux, 3—24 h; (3) 4a: CF3CO2H, dichloromethane, 0 ℃—r. t., 3 h; 4b: guanidine hydrochloride, NEt3, V(CH2Cl2)∶V(MeOH)=1∶2, r. t., 2 h; 4c: BCl3, dichloromethane, -10 ℃, 1 h; 4d: AlBr3/AlCl3, solvent, 0 ℃, 1 h.
| Protecting group | Protection yield(%) | Heck reaction yield*(%) | De-protection yield(%) | Total yield(%) |
|---|---|---|---|---|
| —COOC(CH3)3 | 84 | 22 | 85 | 16 |
| —COCH3 | 96 | 26 | 94 | 23 |
| —CH3 | 100 | 82 | ≤20 | ≤16 |
| —CH2— | 70 | 79 | ≤20 | ≤11 |
Table 1 Choose appropriate protecting group for o-phenolic hydroxyl*
| Protecting group | Protection yield(%) | Heck reaction yield*(%) | De-protection yield(%) | Total yield(%) |
|---|---|---|---|---|
| —COOC(CH3)3 | 84 | 22 | 85 | 16 |
| —COCH3 | 96 | 26 | 94 | 23 |
| —CH3 | 100 | 82 | ≤20 | ≤16 |
| —CH2— | 70 | 79 | ≤20 | ≤11 |
| Entry | Solvent | Temperature/℃ | Time/h | Conversion(%) | Yield(%) | ||
|---|---|---|---|---|---|---|---|
| CAPE | 4-Br catechol | Others | |||||
| 1 | DMF | 100 | 3 | 100 | 26 | 58 | 16 |
| 2 | 1,4-Dioxane | Reflux | 12 | 94 | 9 | 37 | 48 |
| 3 | ACN | Reflux | 12 | 87 | 12 | 45 | 30 |
| 4 | Toluene | Reflux | 24 | 30 | 18 | Race | 12 |
| 5 | Xylene | Reflux | 24 | 36 | 21 | Race | 15 |
| 6 | V(Toluene)∶V(DMF)=4∶1 | Reflux | 24 | 92 | 39 | 41 | 12 |
| 7 | V(Toluene)∶V(DMF)=4∶1 | 90 | 24 | 81 | 44 | 28 | 9 |
| 8 | V(Toluene)∶V(DMF)=4∶1 | 70 | 24 | 24 | 6 | 14 | 4 |
Table 2 Condition optimization of Heck reaction*
| Entry | Solvent | Temperature/℃ | Time/h | Conversion(%) | Yield(%) | ||
|---|---|---|---|---|---|---|---|
| CAPE | 4-Br catechol | Others | |||||
| 1 | DMF | 100 | 3 | 100 | 26 | 58 | 16 |
| 2 | 1,4-Dioxane | Reflux | 12 | 94 | 9 | 37 | 48 |
| 3 | ACN | Reflux | 12 | 87 | 12 | 45 | 30 |
| 4 | Toluene | Reflux | 24 | 30 | 18 | Race | 12 |
| 5 | Xylene | Reflux | 24 | 36 | 21 | Race | 15 |
| 6 | V(Toluene)∶V(DMF)=4∶1 | Reflux | 24 | 92 | 39 | 41 | 12 |
| 7 | V(Toluene)∶V(DMF)=4∶1 | 90 | 24 | 81 | 44 | 28 | 9 |
| 8 | V(Toluene)∶V(DMF)=4∶1 | 70 | 24 | 24 | 6 | 14 | 4 |
| Entry | Product | Heck reaction yield(%) | De-protection yield(%) | Total yield(%) | m.p./℃(ref.) |
|---|---|---|---|---|---|
| 1 | 44 | 94 | 40 | 128—130(126—128[ | |
| (5) | |||||
| 2 | 42 | 93 | 37 | 122—124(121—122[ | |
| 3 | 23 | 79 | 17 | 151—153(150—152[ | |
| 4 | 41 | 75 | 29 | 144—147(147—148[ | |
| 5 | 38 | 95 | 35 | 152—154(150—151[ | |
| 6 | 26 | 87 | 22 | 133—135(132—133[ |
Table 3 Syntheses of several caffeic acid esters
| Entry | Product | Heck reaction yield(%) | De-protection yield(%) | Total yield(%) | m.p./℃(ref.) |
|---|---|---|---|---|---|
| 1 | 44 | 94 | 40 | 128—130(126—128[ | |
| (5) | |||||
| 2 | 42 | 93 | 37 | 122—124(121—122[ | |
| 3 | 23 | 79 | 17 | 151—153(150—152[ | |
| 4 | 41 | 75 | 29 | 144—147(147—148[ | |
| 5 | 38 | 95 | 35 | 152—154(150—151[ | |
| 6 | 26 | 87 | 22 | 133—135(132—133[ |
| [1] | Brumfitt, W. , Hamilton M., J. , Franklin, I. , Microbios., 1990, 62, 19- 22 |
| [2] | Son, S. , Lewis B., A. , J. Agric. Food Chem., 2002, 50, 468- 472 |
