

高等学校化学学报 ›› 2025, Vol. 46 ›› Issue (3): 20240358.doi: 10.7503/cjcu20240358
曾湘楚1,2,3, 丁以宣1,2,3, 武哲1,2,3, 汪艳平1,2,3( ), 刘牧4
), 刘牧4
收稿日期:2024-07-18
出版日期:2025-03-10
发布日期:2024-11-19
通讯作者:
汪艳平
E-mail:wangyanpingdy@163.com
基金资助:
ZENG Xiangchu1,2,3, DING Yixuan1,2,3, WU Zhe1,2,3, WANG Yanping1,2,3( ), LIU Mu4
), LIU Mu4
Received:2024-07-18
Online:2025-03-10
Published:2024-11-19
Contact:
WANG Yanping
E-mail:wangyanpingdy@163.com
Supported by:摘要:
废水中喹诺酮类抗生素作为新污染物引起了广泛的关注, 而选择性净化是解决此问题的有效方法之一. 本文提出了一种铜络合物活化过氧单硫酸盐(PMS)的均相类Fenton氧化体系, 用于去除水体微量喹诺酮(QNs). 在较宽的pH范围内, 99%以上的QNs在60 min内可被降解, 且免受天然有机质(质量分数高达1%)和各种阴离子(质量分数高达20%)的影响. Cu(Ⅱ)-QNs络合物活化PMS伴随Cu(Ⅲ)-QNs络合物的原位生成, 通过分子内电子转移过程促进了QNs的选择性氧化. 所产生的Cu(Ⅲ)和·OH在QNs的降解中起到主要和次要作用.
中图分类号:
TrendMD:
曾湘楚, 丁以宣, 武哲, 汪艳平, 刘牧. 原位Cu络合调控类芬顿氧化强化水体痕量喹诺酮的选择性净化. 高等学校化学学报, 2025, 46(3): 20240358.
ZENG Xiangchu, DING Yixuan, WU Zhe, WANG Yanping, LIU Mu. In situ Cupric Complexation Regulated Fenton-like Oxidation to Enhance the Selective Decontamination of Trace Aqueous Quinolones. Chem. J. Chinese Universities, 2025, 46(3): 20240358.
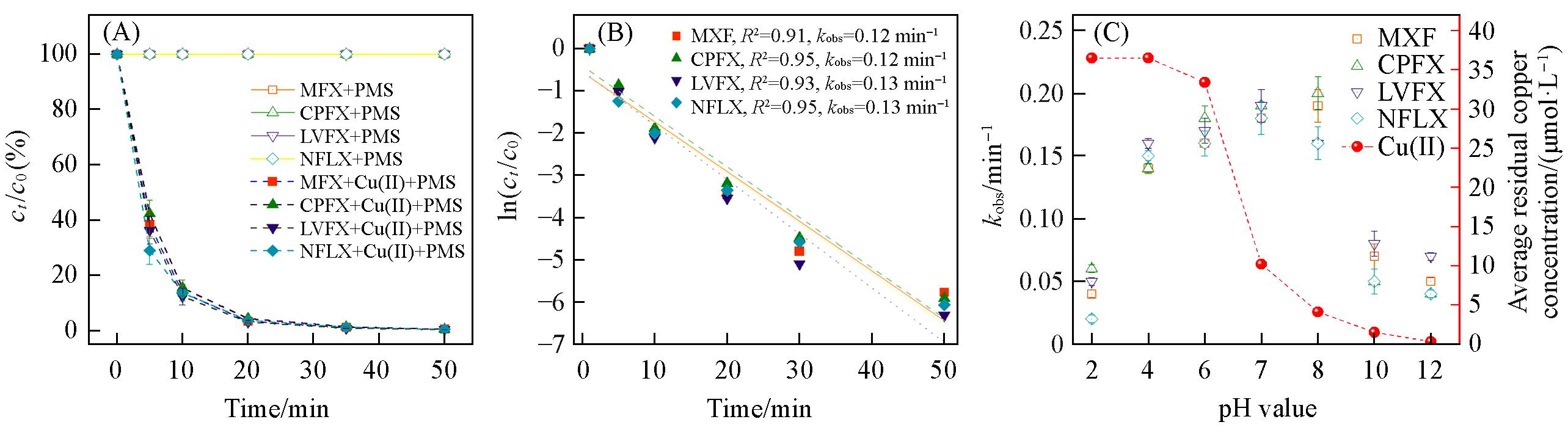
Fig.1 Degradation kinetics(A), degradation kinetics parameters(kobs) of QNs(B) and effect of pH on QNs kobs and average residual copper concentration(C)(B) cCu(Ⅱ)=1 mg/L, cQNs=10 mg/L, VCu(Ⅱ)/VQNs=1∶1, V=50 mL, mPMS=5 mg.
