

Chem. J. Chinese Universities ›› 2014, Vol. 35 ›› Issue (4): 847.doi: 10.7503/cjcu20130724
• Physical Chemistry • Previous Articles Next Articles
Received:2013-07-26
Online:2014-04-10
Published:2013-10-23
Contact:
JIN Baokang
E-mail:bkjinhf@aliyun.com
Supported by:CLC Number:
TrendMD:
LI Tong, JIN Baokang. Electrochemical Redox of Benzoquinone in Ionic Liquids†[J]. Chem. J. Chinese Universities, 2014, 35(4): 847.
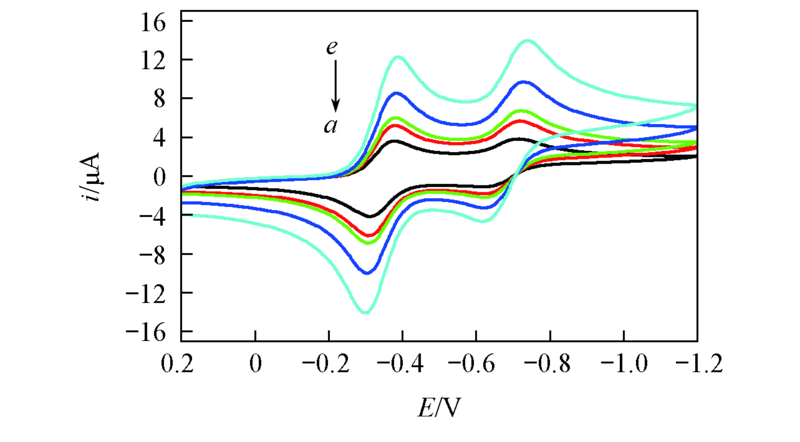
Fig.1 Cyclic voltammogram of 0.005 mol/L BQ in BMIMBF4 at Pt working electrode using various scan ratesScan rate/(mV·s-1): a. 20; b. 40; c. 50; d. 100; e. 200.
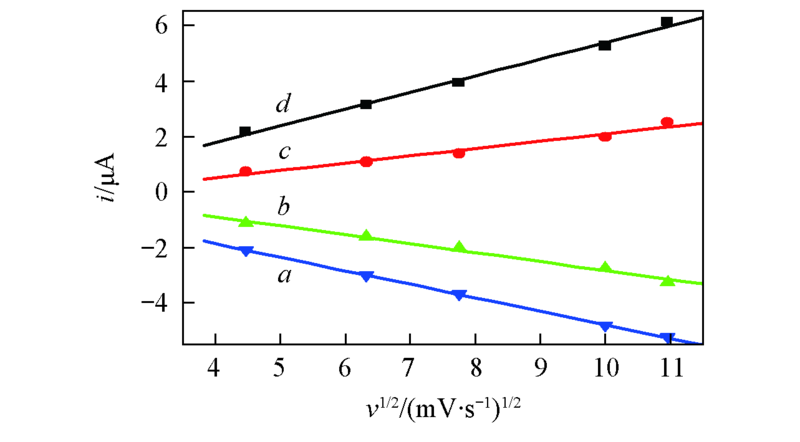
Fig.2 Plots of Ip vs. v1/2 from cyclic vohammogram of 0.005 mol/L BQ in BMIMBF4a. ipa1 vs. v1/2; b. ipa2 vs. v1/2; c. ipc2 vs. v1/2; d. ipc1 vs. v1/2.
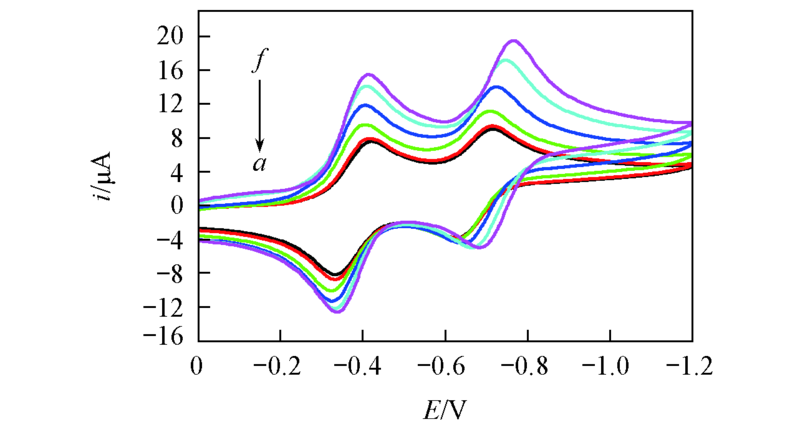
Fig.3 Cyclic voltammogram of BQ at various temperatures in BMIMPF6(scan rate: 100 mV/s)Temperature/K: a. 298.15; b. 303.15; c. 313.15; d. 323.15; e. 333.15; f. 343.15.