| [3] | Mirzoeva O., K. , Calder P., C. , Prostaglandins, Leukotrienes Essent. Fatty Acids, 1997, 55( 6), 441- 449 |
| [4] | Lee Y., J. , Liao P., H. , Chen W., K. , Yang C., C. , Cancer Lett., 2000, 153( 1/2), 51- 56 |
| [5] | Al-Hariri, M. , Gamal Eldin, T. , Abu-Hozaifa, B. , Elnour, A. , Diabetes Metab. Syndr. Obes.: Targets and Therapy, 2011, 4, 377- 384 |
| [6] | Nagaoka, T. , Banskota A., H. , Tezuka, Y. , Saiki, I. , Kadota, S. , Bioorg. Med. Chem., 2002, 10( 10), 3351- 3359 |
| [7] | Lee Y., J. , A One Pot Process for the Preparation of Caffeic Acid Ester Derivatives, EP 1211237A1, 2002- 06-05 |
| [8] | Eisinger, M. , Marko, O. , Shun-Ichiro, O. , Science, 1985, 229( 4717), 984- 986 |
| [9] | Zhang Y., D. , Cheng Z., J. , Ye, S. , Zhang, Y. , J. Jiangxi Chem. Ind., 2012, 1, 65- 68 |
| [10] | Zhou S., Y. , Chen, L. , Huang K., L. , J. Eng. Heilongjiang University, 2013, 4( 2), 27- 32 |
| [11] | Jin L., L. , Jin G., Z. , Chem. Res. Chinese Universities, 2012, 28( 6), 971- 975 |
| [12] | Sun C., W. , Wang, J. , Pang C., C. , Li, J. , Xiao, D. , Chem. Res. Chinese Universities, 2013, 29( 6), 1104- 1109 |
| [13] | Han, Y. , Hao M., H. , Acta, Cryst. , Section E: Structure Reports Online, 2012, 68( 3), o727 |
| [14] | Bankova V., S. , J. Nat. Prod., 1990, 53( 4), 821- 824 |
| [15] | 柳枫. 咖啡酸酯衍生物的制备研究及其生物活性研究, 杭州: 浙江工业大学, 2006) |
| Liu, F. , Preparation of Caffeic Acid Ester Derivatives and Research on Their Biological Activity, Zhejiang Industry University, Hangzhou, 2006( | |
| [16] | Xia C., N. , Hu W., X. , Synth. Chem., 2004, 12, 545- 550 |
| [17] | Hu W., X. , Xia C., N. , Wang G., H. , Zhou, W. , J. Chem. Res., 2006, 9, 586- 588 |
| [18] | Sanderson J., T. , Clabault, H. , Patton, C. , Lassalle-Claux, G. , Jean-Francois, J. , Paré, A. F. , Hé, bert M. J. G. , Surette M., E. , Touaibia, M. , Bioorg. Med. Chem., 2013, 21( 22), 7182- 7188 |
| [19] | Xia C., N. , Li H., B. , Liu, F. , Hua W., X. , Bioorg. Med. Chem. Lett., 2008, 18, 6553- 6557 |
| [20] | Sun S., L. , Yang J., G. , Zhang L., C. , Yu H., N. , Shen S., R. , Chin. J. Cell Biol., 2007, 29, 225- 228 |
| [21] | Clark J., H. , Holland H., L. , Miller J., M. , Tetrahedron Lett., 1976, 17( 38), 3361- 3364 |
| [22] | Castella, M. , Lopec-calahorra, F. , Velasco, D. , Finkelmann, H. , Liq. Cryst., 2002, 29( 4), 559- 565 |
| [23] | Brooks P., R. , Wirtz M., C. , Vetelino M., G. , Rescek D., M. , Woodworth G., F. , Morgan B., P. , Coe J., W. , J. Org. Chem., 1999, 64( 26), 9719- 9721 |
| [24] | Hu Y., Z. , Clive D. L., J. , J. Chem., Soc. , Perkin Trans., 1997, 1, 1421- 1424 |
| [25] | LeBlanc L., M. , Pare A., F. , Jean-Francois, J. , Hebert M. J., G. , Surette M., E. , Touaibia, M. , Mol., 2012, 17( 12), 14637- 14650 |
| [1] | CAO Shujie, LI Hongjun, GUAN Wenli, REN Mengtian, ZHOU Chuanzheng. Progress on the Stereocontrolled Synthesis of Phosphorothioate Oligonucleotides [J]. Chem. J. Chinese Universities, 2022, 43(Album-4): 20220304. |
