

Chem. J. Chinese Universities ›› 2014, Vol. 35 ›› Issue (9): 1919.doi: 10.7503/cjcu20140459
• Physical Chemistry • Previous Articles Next Articles
JIANG Juxing1,2, WANG Jiajun1,2, DUAN Yanqing1,2, LIU Ya1,2, WANG Wenyuan1,2, WU Shaohua3,*
Received:2014-05-14
Online:2014-09-10
Published:2019-08-01
Contact:
WU Shaohua
Supported by:CLC Number:
TrendMD:
JIANG Juxing, WANG Jiajun, DUAN Yanqing, LIU Ya, WANG Wenyuan, WU Shaohua. Theoretical Studies on Water Catalysis of Two Esters Interconversion Reaction†[J]. Chem. J. Chinese Universities, 2014, 35(9): 1919.
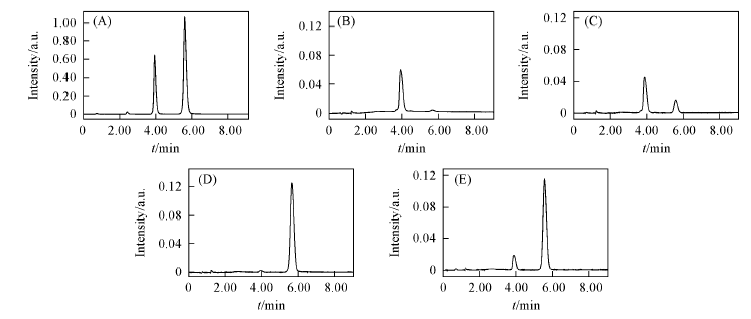
Fig.2 Chromatogram for the interconversion between compounds 1 and 2 (A) Equilibrium sample; (B) compound 2 for 10 min; (C) compound 2 for 153 min; (D) compound 1 for 10 min; (E) compound 1 for 155 min.
| Species | Phase | ΔE/(kJ·mol-1) | ΔG298 K/(kJ·mol-1) | ΔH298 K(kJ·mol-1) | P(%) | 1030 μ/C·m | 1030 μtot/C·m |
|---|---|---|---|---|---|---|---|
| 1* | Gas | 0 | 0 | 0 | 11.34 | 11.34 | |
| 1-a | Gas | 0 | 0.003 | 0 | 90.1 | 11.34 | 10.61 |
| 1-b | Gas | 8.48 | 7.04 | 8.23 | 5.3 | 7.27 | |
| 2-a | Gas | 8.28 | 7.38 | 8.24 | 4.6 | 9.64 | 9.64 |
| 1-a | Diethyl ether | 0 | 0 | 0 | 85.4 | 11.34 | 10.44 |
| 1-b | Diethyl ether | 5.50 | 5.22 | 5.18 | 10.4 | 7.27 | |
| 2-a | Diethyl ether | 5.43 | 7.49 | 5.52 | 4.2 | 14.91 | 14.91 |
| 1-a | Water | 0 | 0 | 0 | 61.6 | 13.77 | 11.04 |
| 1-b | Water | 4.26 | 1.94 | 3.81 | 28.1 | 9.07 | |
| 2-a | Water | 3.28 | 4.46 | 3.40 | 10.3 | 13.27 | 13.27 |
Table 1 Calculated relative electronic energies(ΔE), relative Gibbs free energy(ΔG298 K), relative enthalpy(ΔH298 K), Boltzmann populations(P) and dipole moment(μ) for compounds 1 and 2 at B3LYP/6-311+G(d,p) level in gas phase, diethyl ether phase and water phase
| Species | Phase | ΔE/(kJ·mol-1) | ΔG298 K/(kJ·mol-1) | ΔH298 K(kJ·mol-1) | P(%) | 1030 μ/C·m | 1030 μtot/C·m |
|---|---|---|---|---|---|---|---|
| 1* | Gas | 0 | 0 | 0 | 11.34 | 11.34 | |
| 1-a | Gas | 0 | 0.003 | 0 | 90.1 | 11.34 | 10.61 |
| 1-b | Gas | 8.48 | 7.04 | 8.23 | 5.3 | 7.27 | |
| 2-a | Gas | 8.28 | 7.38 | 8.24 | 4.6 | 9.64 | 9.64 |
| 1-a | Diethyl ether | 0 | 0 | 0 | 85.4 | 11.34 | 10.44 |
| 1-b | Diethyl ether | 5.50 | 5.22 | 5.18 | 10.4 | 7.27 | |
| 2-a | Diethyl ether | 5.43 | 7.49 | 5.52 | 4.2 | 14.91 | 14.91 |
| 1-a | Water | 0 | 0 | 0 | 61.6 | 13.77 | 11.04 |
| 1-b | Water | 4.26 | 1.94 | 3.81 | 28.1 | 9.07 | |
| 2-a | Water | 3.28 | 4.46 | 3.40 | 10.3 | 13.27 | 13.27 |