| T/K | Ipc1/μA | Ipc2/μA | 107D/(cm2·s-1) | T/K | Ipc1/μA | Ipc2/μA | 107D/(cm2·s-1) |
|---|---|---|---|---|---|---|---|
| 298.15 | 6.897 | 3.950 | 2.671 | 323.15 | 9.991 | 5.847 | 6.075 |
| 303.15 | 7.285 | 4.023 | 3.030 | 333.15 | 11.540 | 7.894 | 8.356 |
| 313.15 | 8.524 | 4.505 | 4.285 | 343.15 | 13.140 | 9.535 | 11.160 |
Table 1 Values of diffusion coefficient D of BQ in BMIMPF6 at different temperatures
| T/K | Ipc1/μA | Ipc2/μA | 107D/(cm2·s-1) | T/K | Ipc1/μA | Ipc2/μA | 107D/(cm2·s-1) |
|---|---|---|---|---|---|---|---|
| 298.15 | 6.897 | 3.950 | 2.671 | 323.15 | 9.991 | 5.847 | 6.075 |
| 303.15 | 7.285 | 4.023 | 3.030 | 333.15 | 11.540 | 7.894 | 8.356 |
| 313.15 | 8.524 | 4.505 | 4.285 | 343.15 | 13.140 | 9.535 | 11.160 |
| Ionic liquid | Slope | Ea/(kJ·mol-1) | R* | Ionic liquid | Slope | Ea/(kJ·mol-1) | R* |
|---|---|---|---|---|---|---|---|
| BMIMBF4 | -2401.77 | 19.97 | -0.996 | HMIMBF4 | -2583.76 | 21.48 | -0.990 |
| BMIMPF6 | -3313.19 | 27.55 | -0.999 | HMIMPF6 | -4734.50 | 39.36 | -0.991 |
Table 2 Values of activation energy Ea of BQ in ionic liquids
| Ionic liquid | Slope | Ea/(kJ·mol-1) | R* | Ionic liquid | Slope | Ea/(kJ·mol-1) | R* |
|---|---|---|---|---|---|---|---|
| BMIMBF4 | -2401.77 | 19.97 | -0.996 | HMIMBF4 | -2583.76 | 21.48 | -0.990 |
| BMIMPF6 | -3313.19 | 27.55 | -0.999 | HMIMPF6 | -4734.50 | 39.36 | -0.991 |
| Solution | Epc1/V | Epa1/V | Epc2/V | Epa2/V | ΔEp1/V | ΔEp2/V | (E1/2)1/V | (E1/2)2/V | ΔE1/2/V |
|---|---|---|---|---|---|---|---|---|---|