| [2] | WANG Ruyue, WEI Hehe, HUANG Kai, WU Hui. Freezing Synthesis for Single Atom Materials [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220428. |
| [3] | YAO Qing, YU Zhiyong, HUANG Xiaoqing. Progress in Synthesis and Energy-related Electrocatalysis of Single-atom Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220323. |
| [4] | JIN Ruiming, MU Xiaoqing, XU Yan. Bio-chemical Synthesis of Melanin Precursor—— 5,6-Dihydroxyindole(DHI) [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220134. |
| [5] | WEI Chunhong, JIANG Qian, WANG Panpan, JIANG Chengfa, LIU Yuefeng. Atomic Scale Investigation of Pt Atoms/clusters Promoted Co-catalyzed Fischer-Tropsch Synthesis [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220074. |
| [6] | ZHANG Xinxin, XU Di, WANG Yanqiu, HONG Xinlin, LIU Guoliang, YANG Hengquan. Effect of Mn Promoter on CuFe-based Catalysts for CO2 Hydrogenation to Higher Alcohols [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220187. |
| [7] | ZHOU Zixuan, YANG Haiyan, SUN Yuhan, GAO Peng. Recent Progress in Heterogeneous Catalysts for the Hydrogenation of Carbon Dioxide to Methanol [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220235. |
| [8] | LI Yidi, TIAN Xiaochun, LI Junpeng, CHEN Lixiang, ZHAO Feng. Electron Transfer on the Semiconductor-microbe Interface and Its Environmental Application [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220089. |
| [9] | LU Cong, LI Zhenhua, LIU Jinlu, HUA Jia, LI Guanghua, SHI Zhan, FENG Shouhua. Synthesis, Structure and Fluorescence Detection Properties of a New Lanthanide Metal-Organic Framework Material [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220037. |
| [10] | ZHUANG Jiahao, WANG Dingsheng. Current Advances and Future Challenges of Single-atom Catalysis [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220043. |
| [11] | FENG Li, SHAO Lanxing, LI Sijun, QUAN Wenxuan, ZHUANG Jinliang. Synthesis of Ultrathin Sm-MOF Nanosheets and Their Visible-light Induced Photodegradation of Mustard Simulant [J]. Chem. J. Chinese Universities, 2022, 43(4): 20210867. |
| [12] | ZHANG Zhinan, CHENG Haiming, TENG Shiyong, ZHANG Ying. Synthesis and Optical Properties of RbPb2Cl5 [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220418. |
| [13] | XING Peiqi, LU Tong, LI Guanghua, WANG Liyan. Controllable Syntheses of Two Cd(II) Metal-organic Frameworks Possessing Related Structures [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220218. |
| [14] | WANG Jie, HUO Haiyan, WANG Yang, ZHANG Zhong, LIU Shuxia. General Strategy for In situ Synthesis of NENU-n Series Polyoxometalate-based MOFs on Copper Foil [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210557. |
| [15] | LIANG Yu, LIU Huan, GONG Lige, WANG Chunxiao, WANG Chunmei, YU Kai, ZHOU Baibin. Synthesis and Supercapacitor Properties of Biimidazole-modified {SiW12O40} Hybrid [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210556. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||