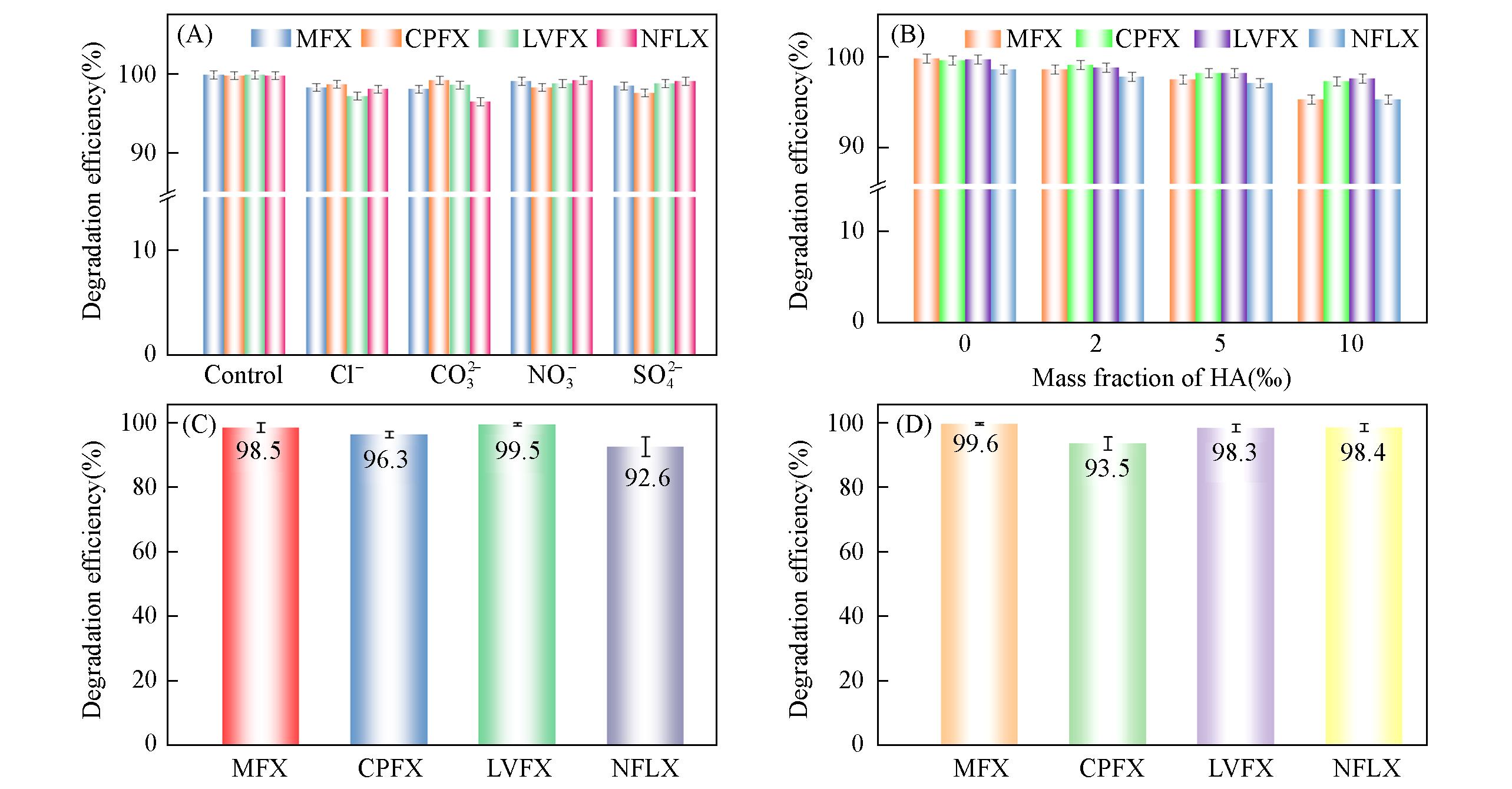
Fig.2 Effect of coexisting anions(20%)(A), and coexisting NOM(B), degradation efficiency of QNs in tap water(C) and lake water(D)pH=8, cCu(Ⅱ)=1 mg/L, cQNs=10 mg/L, VCu(Ⅱ)/VQNs=1∶1, V=50 mL, mPMS=5 mg.
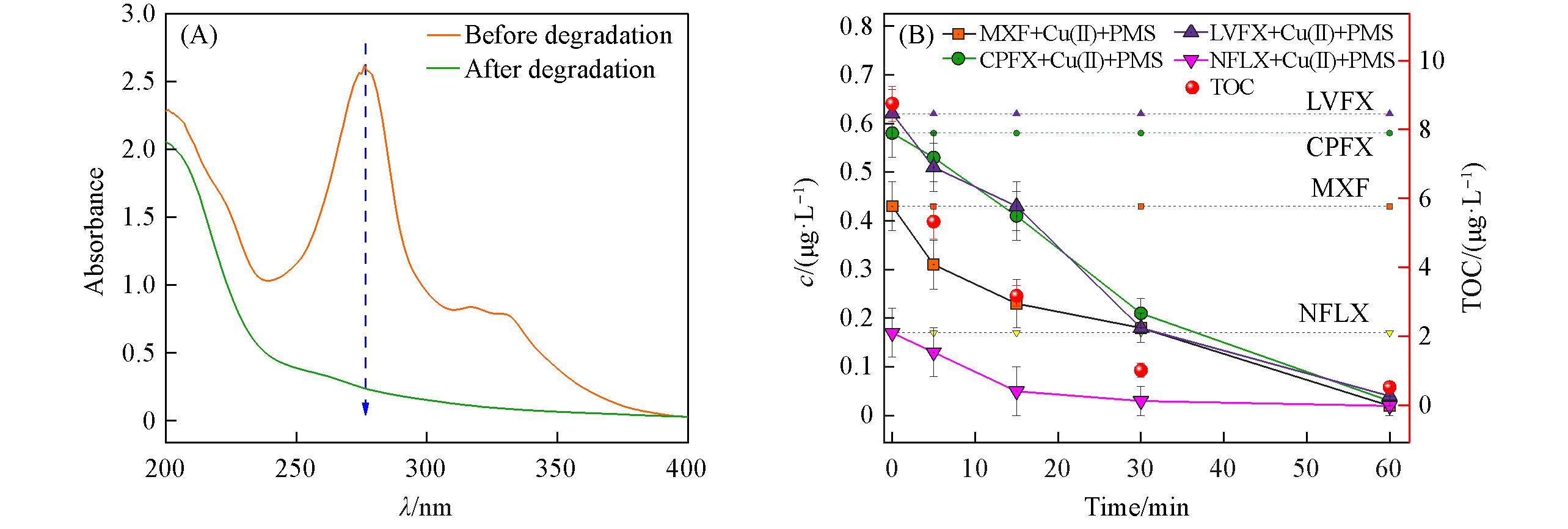
Fig.3 Degradation performance of the mixed 4 antibiotics solution in tap water(cQNs=10 mg/L)(A) and degradation kinetics of QNs, and TOC content change in piggery wastewater(B)
| Material | MXF | CPFX | LVFX | NFLX | Cu(Ⅱ) | TOC |
|---|---|---|---|---|---|---|