| Species | Compd. | ΔH298 K/ (kJ·mol-1) | ΔG298 K/ (kJ·mol-1) | Species | Compd. | ΔH298 K/ (kJ·mol-1) | ΔG298 K/ (kJ·mol-1) | 1030μ/ (C·m) |
|---|---|---|---|---|---|---|---|---|
| Mechanism Aa | 1 | 0 | 0 | Mechanism Dd | 1 | 0 | 0 | 3.67 |
| TS1 | 191.03 | 202.31 | TS1 | 115.18 | 132.40 | 6.60 | ||
| Int. | 49.38 | 61.36 | Int. | 33.83 | 43.14 | 14.34 | ||
| TS2 | 177.27 | 188.82 | TS2 | 113.99 | 129.20 | 9.17 | ||
| 2 | 8.24 | 7.38 | 2 | 3.20 | -3.01 | 3.17 | ||
| Mechanism Bb | 1 | 0 | 0 | Mechanism D | 1 | 0 | 0 | |
| TS1 | 132.14 | 152.34 | in water phasee | TS1 | 108.48 | 128.15 | ||
| Int. | 30.00 | 45.09 | Int. | 32.67 | 43.39 | |||
| TS2 | 127.58 | 153.69 | TS2 | 110.95 | 131.24 | |||
| 2 | 5.00 | 7.39 | 2 | -7.15 | -18.19 | |||
| Mechanism Cc | 1 | 0 | 0 | SPE with mechanism | 1 | 0 | 0 | |
| TS1 | 137.85 | 154.24 | D in water phasef | TS1 | 106.24 | 110.94 | ||
| Int. | 33.83 | 43.14 | Int. | 19.11 | 21.68 | |||
| TS2 | 125.46 | 145.30 | TS2 | 107.37 | 112.22 | |||
| 2 | 3.20 | -3.01 | 2 | 1.05 | -1.58 |
Table 2 Calculated relative Gibbs free energy(ΔG298 K) and relative enthalpy(ΔH298 K) for compounds 1, TSs, intermediate and compound 2
| Species | Compd. | ΔH298 K/ (kJ·mol-1) | ΔG298 K/ (kJ·mol-1) | Species | Compd. | ΔH298 K/ (kJ·mol-1) | ΔG298 K/ (kJ·mol-1) | 1030μ/ (C·m) |
|---|---|---|---|---|---|---|---|---|
| Mechanism Aa | 1 | 0 | 0 | Mechanism Dd | 1 | 0 | 0 | 3.67 |
| TS1 | 191.03 | 202.31 | TS1 | 115.18 | 132.40 | 6.60 | ||
| Int. | 49.38 | 61.36 | Int. | 33.83 | 43.14 | 14.34 | ||
| TS2 | 177.27 | 188.82 | TS2 | 113.99 | 129.20 | 9.17 | ||
| 2 | 8.24 | 7.38 | 2 | 3.20 | -3.01 | 3.17 | ||
| Mechanism Bb | 1 | 0 | 0 | Mechanism D | 1 | 0 | 0 | |
| TS1 | 132.14 | 152.34 | in water phasee | TS1 | 108.48 | 128.15 | ||
| Int. | 30.00 | 45.09 | Int. | 32.67 | 43.39 | |||
| TS2 | 127.58 | 153.69 | TS2 | 110.95 | 131.24 | |||
| 2 | 5.00 | 7.39 | 2 | -7.15 | -18.19 | |||
| Mechanism Cc | 1 | 0 | 0 | SPE with mechanism | 1 | 0 | 0 | |
| TS1 | 137.85 | 154.24 | D in water phasef | TS1 | 106.24 | 110.94 | ||
| Int. | 33.83 | 43.14 | Int. | 19.11 | 21.68 | |||
| TS2 | 125.46 | 145.30 | TS2 | 107.37 | 112.22 | |||
| 2 | 3.20 | -3.01 | 2 | 1.05 | -1.58 |
| Mechanism | Species | O1—Hx/nm | C5—O6/nm | ∠C2C3O4/(°) | ∠C3C2O1/(°) |
|---|---|---|---|---|---|
| A | Compound 1 | 0.09636 | 0.12056 | 103.1 | 111.4 |
| TS1 | 0.13791 | 0.13076 | 101.1 | 107.9 | |
| Difference | 0.04155 | 0.01020 | -2.0 | -3.5 | |
| B | Compound 1 | 0.09712 | 0.12113 | 108.3 | 113.0 |
