

Chem. J. Chinese Universities ›› 2014, Vol. 35 ›› Issue (3): 582.doi: 10.7503/cjcu20131000
• Physical Chemistry • Previous Articles Next Articles
CHENG Xiaoguang1, ZHAO Jigang1,*( ), WANG Lei1,2, REN Ruofan1,2, YANG Henghua2, SHEN Benxian1
), WANG Lei1,2, REN Ruofan1,2, YANG Henghua2, SHEN Benxian1
Received:2013-10-08
Online:2014-03-10
Published:2019-08-01
Contact:
ZHAO Jigang
E-mail:zjg@ecust.edu.cn
Supported by:CLC Number:
TrendMD:
CHENG Xiaoguang, ZHAO Jigang, WANG Lei, REN Ruofan, YANG Henghua, SHEN Benxian. Effect of Surface Oxygen Groups on the Two-component Non-mercury AuCl3-CuCl2/C Catalyst†[J]. Chem. J. Chinese Universities, 2014, 35(3): 582.
| Sample | |||
|---|---|---|---|
| C | 713.8 | 0.0673 | 8.32 |
| HNO3-C | 699.4 | 0.0705 | 8.68 |
| He500-C | 700.6 | 0.0683 | 8.58 |
| He700-C | 695.4 | 0.0679 | 8.95 |
| He1000-C | 694.9 | 0.0676 | 9.58 |
Table 1 Textural data of the active carbons before and after different pre-treatments
| Sample | |||
|---|---|---|---|
| C | 713.8 | 0.0673 | 8.32 |
| HNO3-C | 699.4 | 0.0705 | 8.68 |
| He500-C | 700.6 | 0.0683 | 8.58 |
| He700-C | 695.4 | 0.0679 | 8.95 |
| He1000-C | 694.9 | 0.0676 | 9.58 |
| Sample | Surface functional group(mass fraction, %) | Surface element(mass fraction, %) | ||||||
|---|---|---|---|---|---|---|---|---|
| Phenol | Carbonyl | Carboxyl | Lactone | C—C | O | C | ||
| C | 10.75 | 5.40 | 1.66 | 11.91 | 70.28 | 18.7 | 77.4 | |
| HNO3-C | 23.61 | 6.33 | 6.86 | 3.11 | 60.09 | 25.2 | 69.5 | |
| He500-C | 9.83 | 6.54 | 0.68 | 10.83 | 71.68 | 15.6 | 80.9 | |
| He700-C | 5.03 | 5.99 | 0.45 | 6.43 | 81.90 | 14.8 | 81.8 | |
| He1000-C | 3.21 | 3.32 | 0.08 | 3.28 | 90.11 | 10.6 | 86.8 | |
Table 2 Results of the fits of the C1s region and C, O contents from XPS for the samples
| Sample | Surface functional group(mass fraction, %) | Surface element(mass fraction, %) | ||||||
|---|---|---|---|---|---|---|---|---|
| Phenol | Carbonyl | Carboxyl | Lactone | C—C | O | C | ||
| C | 10.75 | 5.40 | 1.66 | 11.91 | 70.28 | 18.7 | 77.4 | |
| HNO3-C | 23.61 | 6.33 | 6.86 | 3.11 | 60.09 | 25.2 | 69.5 | |
| He500-C | 9.83 | 6.54 | 0.68 | 10.83 | 71.68 | 15.6 | 80.9 | |
| He700-C | 5.03 | 5.99 | 0.45 | 6.43 | 81.90 | 14.8 | 81.8 | |
| He1000-C | 3.21 | 3.32 | 0.08 | 3.28 | 90.11 | 10.6 | 86.8 | |
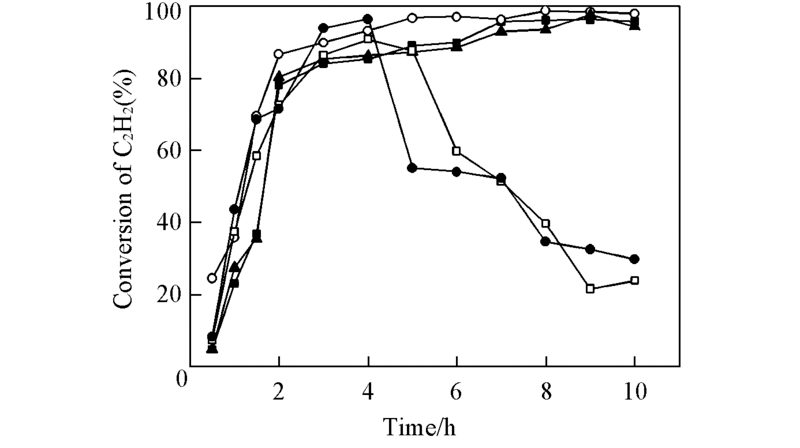
Fig.3 Variation curves of the C2H2 conversion with the reaction time for different catalysts —■— AuCl3-CuCl2/C; —○— AuCl3-CuCl2/HNO3-C; —▲— AuCl3-CuCl2/He500-C; —□— AuCl3-CuCl2/He700-C; —●— AuCl3-CuCl2/He1000-C.