| Content/(μg‧L‒1) | 0.43 | 0.58 | 0.62 | 0.17 | 372.46 | 8.75 |
Table 1 Content of MXF, CPFX, LVFX, NFLX, Cu(Ⅱ) and the TOC in real breeding wastewater
| Material | MXF | CPFX | LVFX | NFLX | Cu(Ⅱ) | TOC |
|---|---|---|---|---|---|---|
| Content/(μg‧L‒1) | 0.43 | 0.58 | 0.62 | 0.17 | 372.46 | 8.75 |
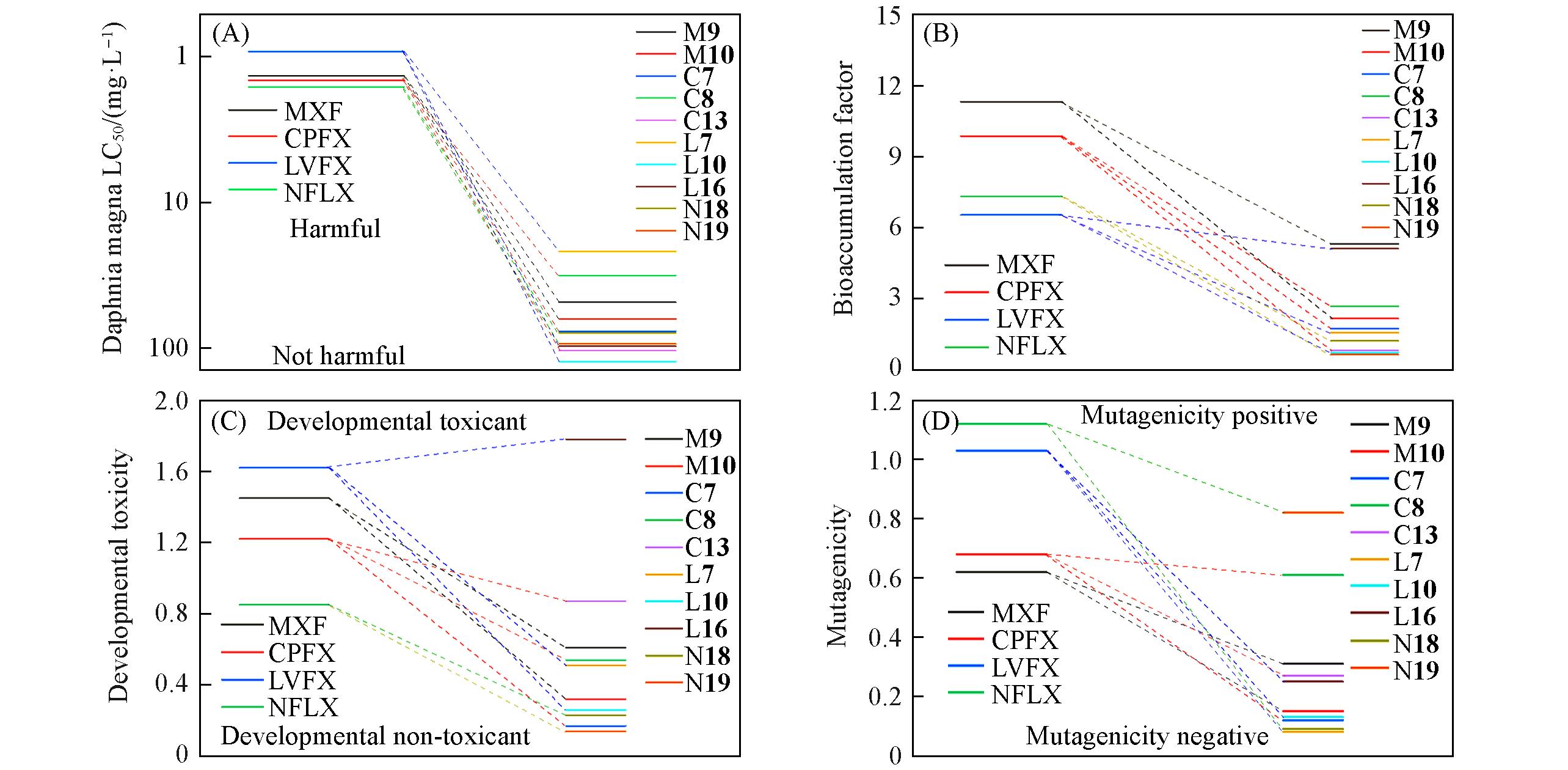
Fig.4 Toxicity assessment of QNs and its intermediates(A) Daphnia magna LC50; (B) bioaccumulation factor; (C) developmental toxicity value; (D) mutagenic developmental toxicity.