| TS1 | 0.12379 | 0.12888 | 100.9 | 105.5 | |
| Difference | 0.02667 | 0.00775 | -7.4 | -7.5 | |
| C | Compound 1 | 0.09733 | 0.12036 | 108.1 | 109.7 |
| TS1 | 0.13549 | 0.12883 | 101.9 | 104.2 | |
| Difference | 0.03816 | 0.00847 | -6.2 | -5.5 | |
| D | Compound 1 | 0.09733 | 0.12036 | 108.1 | 109.7 |
| TS1 | 0.12818 | 0.12662 | 102.9 | 103.6 | |
| Difference | 0.03085 | 0.00626 | -5.2 | -6.1 |
Table 3 Structural parameters of compound 1, TS1 and their differences for mechanism A—D at B3LYP/6-311+G(d,p) level in gas phase
| Mechanism | Species | O1—Hx/nm | C5—O6/nm | ∠C2C3O4/(°) | ∠C3C2O1/(°) |
|---|---|---|---|---|---|
| A | Compound 1 | 0.09636 | 0.12056 | 103.1 | 111.4 |
| TS1 | 0.13791 | 0.13076 | 101.1 | 107.9 | |
| Difference | 0.04155 | 0.01020 | -2.0 | -3.5 | |
| B | Compound 1 | 0.09712 | 0.12113 | 108.3 | 113.0 |
| TS1 | 0.12379 | 0.12888 | 100.9 | 105.5 | |
| Difference | 0.02667 | 0.00775 | -7.4 | -7.5 | |
| C | Compound 1 | 0.09733 | 0.12036 | 108.1 | 109.7 |
| TS1 | 0.13549 | 0.12883 | 101.9 | 104.2 | |
| Difference | 0.03816 | 0.00847 | -6.2 | -5.5 | |
| D | Compound 1 | 0.09733 | 0.12036 | 108.1 | 109.7 |
| TS1 | 0.12818 | 0.12662 | 102.9 | 103.6 | |
| Difference | 0.03085 | 0.00626 | -5.2 | -6.1 |
| [1] | Wu S. H., Chen Y. W., Shao S. C., Wang L. D., Li Z. Y., Yang L. Y., Li S. L., Huang R., J. Nat. Prod., 2008, 71, 731—734 |
| [2] | Umehara M., Takada Y., Nakao Y., Kimura J., Tetrahedron Lett., 2009, 50, 840—843 |
| [3] | Frisch M.J., Trucks G. W., Schlegel H. B., Scuseria G. E., Robb M. A., Cheeseman J. R., Scalmani G., Barone V., Mennucci B., Petersson G. A., Nakatsuji H., Caricato M., Li X., Hratchian H. P., Izmaylov A. F., Bloino J., Zheng G., Sonnenberg J. L., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Vreven T., Montgomery J. A. Jr., Peralta J. E., Ogliaro F., Bearpark M., Heyd J. J., Brothers E., Kudin K. N., Staroverov V. N., Kobayashi R., Normand J., Raghavachari K., Rendell A., Burant J. C., Iyengar S. S., Tomasi J., Cossi M., Rega N., Millam J. M., Klene M., Knox J. E., Cross J. B., Bakken V., Adamo C., Jaramillo J., Gomperts R., Stratmann R. E., Yazyev O., Austin A. J., Cammi R., Pomelli C., Ochterski J. W., Martin R. L., Morokuma K., Zakrzewski V. G., Voth G. A., Salvador P., Dannenberg J. J., Dapprich S., Daniels A. D., Farkas Ö., Foresman J. B., Ortiz J. V., Cioslowski J., Fox D. J., Gaussian 09, Revision A.02, Gaussian Inc., Wallingford CT, 2009 |
| [4] | Siddiqui I. N., Zahoor A., Hussain H., Ahmed I., Ahmad V. U., Padula D., Draeger S., Schulz B., Meier K., Steinert M., J. Nat. Prod., 2011, 74, 365—373 |