| Sample | Fresh | Used | |||||
|---|---|---|---|---|---|---|---|
| SBET/(m2·g-1) | Vp/(cm3·g-1) | Dp/nm | SBET/(m2·g-1) | Vp/(cm3·g-1) | Dp/nm | ||
| AuCl3-CuCl2/C | 711.5 | 0.0662 | 8.32 | 650.6 | 0.0641 | 8.21 | |
| AuCl3-CuCl2/HNO3-C | 698.1 | 0.0683 | 8.68 | 661.8 | 0.0647 | 8.49 | |
| AuCl3-CuCl2/He500-C | 701.3 | 0.0683 | 8.58 | 647.7 | 0.0678 | 8.65 | |
| AuCl3-CuCl2/He700-C | 690.9 | 0.0679 | 8.95 | 620.5 | 0.0620 | 8.75 | |
| AuCl3-CuCl2/He1000-C | 692.0 | 0.0659 | 9.37 | 613.7 | 0.0608 | 8.92 | |
Table 3 Textural data of the as-prepared AuCl3-CuCl2/C catalysts
| Sample | Fresh | Used | |||||
|---|---|---|---|---|---|---|---|
| SBET/(m2·g-1) | Vp/(cm3·g-1) | Dp/nm | SBET/(m2·g-1) | Vp/(cm3·g-1) | Dp/nm | ||
| AuCl3-CuCl2/C | 711.5 | 0.0662 | 8.32 | 650.6 | 0.0641 | 8.21 | |
| AuCl3-CuCl2/HNO3-C | 698.1 | 0.0683 | 8.68 | 661.8 | 0.0647 | 8.49 | |
| AuCl3-CuCl2/He500-C | 701.3 | 0.0683 | 8.58 | 647.7 | 0.0678 | 8.65 | |
| AuCl3-CuCl2/He700-C | 690.9 | 0.0679 | 8.95 | 620.5 | 0.0620 | 8.75 | |
| AuCl3-CuCl2/He1000-C | 692.0 | 0.0659 | 9.37 | 613.7 | 0.0608 | 8.92 | |
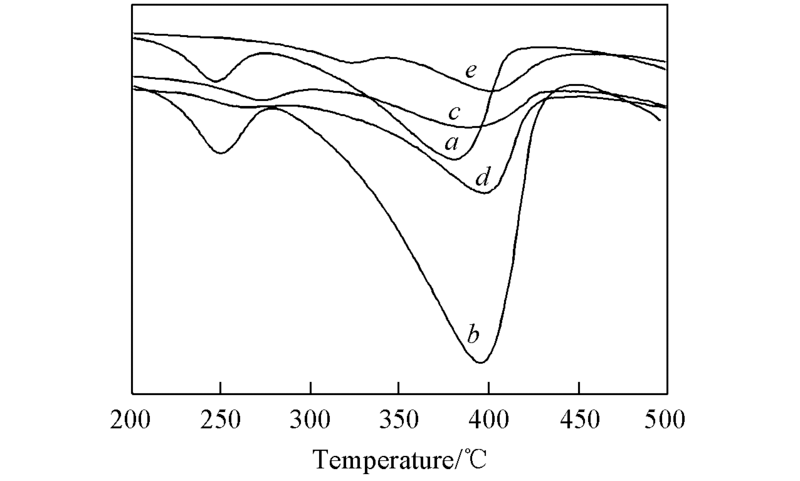
Fig.5 H2-TPR spectra for five fresh catalystsa. AuCl3-CuCl2/C; b. AuCl3-CuCl2/HNO3-C; c. AuCl3-CuCl2/He500-C; d. AuCl3-CuCl2/He700-C; e. AuCl3-CuCl2/He1000-C.