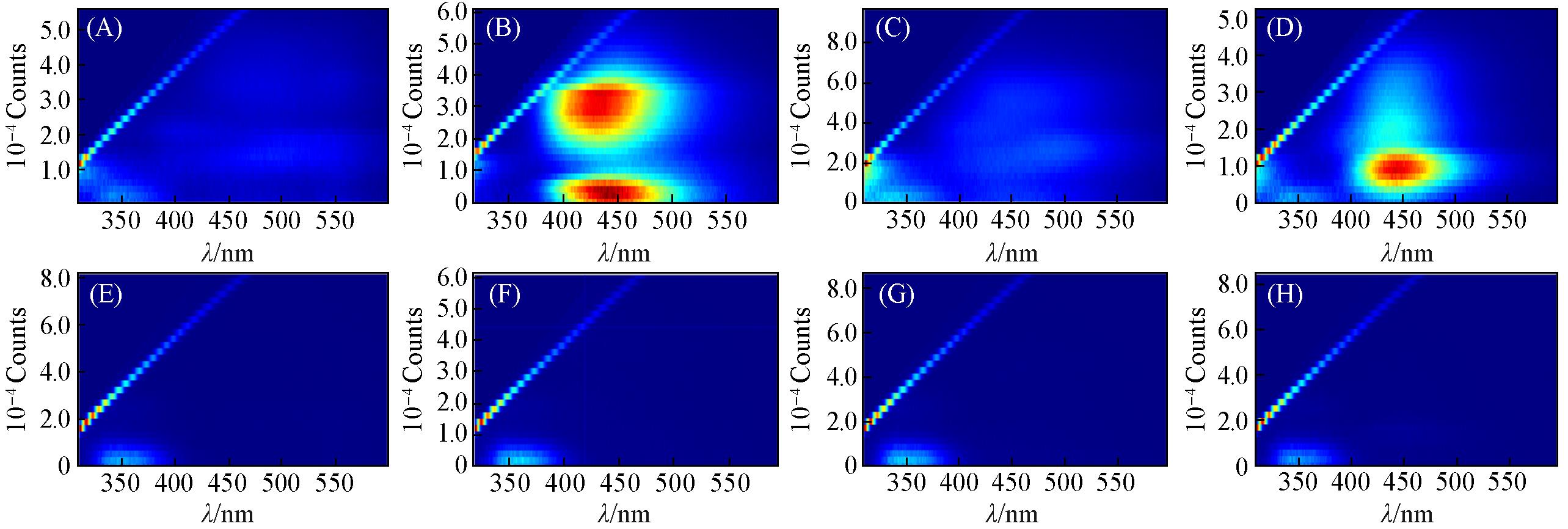
Fig.5 Three⁃dimensional fluorescence spectra of QNs before and after degradation for moxifloxacin(A, E), ciprofloxacin(B, F), levofloxacin(C, G) and norfloxacin(D, H)
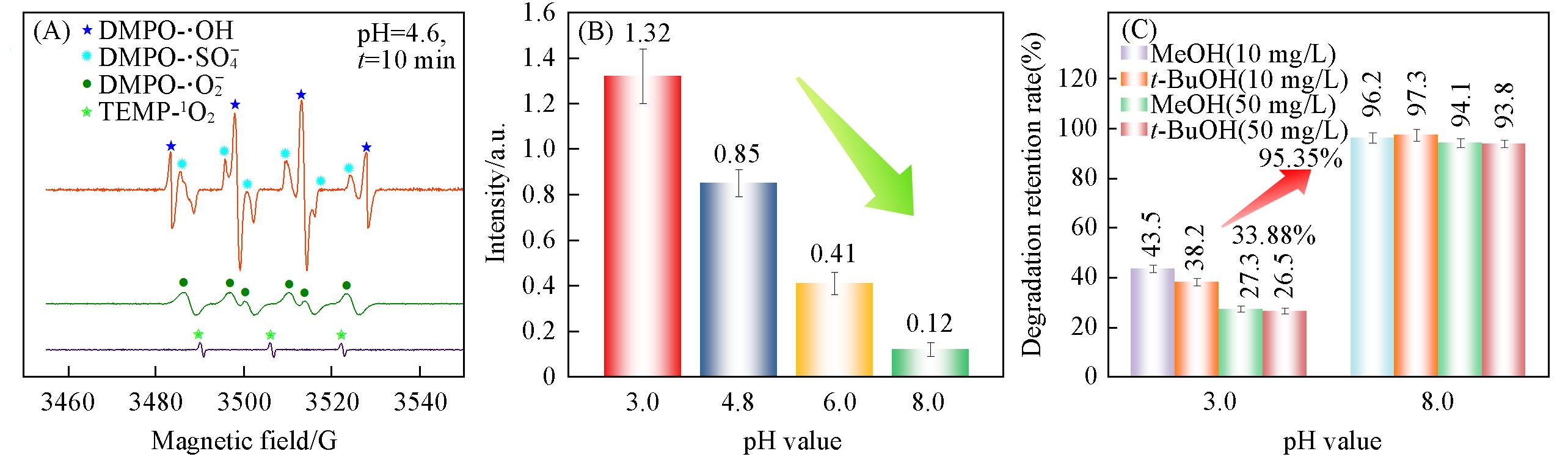
Fig.6 DMPO⁃·OH/·SO4‒ /·O2‒ and TEMP⁃1O2 analyses by EPR(A), DMPO⁃·OH intensity at different pH(B), degradation retention rate of QNs after adding scavengers(MeOH and t⁃BuOH with concentration of 10 and 50 mg/L) under acidic and alkaline condition(C)
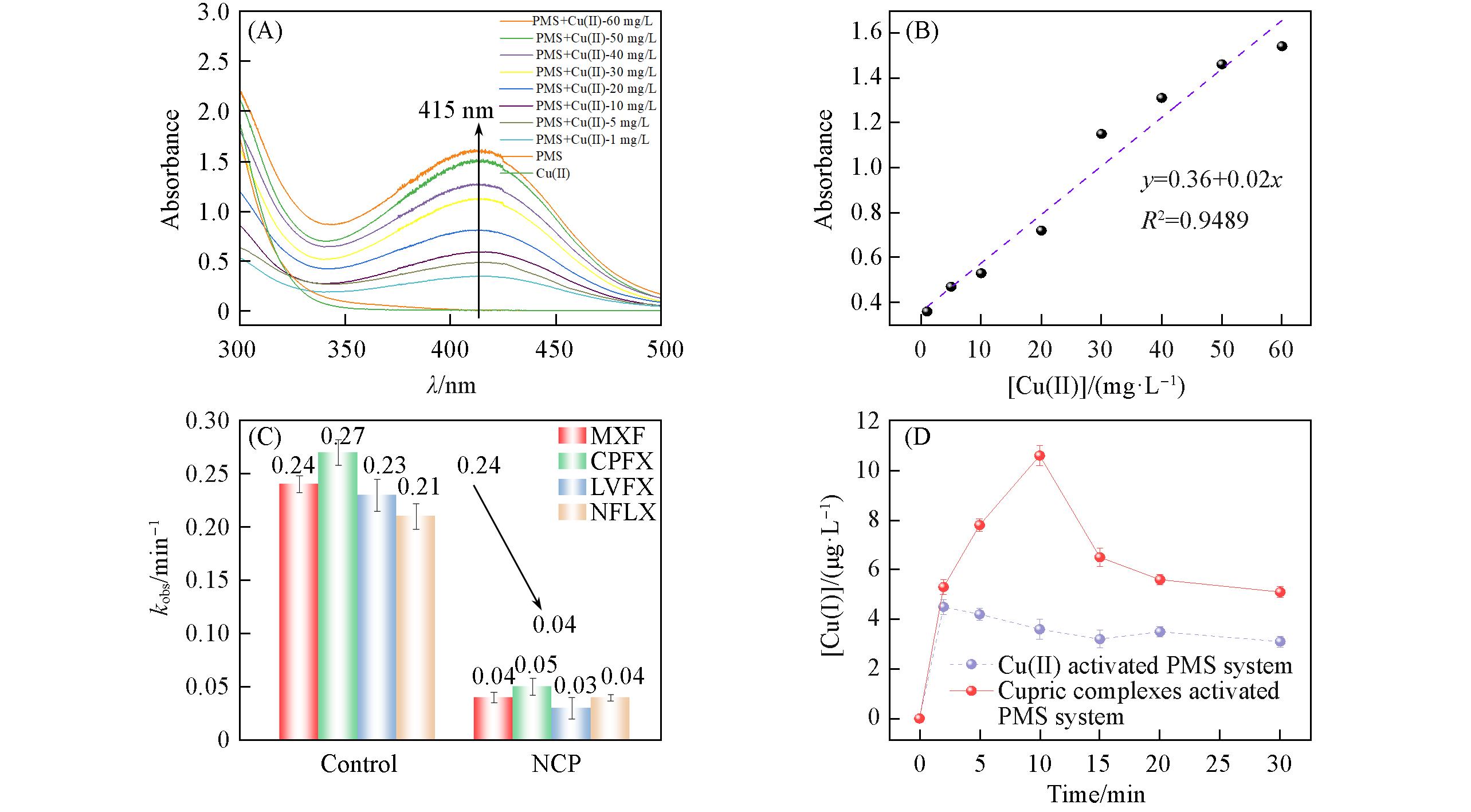
Fig.7 UV⁃Vis spectra of Cu(Ⅲ)⁃periodate complexes(A), influence of the concentration of Cu(Ⅱ) in the absorbance of periodate Cu(Ⅲ) at 415 nm(B), influence of Cu(Ⅰ)⁃chelating agent neocuproine(NCP) on QNs degradation kinetics(C), consumption kinetic of Cu(Ⅰ) in Cu(Ⅱ) and cupric complexes activated PMS systems(D)cCu(Ⅱ)=1—60 mg/L, cPMS=10 mg/L, pH=8, [NaIO4]=20 mg/L, 25 ℃.
| Species | E/(kJ·mol-1) | Gcorr303 K /(kJ·mol-1) | Gcorr353 K/(kJ·mol-1) | G303 K/(kJ·mol-1) | G353 K/(kJ·mol-1) |
|---|---|---|---|---|---|
| Cu(H2O)42+ | -1.3195×106 | 17.6896 | 155.4664 | -1.3194×106 | -1.3194×106 |
| H2O | -2.0055×106 | 6.7843 | -3.0981 | -2.0050×105 | -2.0055×106 |
| H+ | 0 | 26.7801 | -32.3199 | -1.1703×103 | -1.1759×103 |
| H3O | -2.0164×106 | 41.4566 | 31.1883 | -2.0164×105 | -2.0165×105 |
| MXF | -3.6194×106 | 1001.6886 | 962.9651 | -3.6184×106 | -3.6184×106 |
| CPFX | -4.5367×106 | 1092.5257 | 1047.7872 | -4.5356×106 | -4.5357×106 |
| LVFX | -3.0128×106 | 761.3897 | 728.1063 | -3.0120×106 | -3.0121×106 |
| NFLX | -3.9301×106 | 851.0007 | 811.5053 | -3.9292×106 | -3.9293×106 |
| [Cu(Ⅱ)-MXF]+ | -3.3133×106 | 850.6436 | 816.1997 | -3.3125×106 | -3.3125×106 |
| [Cu(Ⅱ)-CPFX]+ | -4.2307×106 | 940.9031 | 900.4179 | -4.2297×106 | -4.2297×106 |
| [Cu(Ⅱ)-LVFX]+ | -2.9129×106 | 751.8198 | 719.3949 | -2.9122×106 | -2.9122×106 |
| [Cu(Ⅱ)-NFLX]+ | -3.8302×106 | 839.0074 | 799.9426 | -3.8294×106 | -3.8294×106 |
Table 2 Free energy of different species
| Species | E/(kJ·mol-1) | Gcorr303 K /(kJ·mol-1) | Gcorr353 K/(kJ·mol-1) | G303 K/(kJ·mol-1) | G353 K/(kJ·mol-1) |
|---|---|---|---|---|---|
| Cu(H2O)42+ | -1.3195×106 | 17.6896 | 155.4664 | -1.3194×106 | -1.3194×106 |
| H2O | -2.0055×106 | 6.7843 | -3.0981 | -2.0050×105 | -2.0055×106 |