| [5] | Andres G. O., Pierini A. B., de Rossi R. H., J. Org. Chem., 2006, 71 , 7650—7656 |
| [6] | Yang Y., J. Physical Chem. A, 2012, 116, 10150—10159 |
| [7] | Ni Z. M., Shi W., Xia M. Y., Xue J. L., Chem. J. Chinese Universities,2013, 34(10), 2353—2362 |
| (倪哲明, 施炜, 夏明玉, 薛继龙. 高等学校化学学报, 2013, 34(10), 2353—2362) | |
| [8] | Chen J., Wang M., Chem. Res. Chinese Universities,2013, 29(3), 584—588 |
| [9] | Jin X. H., Hu B. C., Jia H. Q., Liu Z. L., Lü C. X., Chem. J. Chinese Universities,2013, 34(12), 2821—2826 |
| (金兴辉, 胡炳成, 贾欢庆, 刘祖亮, 吕春绪. 高等学校化学学报, 2013, 34(12), 2821—2826) | |
| [10] | Ramirez A., Mudryk B., Rossano L., Tummala S., J. Org. Chem., 2012, 77, 775—779 |
| [11] | Burt M. B., Fridgen T. D., J. Phys. Chem. A,2012, 117, 1283—1290 |
| [12] | Han I. S., Kim C. K., Sohn C. K., Ma E. K., Lee H. W., Kim C. K., J. Phys.Chem. A,2011, 115, 1364—1370 |
| [13] | Bender M. L., J. Am. Chem. Soc., 1951, 73, 1626—1629 |
| [14] | Cox R. A., Int. J. Mol. Sci., 2011, 12, 8316—8332 |
| [15] | Yates K., McClelland R. A., J. Am. Chem. Soc., 1967, 89, 2686—2692 |
| [16] | Yang W., Drueckhammer D. G., Org. Lett., 2000, 2, 4133—4136 |
| [17] | Hao J. J., Wang C. S., Acta Chimica Sinica,2009, 67(12), 1285—1290 |
| (郝娇娇, 王长生. 化学学报, 2009, 67(12), 1285—1290) | |
| [18] | Jiang J. X., Li L. C., Wang M. F., Xia J. J., Wang W. Y., Xie X. G., Bull. Korean Chem. Soc., 2012, 33, 1722—1728 |
| [19] | Roohi H., Gholipour Y., J. Quantum Chem., 2008, 108, 462—471 |
| [20] | Kaczor A., Reva I., Fausto R., J. Phys.Chem. A,2013, 117, 888—897 |
| [21] | Jaeqx S., Du W., Meijer E. J., Oomens J., Rijs A. M., J. Phys.Chem. A,2012, 117, 1216—1227 |
| [1] | ZHAO Dongxia, ZHANG Haixia, FENG Wenjuan, YANG Zhongzhi. Molecular Face Guiding the Proton Transfer Reactions of Hydroxyl Carbene and Its Derivatives [J]. Chem. J. Chinese Universities, 2021, 42(7): 2187. |
| [2] | CAI Lihai,GUO Baohua,ZHANG Cheng,XU Jun,HUANG Zhongyao. Long-term Stress Relaxation Prediction for Nylon 1010 Using Time-temperature Superposition Method† [J]. Chem. J. Chinese Universities, 2019, 40(4): 832. |
| [3] | KUANG Jingzhong, YUAN Weiquan, XU Liyong, LI Lin, HUANG Zhen. Effect of La(NO3)3 and Pr(NO3)3 on Kinetic of Dehydroxylation of Kaolinite† [J]. Chem. J. Chinese Universities, 2015, 36(7): 1395. |
| [4] | LI Tong, JIN Baokang. Electrochemical Redox of Benzoquinone in Ionic Liquids† [J]. Chem. J. Chinese Universities, 2014, 35(4): 847. |