| [1] | Hutchings G. J., J. Catal., 1985, 9(1), 292—295 |
| [2] | Conte M., Carley A. F., Hutchings G. J., Catal. Lett., 2008, 124(3/4), 165—167 |
| [3] | Mitchenko S. A., Khomutov E. V., Shubin A. A., Shulga Y. M., J. Mol. Catal. A: Chem., 2004, 212(1/2), 345—352 |
| [4] | Wang S. J., Shen B. X., Xiao W. G., Song Q. L., Acta Petrolei Sinica(Petroleum Processing Section), 2010, 26(2), 201—207 |
| (王声洁, 沈本贤, 肖卫国, 宋庆雷. 石油学报(石油加工), 2010, 26(2), 201—207) | |
| [5] | Zhang H. Y., Dai B., Wang X. G., Xu L. L., Zhu M. Y., J. Ind. Eng. Chem., 2012, 18, 49—54 |
| [6] | Krasnyakova T. V., Zhikharev I. V., Mitchenko R. S., Burkhovetski V. I., Korduban A. M., Kryshchuk T. V., Mitchenko S. A., J. Catal., 2012, 288, 33—43 |
| [7] | Conte M., Carley A. F., Heirene C., Willock D. J., Johnston P., Herzing A. A., Kiely C. J., Hutchings G. J., J. Catal., 2007, 250(2), 231—239 |
| [8] | Wang S.J., Study on Non-mercury Catalytic Acetylene Hydrochlorination to Produce Vinyl Chloride Monomer(VCM), East China University of Science and Technolpgy, Shanghai, 2010 |
| (王声洁. 乙炔氢氯化非汞催化反应制取氯乙烯单体研究, 上海: 华东理工大学, 2010) | |
| [9] | Wang F. C., Gao G. J., Hu R. S., Yang X. Z., Su H. Q., Chem. Ind. Eng. Prog., 2010, 29(12), 2304—2308 |
| (王芳超, 高官俊, 胡瑞生, 杨绪壮, 苏海全. 化工进展, 2010, 29(12), 2304—2308) | |
| [10] | Xu L. L., Wang X. G., Zhang H. Y., Dai B., Liu Z. Y., Zhang Q. F., Chem. Ind. Eng. Prog., 2011, 30(3), 536—541 |
| (徐龙龙, 王绪根, 张海洋, 代斌, 刘志勇, 张清峰. 化工进展, 2011, 30(3), 536—541) | |
| [11] | Li X. Y., Ma D., Bao X. H., Chin. J. Catal., 2008, 29(3), 259—263 |
| (李晓芸, 马丁, 包信和. 催化学报, 2008, 29(3), 259—263) | |
| [12] | Boehm H. P., Carbon, 2002, 40(2), 145—149 |
| [13] | Wang S. B., Zhu Z. H., Dyes and Pigments,2007, 75(20), 306—314 |
| [14] | Aksoylu A. E., Madalena M., Freitas A., Fernando M., Pereira R, Figueiredo J. L., Carbon,2001, 39(2), 175—182 |
| [15] | Pereira M. F. R., Soares S. F., Jose Orfao J. J. M., Figueiredo J. L., Carbon,2003, 41(4), 811—821 |
| [16] | Du H., Wang C. Y., Shi Z. Q., Guo C. Y., Journal of Tianjin University,2006, 39(12), 1479—1484 |
| (杜娠, 王成扬, 时志强, 郭春雨. 天津大学学报, 2006, 39(12), 1479—1484) | |
| [17] | Da Y. X., Acta Materiae Compositae Sinica, 1994, 11(4), 14—19 |
| (笪有仙. 复合材料学报, 1994, 11(4), 14—19) | |
| [18] | Strelko J. V., Malik D. J., Streat M., Carbon., 2002, 40(1), 95—104 |
| [19] | Cordero T., Rodriguez M. J., Ind. Eng. Chem. Res., 2002, 41(24), 6042—6048 |
| [20] | Castilla C. M., Carbon, 2003, 41(9), 1157—116 |
| [21] | Moreno C. C., Lopez R. M. V., Carraseo M. F., Dyes and Pigments,2007, 75(4), 306—314 |
| [22] | Torres G. C., Jablonski E. L., Baronetti G. T., Castro A. A., Miguel S. R., Scelza O. A., Blanco M. D., Pena Jimenez M. A., Fierro J. L. G., Appl. Catal. A,1997, 161(1/2) : 213—226 |
| [23] | Hao X. Z., Zhao T. M., Jiang B., Yin M., Si F. Z., Liu C. P., Yang W. S., Chem. Res. Chinese Universities,2012, 28(6), 1074—1077 |
| [24] | Conte M., Davies C. J., Morgan D. J. A., Davies T. E., Elias D. J., Carley A. F., Johnsto P., Hutchings G. J., J. Catal., 2013, 297, 128—136 |
| [25] | Prado-Burguete C., Linares-Solano A., Rodriguez-Reinoso F., Lecea C. S. M., J. Catal., 1989, 115(1), 98—107 |
| [26] | Fung A. S., Kelley M. J., Koningsberger D. C., Gates B. C., J. Am. Chem. Soc., 1997, 119(25), 5877—5887 |
| [27] | Han W. F., Zhao B., Huo C., Liu H. Z., Chin. J. Catal., 2008, 25(3), 194—198 |
| (韩文锋, 赵波, 霍超, 刘化章. 催化学报, 2008, 25(3), 194—198) |
