

高等学校化学学报 ›› 2025, Vol. 46 ›› Issue (1): 20240245.doi: 10.7503/cjcu20240245
收稿日期:2024-05-20
出版日期:2025-01-10
发布日期:2024-08-13
通讯作者:
刘轶
E-mail:yiliuchem@jlu.edu.cn
基金资助:
CUI Yanqi, FENG Wenjie, LIU Yi( )
)
Received:2024-05-20
Online:2025-01-10
Published:2024-08-13
Contact:
LIU Yi
E-mail:yiliuchem@jlu.edu.cn
Supported by:摘要:
纳米疫苗是肿瘤免疫治疗的重要组成部分, 主要通过激活机体免疫系统抑制并清除肿瘤; 但是肿瘤组织普遍存在的免疫抑制微环境会大大降低纳米疫苗的治疗效果. 因此,设计制备既可以逆转肿瘤组织免疫抑制微环境, 又可以激活机体抗肿瘤免疫响应的纳米疫苗, 对肿瘤免疫治疗的发展具有重要意义. 本文利用铁离子(Fe3+)和紫草素(Shik)之间的配位相互作用构筑了Fe/Shik超分子纳米结构, 并通过负载鸡卵白蛋白(OVA, B16-OVA肿瘤抗原)和R837(TLR7激动剂, 佐剂)制备了OVA/R837@Fe/Shik纳米疫苗. 该纳米疫苗具有良好的胶体稳定性及抗肿瘤活性, 可以在肿瘤微环境中特异性拆解并释放Fe2+, Shik, OVA和R837, 通过诱导肿瘤细胞铁死亡和程序性坏死引发免疫原性细胞死亡并释放细胞裂解物, 细胞裂解物协同OVA以及R837共同诱导树突细胞成熟, 促进细胞毒性T淋巴细胞的激活与浸润以及巨噬细胞向M1表型极化, 从而在逆转肿瘤组织免疫抑制微环境的同时激活机体的抗肿瘤免疫反应.