| [5] | TANG Haiyan, ZHAO Maoshuang, FENG Li, CAO Zexing. Theoretical Studies on the Taking off of Oxygen-containing Functional Groups in Lignite Model Compounds† [J]. Chem. J. Chinese Universities, 2014, 35(11): 2370. |
| [6] | LI Heng-Dong, SU Xiao-Long, CHEN Ping-Hua, XIE Li-Li, YUAN Yao-Feng. Synthesis and Properties of 5-Ferrocenyl-pyrazoline Derivatives [J]. Chem. J. Chinese Universities, 2013, 34(7): 1653. |
| [7] | GUO Lai-Hui, FANG Xing-Zhong*, WANG Gui-Bin, WU Zhong-Wen. Isothermal Crystallization Kinetics of Thermoplastic Polyimide and Poly(ether ether ketone) Blends [J]. Chem. J. Chinese Universities, 2011, 32(12): 2908. |
| [8] | SUN Ying, REN Ai-Min*, LI Zuo-Sheng, MIN Chun-Gang, REN Xue-Feng, FENG Ji-Kang. Theoretical Investigation of the Reaction Mechanism of Cypridina Luciferin Analogues [J]. Chem. J. Chinese Universities, 2011, 32(11): 2586. |
| [9] | YAO Tong-Wei, DU Li-Bo, YANG Yi, XU Yuan-Chao, JIA Hong-Ying, LIU Yang*. Studies on Intermolecular Synergistic Antioxidant Activity in Glyceride Tri-ferulate [J]. Chem. J. Chinese Universities, 2009, 30(7): 1431. |
| [10] | ZHANG Da-Li, KE Jia-Jun, LU Li-Zhu. Action of Molecular Kinetic Energy on a Gas-solid Chromatography [J]. Chem. J. Chinese Universities, 2009, 30(4): 777. |
| [11] |
XU Xiu-Fang*, SHANG Zhen-Feng, LI Rui-Fang, ZHAO Xue-Zhuang.
Theoretical Study on the Reaction Mechanism for Diels-Alder Cycloaddition of C50(D5h) with 1,3-Butadiene and 2,3-Disubstituted 1,3-Butadienes [J]. Chem. J. Chinese Universities, 2009, 30(11(1)): 16. |
| [12] | WANG Yi, WANG Yong, HAN Ke-Li*. Comparison Between [FeⅣ(O)(TMC)(NCMe)]2+ and [FeⅣ(O)(TMCS)]+ Non-heme Complexes of Geometric, Electronic Structures, Bonding and Reactivities [J]. Chem. J. Chinese Universities, 2008, 29(12): 2469. |
| [13] | YANG Jia-Zhen, JIN Yi, PAN Wei, ZANG Shu-Liang. Measurement of Diffusion Coefficient of Fe3+ in Ionic Liquid BPBF4 by Chronoampermetry [J]. Chem. J. Chinese Universities, 2005, 26(6): 1146. |
| [14] | ZHANG Qi-Zhen, LIU Jian-Qiang, YIN Xiao-Ying, ZHANG Jing-Zhi. Photoresponse Behaviour of a New Photochromic Liquid Crystalline Carbosilane Dendrimer of the First Generation Containing Hexyloxyazobenzene Moieties Groups in Its Periphery [J]. Chem. J. Chinese Universities, 2004, 25(7): 1368. |
| [15] | WANG Ying-Jun, WANG Xin-Ping, CHAI Song-Hai, CAI Tian-Xi . n-Heptane Isomerization over Ni Doped MoOx Catalyst [J]. Chem. J. Chinese Universities, 2003, 24(7): 1281. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||