| [1] | CHU Yuyi, LAN Chang, LUO Ergui, LIU Changpeng, GE Junjie, XING Wei. Single-atom Cerium Sites Designed for Durable Oxygen Reduction Reaction Catalyst with Weak Fenton Effect [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220294. |
| [2] | ZHENG Anni, JIN Lei, YANG Jiaqiang, WANG Zhaoyun, LI Weiqing, YANG Fangzu, ZHAN Dongping, TIAN Zhongqun. Effects of 5,5-Dimethylhydantoin on Electroless Copper Plating [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220191. |
| [3] | WEI Chunhong, JIANG Qian, WANG Panpan, JIANG Chengfa, LIU Yuefeng. Atomic Scale Investigation of Pt Atoms/clusters Promoted Co-catalyzed Fischer-Tropsch Synthesis [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220074. |
| [4] | XU Siran, YIN Hengbo, XUE Dongping, XIA Huicong, ZHAO Shuyan, YAN Wenfu, MU Shichun, ZHANG Jianan. Atomically Dispersed Metal-Nitrogen-Carbon Catalysts for Oxygen Reduction Reaction [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220028. |
| [5] | WANG Hongning, HUANG Li, QING Jiang, MA Tengzhou, JIANG Wei, HUANG Weiqiu, CHEN Ruoyu. Activation of Biochar from Cattail and the VOCs Adsorption Application [J]. Chem. J. Chinese Universities, 2022, 43(4): 20210824. |
| [6] | LI Weihui, LI Haobo, ZENG Cheng, LIANG Haoyue, CHEN Jiajun, LI Junyong, LI Huiqiao. Hot-pressed PVDF-based Difunctional Protective Layer for Lithium Metal Anodes [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210629. |
| [7] | CHANG Sihui, CHEN Tao, ZHAO Liming, QIU Yongjun. Thermal Degradation Mechanism of Bio-based Polybutylactam Plasticized by Ionic Liquids [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220353. |
| [8] | XU Yan, YANG Hongguo, NIU Huibin, TIAN Hailin, PIAO Hongguang, HUANG Yingping, FANG Yanfen. Preparation Mechanism and Application of Alcohol⁃modified Fe3O4 Magnetic Nanoparticles [J]. Chem. J. Chinese Universities, 2021, 42(8): 2564. |
| [9] | YUE Shengli, WU Guangbao, LI Xing, LI Kang, HUANG Gaosheng, TANG Yi, ZHOU Huiqiong. Research Progress of Quasi-two-dimensional Perovskite Solar Cells [J]. Chem. J. Chinese Universities, 2021, 42(6): 1648. |
| [10] | WANG Hongning, HUANG Li, SONG Fujiao, ZHU Ting, HUANG Weiqiu, ZHONG Jing, CHEN Ruoyu. Synthesis and VOCs Adsorption Properties of Hollow Carbon Nanospheres [J]. Chem. J. Chinese Universities, 2021, 42(6): 1704. |
| [11] | WANG Kunhua, YAO Jisong, YANG Junnan, SONG Yonghui, LIU Yuying, YAO Hongbin. Synthesis and Device Optimization of Highly Efficient Metal Halide Perovskite Light-emitting Diodes [J]. Chem. J. Chinese Universities, 2021, 42(5): 1464. |
| [12] | LIU Yao, DENG Zhengtao. Fast Synthesis of Highly Luminescent Two-dimensional Tin-halide Perovskites by Anti-solvent Method [J]. Chem. J. Chinese Universities, 2021, 42(12): 3774. |
| [13] | LI Boxin, YANG Junge, YIN Dezhong, GAO Chengqian, ZHANG Qiuyu. Preparation of Large-sized Microencapsulated Phase Change Materials Through Pickering Emulsion Stabilized by Monodisperse Polymer Microspheres [J]. Chem. J. Chinese Universities, 2020, 41(9): 2085. |
| [14] | ZHANG Jun, WANG Bin, PAN Li, MA Zhe, LI Yuesheng. Synthesis and Properties of Imidazolium-based Polyethylene Ionomer [J]. Chem. J. Chinese Universities, 2020, 41(9): 2070. |
| [15] | WANG Tingting, LEI Yuhan, LIN Yujuan, HUANG Jialing, LIU Cuie, ZHENG Fengying, LI Shunxing. Preparation of Liposome-terminated CsPbX3(X=Cl,Br,I) Nanocrystals and Applications in Light-emitting Diode Devices [J]. Chem. J. Chinese Universities, 2020, 41(8): 1896. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||