| H+ | 0 | 26.7801 | -32.3199 | -1.1703×103 | -1.1759×103 |
| H3O | -2.0164×106 | 41.4566 | 31.1883 | -2.0164×105 | -2.0165×105 |
| MXF | -3.6194×106 | 1001.6886 | 962.9651 | -3.6184×106 | -3.6184×106 |
| CPFX | -4.5367×106 | 1092.5257 | 1047.7872 | -4.5356×106 | -4.5357×106 |
| LVFX | -3.0128×106 | 761.3897 | 728.1063 | -3.0120×106 | -3.0121×106 |
| NFLX | -3.9301×106 | 851.0007 | 811.5053 | -3.9292×106 | -3.9293×106 |
| [Cu(Ⅱ)-MXF]+ | -3.3133×106 | 850.6436 | 816.1997 | -3.3125×106 | -3.3125×106 |
| [Cu(Ⅱ)-CPFX]+ | -4.2307×106 | 940.9031 | 900.4179 | -4.2297×106 | -4.2297×106 |
| [Cu(Ⅱ)-LVFX]+ | -2.9129×106 | 751.8198 | 719.3949 | -2.9122×106 | -2.9122×106 |
| [Cu(Ⅱ)-NFLX]+ | -3.8302×106 | 839.0074 | 799.9426 | -3.8294×106 | -3.8294×106 |
| Reaction | ΔG303 K/(kJ·mol-1) | ΔG353 K/(kJ·mol-1) |
|---|---|---|
Cu(H2O)24 + + MXF → [Cu(Ⅱ)-MXF]+ + H2O + H3O+ Cu(H2O)24 + + CPFX → [Cu(Ⅱ)-CPFX]+ + H2O + H3O+ Cu(H2O)24 + + LVFX → [Cu(Ⅱ)-LVFX]+ + H2O + H3O+ Cu(H2O)24 + + NFLX → [Cu(Ⅱ)-NFLX]+ + H2O + H3O+ | -37.2117 | -41.9496 |
| -39.1593 | -44.0952 | |
| -36.6008 | -41.3660 | |
| -42.3994 | -47.7637 | |
Cu(H2O)24 + + MXF → [Cu(Ⅱ)-MXF]+ + 2H2O + H+ Cu(H2O)24 + + CPFX → [Cu(Ⅱ)-CPFX]+ + 2H2O + H+ Cu(H2O)24 + + LVFX → [Cu(Ⅱ)-LVFX]+ + 2H2O + H+ Cu(H2O)24 + + NFLX → [Cu(Ⅱ)-NFLX]+ + 2H2O + H+ | -103.2055 | -113.1061 |
| -105.1540 | -115.2516 | |
| -102.5954 | -112.5224 | |
| -108.3945 | -118.9202 |
Table 3 Free energy variation of the reactions
| Reaction | ΔG303 K/(kJ·mol-1) | ΔG353 K/(kJ·mol-1) |
|---|---|---|
Cu(H2O)24 + + MXF → [Cu(Ⅱ)-MXF]+ + H2O + H3O+ Cu(H2O)24 + + CPFX → [Cu(Ⅱ)-CPFX]+ + H2O + H3O+ Cu(H2O)24 + + LVFX → [Cu(Ⅱ)-LVFX]+ + H2O + H3O+ Cu(H2O)24 + + NFLX → [Cu(Ⅱ)-NFLX]+ + H2O + H3O+ | -37.2117 | -41.9496 |
| -39.1593 | -44.0952 | |
| -36.6008 | -41.3660 | |
| -42.3994 | -47.7637 | |
Cu(H2O)24 + + MXF → [Cu(Ⅱ)-MXF]+ + 2H2O + H+ Cu(H2O)24 + + CPFX → [Cu(Ⅱ)-CPFX]+ + 2H2O + H+ Cu(H2O)24 + + LVFX → [Cu(Ⅱ)-LVFX]+ + 2H2O + H+ Cu(H2O)24 + + NFLX → [Cu(Ⅱ)-NFLX]+ + 2H2O + H+ | -103.2055 | -113.1061 |
| -105.1540 | -115.2516 | |
| -102.5954 | -112.5224 | |
| -108.3945 | -118.9202 |
| Complex | Bond length/nm | Mayer bond order | NPA charge | Ionization potential/eV | ||||
|---|---|---|---|---|---|---|---|---|
| Cu—Ow | Cu—Ocarboxyl | Cu—Ocarbonyl | Cu—Ow | Cu—Ocarboxyl | Cu—Ocarbonyl | Cu(Ⅱ) | ||