| BMIMBF4 | -0.542 | -0.102 | -0.753 | -0.468 | 0.440 | 0.280 | -0.322 | -0.611 | 0.289 |
| BMIMPF6 | -0.578 | -0.078 | -0.804 | -0.409 | 0.500 | 0.395 | -0.328 | -0.607 | 0.279 |
| CH3CN | -0.563 | -0.389 | -0.959 | -0.747 | 0.174 | 0.212 | -0.476 | -0.853 | 0.377 |
Table 3 Parameters ofcyclic voltammogram for 0.025 mol/L BQ in ionic liquids and CH3CN solution
| Solution | Epc1/V | Epa1/V | Epc2/V | Epa2/V | ΔEp1/V | ΔEp2/V | (E1/2)1/V | (E1/2)2/V | ΔE1/2/V |
|---|---|---|---|---|---|---|---|---|---|
| BMIMBF4 | -0.542 | -0.102 | -0.753 | -0.468 | 0.440 | 0.280 | -0.322 | -0.611 | 0.289 |
| BMIMPF6 | -0.578 | -0.078 | -0.804 | -0.409 | 0.500 | 0.395 | -0.328 | -0.607 | 0.279 |
| CH3CN | -0.563 | -0.389 | -0.959 | -0.747 | 0.174 | 0.212 | -0.476 | -0.853 | 0.377 |
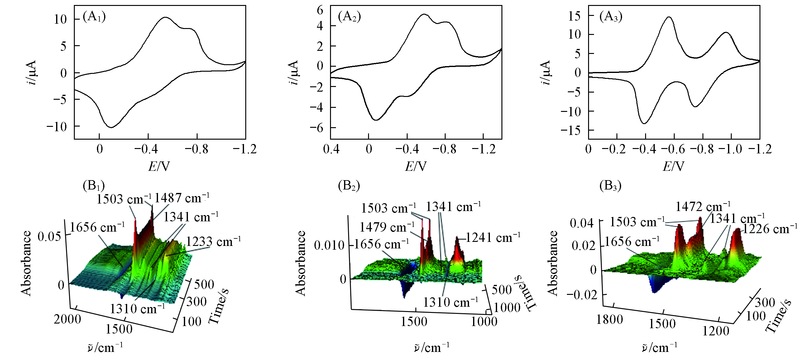
Fig.5 Cyclic voltammogram(A1—A3) and the corresponding 3D spectra(B1—B3) of 0.025 mol/L BQ in BMIMBF4(A1,B1), BMIMPF6(A2,B2) and CH3CN containing 0.1 mol/L TBAP(A3,B3)(scan rate: 5 mV/s)
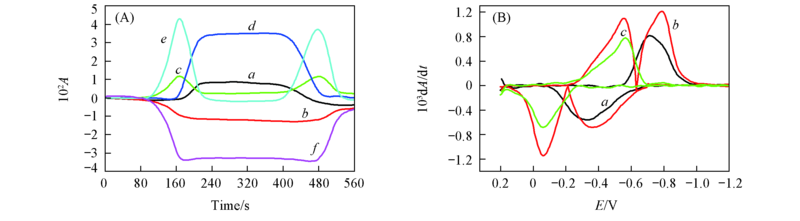
Fig.6 CVAs(A) and selected DCVAs(B)(smoothing with a fast Fourier transform smoothing algorithm) for the BQ electrochemical reaction in BMIMBF4 ν˙/cm-1: (A) a. 1233, b. 1310, c. 1341, d. 1487, e. 1503, f. 1656; (B) a. 1487, b. 1503, c. 1656.
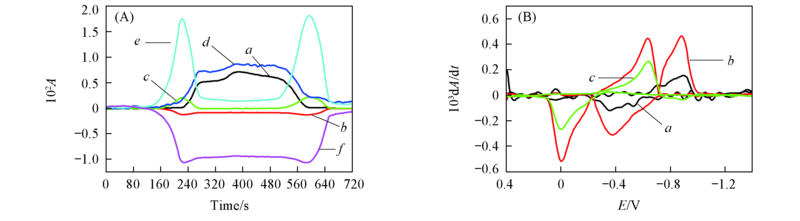
Fig.7 CVAs(A) and selected DCVAs(B)(smoothing with a fast Fourier transform smoothing algorithm) for the BQ electrochemical reaction in BMIMPF6 ν˙/cm-1: (A) a. 1241, b. 1310, c. 1341, d. 1479, e. 1503, f. 1656; (B) a. 1479, b. 1503, c. 1656.