| Cu(H2O)24 + | 0.1981 | — | — | 0.434 | — | — | 1.242 | 10.03 |
| [Cu(Ⅱ)⁃MXF]+ | 0.2014 | 0.1877 | 0.1893 | 0.403 | 0.627 | 0.571 | 1.070 | 5.43 |
| [Cu(Ⅱ)⁃CPFX]+ | 0.2010 | 0.1877 | 0.1894 | 0.402 | 0.626 | 0.568 | 1.072 | 5.25 |
| [Cu(Ⅱ)⁃LVFX]+ | 0.2012 | 0.1877 | 0.1895 | 0.405 | 0.625 | 0.565 | 1.073 | 5.03 |
| [Cu(Ⅱ)⁃NFLX]+ | 0.2013 | 0.1877 | 0.1898 | 0.404 | 0.627 | 0.570 | 1.071 | 5.26 |
Table 4 DFT calculation properties of different cupric complexes
| Complex | Bond length/nm | Mayer bond order | NPA charge | Ionization potential/eV | ||||
|---|---|---|---|---|---|---|---|---|
| Cu—Ow | Cu—Ocarboxyl | Cu—Ocarbonyl | Cu—Ow | Cu—Ocarboxyl | Cu—Ocarbonyl | Cu(Ⅱ) | ||
| Cu(H2O)24 + | 0.1981 | — | — | 0.434 | — | — | 1.242 | 10.03 |
| [Cu(Ⅱ)⁃MXF]+ | 0.2014 | 0.1877 | 0.1893 | 0.403 | 0.627 | 0.571 | 1.070 | 5.43 |
| [Cu(Ⅱ)⁃CPFX]+ | 0.2010 | 0.1877 | 0.1894 | 0.402 | 0.626 | 0.568 | 1.072 | 5.25 |
| [Cu(Ⅱ)⁃LVFX]+ | 0.2012 | 0.1877 | 0.1895 | 0.405 | 0.625 | 0.565 | 1.073 | 5.03 |
| [Cu(Ⅱ)⁃NFLX]+ | 0.2013 | 0.1877 | 0.1898 | 0.404 | 0.627 | 0.570 | 1.071 | 5.26 |
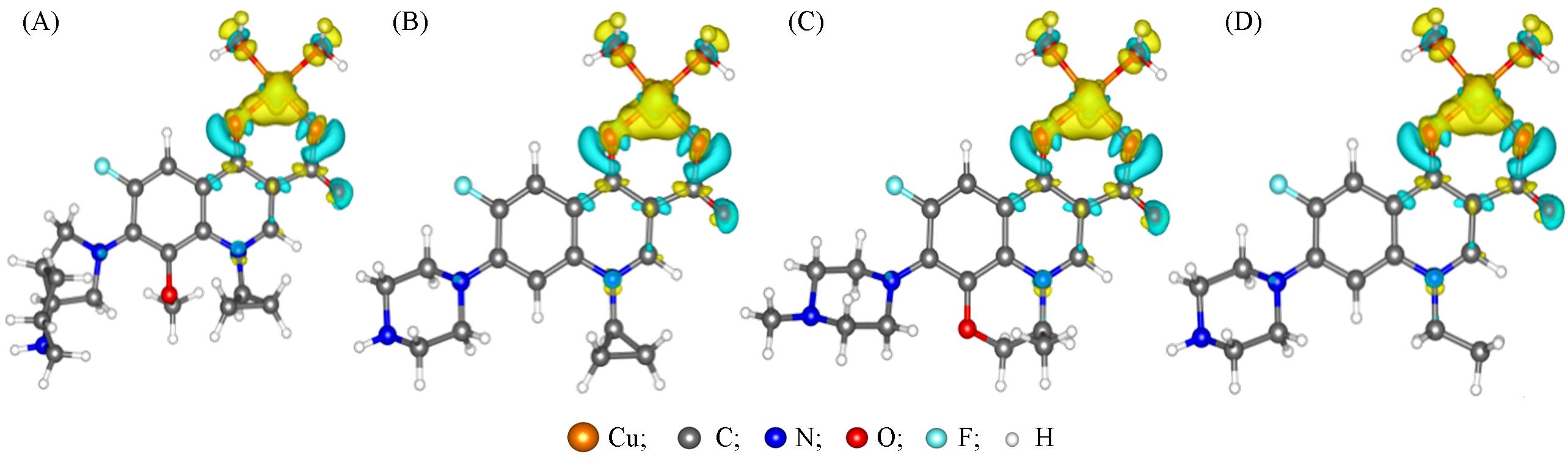
Fig.10 Differential electron density of the cupric complexes(A) [Cu(II)-MXF]+; (B) [Cu(II)-CPFX]+; (C) [Cu(II)-LVFX]+; (D) [Cu(II)-NFLX]+. The yellow and blue isosurfaces(+0.005 and ‒0.005 a.u.) indicate increased and decreased electon densily, respectively.
| 1 | Zeng X. C., Zhu J. F., Zhang G. H., Wu Z., Lu J. Y., Ji H. D., Chem. Eng. J., 2023, 468, 143536 |
| 2 | Schwarzenbach R. P., Escher B. I., Fenner K., Hofstetter T. B., Johnson C. A., von Gunten U., Wehrli B., Science, 2006, 313(5790), 1072—1077 |
| 3 | Yang X. B., Zeng X. C., Chen H. C., Xin L., Pan J. J., Ji H. D., Cheng K. J., Chem. Eng. J., 2024, 483, 148697 |
| 4 | Mulchandani R., Wang Y., Gilbert M., Van Boeckel T. P., Nature News, 2023, 3, e0001305 |
| 5 | Wu J. Y., Gao J. M., Guo J. S., Hou X. Y., Wang D. R., Wu J. C., Li X. J., Jia C. Y., J. Hazard. Mater., 2023, 445, 130570 |
| 6 | Zhang Q. Q., Ji X. M., Tian G. M., Jin R. C., Bioresource Technol., 2021, 319, 124106 |
| 7 | Li J. Y., Pham A. N., Dai R., Wang Z. W., Waite T. D., J. Hazard. Mater., 2020, 392, 122261 |
| 8 | Zeng X. C., Zhang G. H., Wen J., Li X. L., Zhu J. F., Wu Z., Chemosphere, 2023, 318, 137869 |
| 9 | Chen J. B., Zhou X. F., Sun P. Z., Zhang Y. L., Huang C. H., Environ. Sci. Technol., 2019, 53(20), 11774—11782 |
| 10 | Miao J., Song J., Lang J. Y., Zhu Y., Dai J., Wei Y., Long M. C., Zhou B. X., Alvarez P. J. J., Zhang L. Z., Environ. Sci. Technol., 2023, 57, 4266—4275 |
| 11 | Wang S. C., Deng Y., Shao B. B., Zhu J. H., Hu Z. X., Guan X. H., Environ. Sci. Technol., 2021, 55, 11338—11347 |
| 12 | Zhu J. H., Yu F. L., Meng J. R., Shao B. B., Dong H. Y., Chu W. H., Cao T. C., Wei G.F., Wang H. J., Guan X. H., Environ. Sci. Technol., 2021, 54, 9702—9710 |
| 13 | Sun S. H., Shan C., Yang Z. C., Wang S., Pan B. C., Environ. Sci. Technol., 2022, 56, 637—641 |
| 14 | Ahmed Y., Zhong J. X., Yuan Z. G., Guo J. H., Water Res., 2021, 197, 117075 |
| 15 | Wang L. H., Xu H. D., Jiang N., Wang Z. M., Jiang J., Zhang T., Environ. Sci. Technol., 2020, 54, 4686—4694 |
| 16 | Lee H. S., Lee H. J., Seo J. W., Kim H. E., Shin Y. K., Kim J. H., Lee C. H., Environ. Sci. Technol., 2016, 50, 8231—8238 |
| 17 | Yang Y. X., Zhu J. F., Zeng Q. Z., Zeng X. C., Zhang G. H., Niu Y. H., J. Taiwan Inst. Chem. E, 2023, 145, 104775 |
| 18 | Zeng X. C., Zhang G. H., Zhu J. F., J. Environ. Manage., 2022, 314, 114979 |
| 19 | Sun J. S., Chen P., Jing L. P., Sun F. X., Liu J., Chem. J. Chinese Universities, 2022, 43(10), 20220171 |
| 孙金时, 陈鹏, 景丽萍, 孙福兴, 刘佳. 高等学校化学学报, 2022, 43(10), 20220171 | |
| 20 | Zeng Q. Z., Zhu J. F., Xiong J. J., Zha W., Liu J. H., Zeng X. C., Zhang G. H., J. Environ. Chem. Eng., 2024, 12, 114302 |
| 21 | Liu J. H., Zhu J. F., Ma X., Zeng X. C., Zhang G. H., Sun Y. H., Fan G. D., Appl. Surf. Sci., 2025, 680, 161416 |
| 22 | Li L. L., Liu Y., Song S. Y., Zhang H. J., Acta Chim. Sinica, 2022, 80, 16 |
| 李玲玲, 刘宇, 宋术岩, 张洪杰, 化学学报, 2022, 80, 16 | |
| 23 | Yuan Z. X., Zhang H., Hu S. J., Zhang B. T., Zhang J. J., Cui G. L., Acta Chim. Sinica, 2023, 81, 10649 |
| 苑志祥, 张浩, 胡思伽, 张波涛, 张建军, 崔光磊, 化学学报, 2023, 81, 1064 | |
| 24 | Zeng X. C., Qin Y., Yang X. B., Zhou J. M., Pan J. J., Luo S. M., Cheng K. J. J., Hazard. Mater., 2024, 1368, 136266 |
| 25 | Sun S. H., Wang S., Ye Y. X., Pan B. C., Water Res., 2019, 153, 21—28 |
| 26 | Wu S. Y., Wu W., Fan J. N., Zhang L. P., Zhong Y., Xu H., Mao Z. P., Water Res., 2023, 233, 119725 |