| [1] | Welton T., Chem. Rev., 1999, 99(8), 2071—2084 |
| [2] | Yang J. Z., Jin Y., Cao Y. H., Sun L. X., Tang Z. C., Chem. J. Chinese Universities,2004, 25(9), 1733—1735 |
| (杨家振, 金一, 曹英华, 孙立贤,谭志诚. 高等学校化学学报, 2004, 25(9), 1733—1735) | |
| [3] | Giridhar P., Venkatesan K. A., Srinivasan T. G., Rao P. R. V., Electrochim. Acta,2007, 52(9), 3006—3012 |
| [4] | Chiappe C., Pieraccini D., J. Phys. Org. Chem., 2005, 18(4), 275—297 |
| [5] | Shangguan X. D., Tang H. S., Liu R. X., Zheng J. B., Chinese J. Anal. Chem., 2010, 38(10), 1510—1516 |
| (上官小东, 汤宏胜, 刘锐晓, 郑建斌. 分析化学评述与进展, 2010, 38(10), 1510—1516) | |
| [6] | Sun W., Gao R. F., Jiao K., Chinese J. Anal. Chem., 2007, 35(12), 1813—1819 |
| (孙伟, 高瑞芳, 焦奎. 分析化学评述与进展, 2007, 35(12), 1813—1819) | |
| [7] | Shim Y. B., Park S. M., J. Electroanal. Chem., 1997, 425(1), 201—207 |
| [8] | Kim Y. O., Jung Y. M., Kim S. B., Park S. M., Anal. Chem., 2004, 76(17), 5236—5240 |
| [9] | Jin B. K., Huang J. L., Zhao A. K., Zhang S. Y., Tian Y. P., Yang J. X., J. Electroanal. Chem., 2010, 650(1), 116—126 |
| [10] | Wang Y. J., Rogers E. I., Belding S. R., Compton R. G., J. Electroanal. Chem., 2010, 648(2), 134—142 |
| [11] | Rees N. V., Clegg A. D., Klymenko O. V., Coles B. A., Compton R. G., J. Phys. Chem. B,2004, 108(34), 13047—13051 |
| [12] | Enache T. A., Oliveira-Brett A. M., J. Electroanal. Chem., 2011, 655(1), 9—16 |
| [13] | Bielicka-Daszkiewicz K., Krawczyk P., Nowicka K., Electrochim. Acta,2012, 80, 22—26 |
| [14] | Wang J. J., Xie H., Jin B. K., Chinese J. Anal. Chem., 2013, 41(7), 1006—1012 |
| [15] | Ji X. B., Banks C. E., Silvester D. S., Wain A. J., Compton R. G., J. Phys. Chem. C,2007, 111(3), 1496—1504 |
| [16] | Aoki K., Chen J. Y., Zhang H., J. Electroanal. Chem., 2007, 610(2), 211—217 |
| [17] | Batanero B., Saez R., Barba F., Electrochim. Acta,2009, 54(21), 4872—4879 |
| [18] | Bhat M. A., Electrochim. Acta, 2012, 81, 275—282 |
| [19] | Liu X. H., Dong C. W., Yang J., Zhang K., Lu X. Q., Chem. J. Chinese Universities,2008, 29(6), 1216—1219 |
| (刘秀辉, 董存武, 杨俊, 张凯, 卢小泉. 高等学校化学学报, 2008, 29(6), 1216—1219) | |
| [20] | Yang J. Z., Jin Y., Pan W., Zang S. L., Chem. J. Chinese Universities,2005, 26(6), 1146—1148 |
| (杨家振, 金一, 潘伟, 臧树良. 高等学校化学学报, 2005, 26(6), 1146—1148) | |
| [21] | Liu Y. Z., Xiao L. P., Zhang K., Zhao S. F., Zhang J. B., Lu J. X., Chem. J. Chinese Universities,2008, 29(10), 2059—2064 |
| (柳英姿, 肖丽平, 张凯, 赵淑凤, 张静波, 陆嘉星. 高等学校化学学报, 2008, 29(10), 2059—2064) | |
| [22] | Zhu Y. H., Zeng H. Y., Li S. S., Lu Z. X., Ma C. A., Acta Phys. Chim. Sin., 2012, 28(2), 421—426 |
| (朱英红, 曾红燕, 李姗姗, 陆在祥, 马淳安. 物理化学学报, 2012, 28(2), 421—426) | |
| [23] | Wang H., Xue T., Zhang A. J., Zhang L., Lu J. X., Chem. J. Chinese Universities,2006, 27(6), 1135—1137 |
| (王欢, 薛腾, 张爱健, 张丽, 陆嘉星. 高等学校化学学报, 2006, 27(6), 1135—1137) | |
| [24] | Ma C. A., Wang X. J., Li G. H., Li M. C., Chen S., Acta Phys. Chim. Sin., 2007, 23(11), 1719—1722 |
| (马淳安, 王晓娟, 李国华, 李美超, 陈松. 物理化学学报, 2007, 23(11), 1719—1722) | |
| [25] | Huddleston J. G., Visser A. E., Reichert W. M., Willauer H. D., Broker G. A., Rogers R. D., Green Chem., 2001, 3(4), 156—164 |
| [26] | Visser A. E., Swatloski R. P., Reichert W. M., Griffin S. T., Rogers R. D., Ind. Eng. Chem. Res., 2000, 39(10), 3596—3604 |
| [27] | Caban K., Donten M., Stojek Z., J. Phys. Chem. B,2004, 108(3), 1153—1159 |