| 27 | Zhang J., Shan C., Zhang W. M., Pan B. C., PNAS, 2023, 120, e2305255120 |
| 28 | Cai X. L., Wu L. Y., Li Y. M., Lei S. H., Xu J., Lyu H., Li J. D., Wang H. J., Dong X. Z., Zhu Y. X., Wang G. L., J. Hazard. Mater., 2023, 459, 132080 |
| 29 | Zhang H. Q., Quan H. T., Yin S. Z., Sun L. P., Lu H., Environ. Sci. Technol., 2022, 56, 15941—15952 |
| 30 | He L. Y., Yang S. D., Shen S. T., Ma Y. F., Chen Y. L., Xue J. M, Wang J., Zheng L., Wu L., Zhang Z. L., Yang L., J. Hazard. Mater., 2022, 434, 128860 |
| 31 | Zheng J. L., Lin Q. T., Liu Y. X., Deng Y. R., Fan X. D., Xu K. H., Ma Y. J., He J., J. Hazard. Mater., 2024, 462, 132753 |
| 32 | Ma S. Q., Chen D. M., Zhong Y., Feng Y. M., He Z. T., He We. B., Zhang Y. M., Ding H., Wu X. F., Chem. Eng. J., 2023, 467, 143385 |
| 33 | Wang Y. S., Jiao H., Liu Z. J., Yang S. J., Chen R. Z., Liu C. G., Dai J., Ding D. H., J. Hazard. Mater., 2024, 472, 134530 |
| 34 | Wang Z., Jiang J., Pang S. Y., Zhou Y., Guan C. T., Gao Y., Li J., Yang Y., Qiu W., Jiang C. C., Environ. Sci. Technol., 2018, 52, 11276—11284 |
| 35 | Dong Y. W., Huang W. Y., Liang C., Gao Y. F., Wei Z. S., Meng L. J., Zhong F., Zhang J., Zhou L., Xu J. J., Water Process. Eng., 2024, 58, 104929 |
| 36 | Pham A. N., Xing G. W., Miller C. J., Waite T. D., J. Catal., 2013, 301, 54—64 |
| 37 | Lee H. S., Lee H. J., Sedlak D. L., Lee C. H., Chemosphere, 2013, 92, 652—658 |
| 38 | Wang J. L., Wang S. Z., Chem. Eng. J., 2018, 334, 1502—1517 |
| 39 | Zeng X. C., Zhang G. H., Zhu J. F., Wu Z., Environ. Sci. Wat. Res., 2022, 8, 907—925 |
| 40 | Duan R., Ma S. L., Xu,S. J., Wang B. B., He M. F., Li G. X., Fu H. C., Zhao P., Water Res., 2022, 218, 118489 |
| 41 | Bhatt S., Chatterjee S., Environ. Pollut., 2022, 315, 120440 |
| 42 | Ren Y., Shi M. Q., Zhang W. M., Dionysiou D. D., Lu J. H., Shan C., Zhang Y. Y., Lv L., Pan B. C., Environ. Sci. Technol., 2020, 54, 5258—5267 |
| [1] | 彭小明, 吴健群, 戴红玲, 杨展宏, 许莉, 许高平, 胡锋平. Ni-N-C单原子催化剂活化过硫酸盐降解苯酚[J]. 高等学校化学学报, 2021, 42(8): 2581. |
| [2] | 刘子乐, 曾泽泉, 杨洁杨, 崔燕, 武爱莲, 李哲, 黄张根. 表面改性活性炭活化过硫酸盐降解苯酚[J]. 高等学校化学学报, 2017, 38(7): 1241. |
| [3] | 林娟娟, 高庆宇, 宋浩, 黄振炎, 赵学庄. 一种新的ⅥA族化学反应振荡器:Na2S2O8-KSCN-CuSO4体系[J]. 高等学校化学学报, 1996, 17(8): 1292. |
| [4] | 郭新秋, 丘坤元, 冯新德. 过硫酸盐与吡咯烷体系引发丙烯酰胺聚合反应机理的研究[J]. 高等学校化学学报, 1989, 10(10): 1068. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||