| [1] | CUI Wei, ZHAO Deyin, BAI Wenxuan, ZHANG Xiaodong, YU Jiang. CO2 Absorption in Composite of Aprotic Solvent and Iron-based Ionic Liquid [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220120. |
| [2] | PENG Kuilin, LI Guilin, JIANG Chongyang, ZENG Shaojuan, ZHANG Xiangping. Research Progress for the Role of Electrolytes in the CO2 Electrochemical Reduction [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220238. |
| [3] | JI Shuangqi, JIN Zhao, GUAN Wenna, PAN Xiangyu, GUAN Tong. Preparation and Chromatographic Performance of Mixed-mode Silica Stationary Phase Modified by Double Cationic Ionic Liquid and Octadecyl Group [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220008. |
| [4] | CHANG Sihui, CHEN Tao, ZHAO Liming, QIU Yongjun. Thermal Degradation Mechanism of Bio-based Polybutylactam Plasticized by Ionic Liquids [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220353. |
| [5] | ZHAO Dongxia, ZHANG Haixia, FENG Wenjuan, YANG Zhongzhi. Molecular Face Guiding the Proton Transfer Reactions of Hydroxyl Carbene and Its Derivatives [J]. Chem. J. Chinese Universities, 2021, 42(7): 2187. |
| [6] | WANG Man, WANG Xin, ZHOU Jing, GAO Guohua. Efficient Synthesis of Dimethyl Carbonate via Transesterification of Methanol and Ethylene Carbonate Catalyzed by Poly(ionic liquid)s [J]. Chem. J. Chinese Universities, 2021, 42(12): 3701. |
| [7] | WAN Ren, SONG Fan, PENG Changjun, LIU Honglai. Group Contribution Method for Infinite Dilution Molar Conductivity of Unconventional Ions in Water [J]. Chem. J. Chinese Universities, 2021, 42(12): 3672. |
| [8] | ZHOU Molin, JIANG Xin, YI Ting, YANG Xiangguang, ZHANG Yibo. Improvement of Interface Stability Between Sulfide Solid Electrolyte Li10GeP2S12 and Lithium Metal [J]. Chem. J. Chinese Universities, 2020, 41(8): 1810. |
| [9] | GAO Chong,YU Fengli,XIE Congxia,YU Shitao. Baeyer-Villiger Oxidation of Cyclic Ketones Catalyzed by Amino Alcohol Heteropoly Acid Ionic Liquid † [J]. Chem. J. Chinese Universities, 2020, 41(5): 1101. |
| [10] | GAO Naiwei, MA Qiang, HE Yonglin, WANG Yapei. Green Electronic Devices Based on Ionic Liquids † [J]. Chem. J. Chinese Universities, 2020, 41(5): 901. |
| [11] | CHENG Shifu,HU Hao,CHEN Bihua,WU Haihong,GAO Guohua,HE Mingyuan. Preparation and Electrochemical Performance of Porous Carbons Prepared from Binary Ionic Liquids † [J]. Chem. J. Chinese Universities, 2020, 41(5): 1048. |
| [12] | PIAO Huilan,MA Pinyi,QIN Zucheng,JIANG Yanxiao,SUN Ying,WANG Xinghua,SONG Daqian. Determination of Triazine Herbicides from Fruit Juice Samples Using Effervescence Assisted Microextraction Method Based on Acidic Ionic Liquid Packed Syringe [J]. Chem. J. Chinese Universities, 2020, 41(2): 228. |
| [13] | ZHANG Li,QIAN Mingchao,LIU Xueke,Gao Shuaitao,YU Jiang,XIE Haishen,WANG Hongbin,SUN Fengjiang,SU Xianghong. Dynamic Study of Oxidative Desulfurization by Iron-based Ionic Liquids/NHD † [J]. Chem. J. Chinese Universities, 2020, 41(2): 317. |
| [14] | WANG Nan,YAO Kaisheng,ZHAO Chenchen,LI Tianjin,LU Weiwei. Ionic Liquid-assisted Synthesis of AuPd Nanosponges and Their Catalytic Performance † [J]. Chem. J. Chinese Universities, 2020, 41(1): 62. |
| [15] | LI Chenguang, HUA Er, LIU Tianxia. Tribological Behaviour of Protic Ionic Liquid Composed of 2-Ethylhexylethylenediaminium Cation and Trifluoromethanesulfonate Anion as Liquid Paraffin Additive† [J]. Chem. J. Chinese Universities, 2019, 40(7): 1411. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