中图分类号:
TrendMD:
崔彦琪, 封文洁, 刘轶. 超分子纳米疫苗用于黑色素瘤免疫治疗. 高等学校化学学报, 2025, 46(1): 20240245.
CUI Yanqi, FENG Wenjie, LIU Yi. Supramolecular Nanovaccines for Melanoma Immunotherapy. Chem. J. Chinese Universities, 2025, 46(1): 20240245.
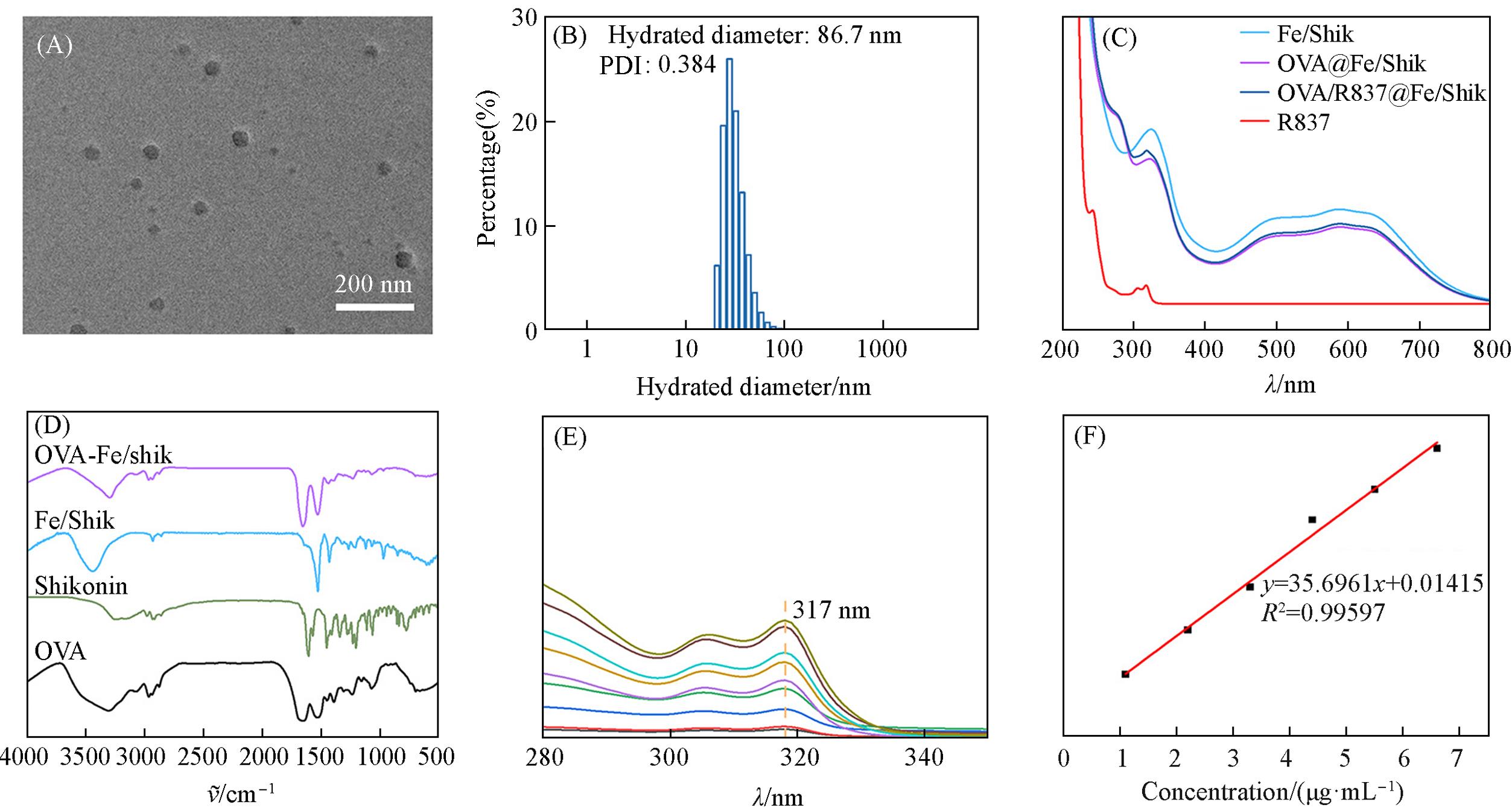
Fig.1 TEM image of OVA/R837@Fe/Shik(A), DLS hydrodynamic diameter of OVA/R837@Fe/Shik(B), UV⁃Vis absorption spectra of R837, Fe/Shik, OVA@Fe/Shik and OVA/R837@Fe/Shik(C), FTIR spectra of OVA, Shik, Fe/Shik and OVA/R837@Fe/Shik(D), UV⁃Vis absorption spectra of R837(E) and the standard absorption curve of R837(F)
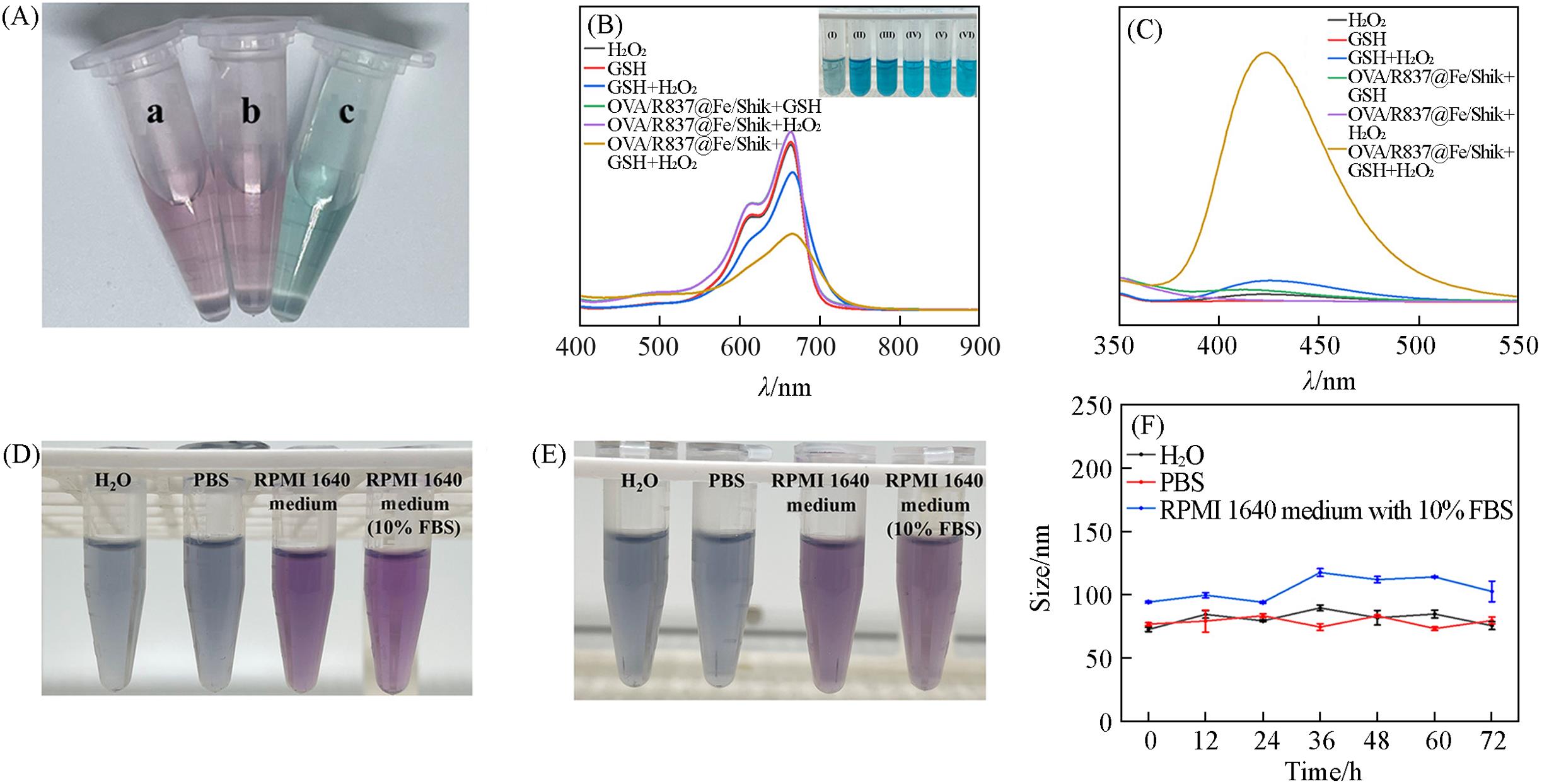
Fig.2 Color changes of the supernatant(A), UV⁃Vis absorption spectra of MB solution under different treatments(B), fluorescence spectra of disodium terephthalate under different treatments(C), physiological stability of OVA/R837@Fe/Shik in H2O, PBS, and RPMI 1640 medium with or without 10% FBS before(D) and after(E) 72 h, time⁃dependent hydrated diameter of OVA/R837@Fe/Shik in H2O, PBS and RPMI 1640 medium with 10% FBS(F)Inset of (A): color changes of the supernatant with the addition of water(a), potassium thiocyanate(b), and potassium ferricyanide(c). Inset of (B): photographs of MB solution under different treatments, I—VI: OVA/R837@Fe/Shik+GSH+H2O2, OVA/R837@Fe/Shik+H2O2, OVA/R837@Fe/Shik+GSH, GSH+H2O2, GSH, H2O2.
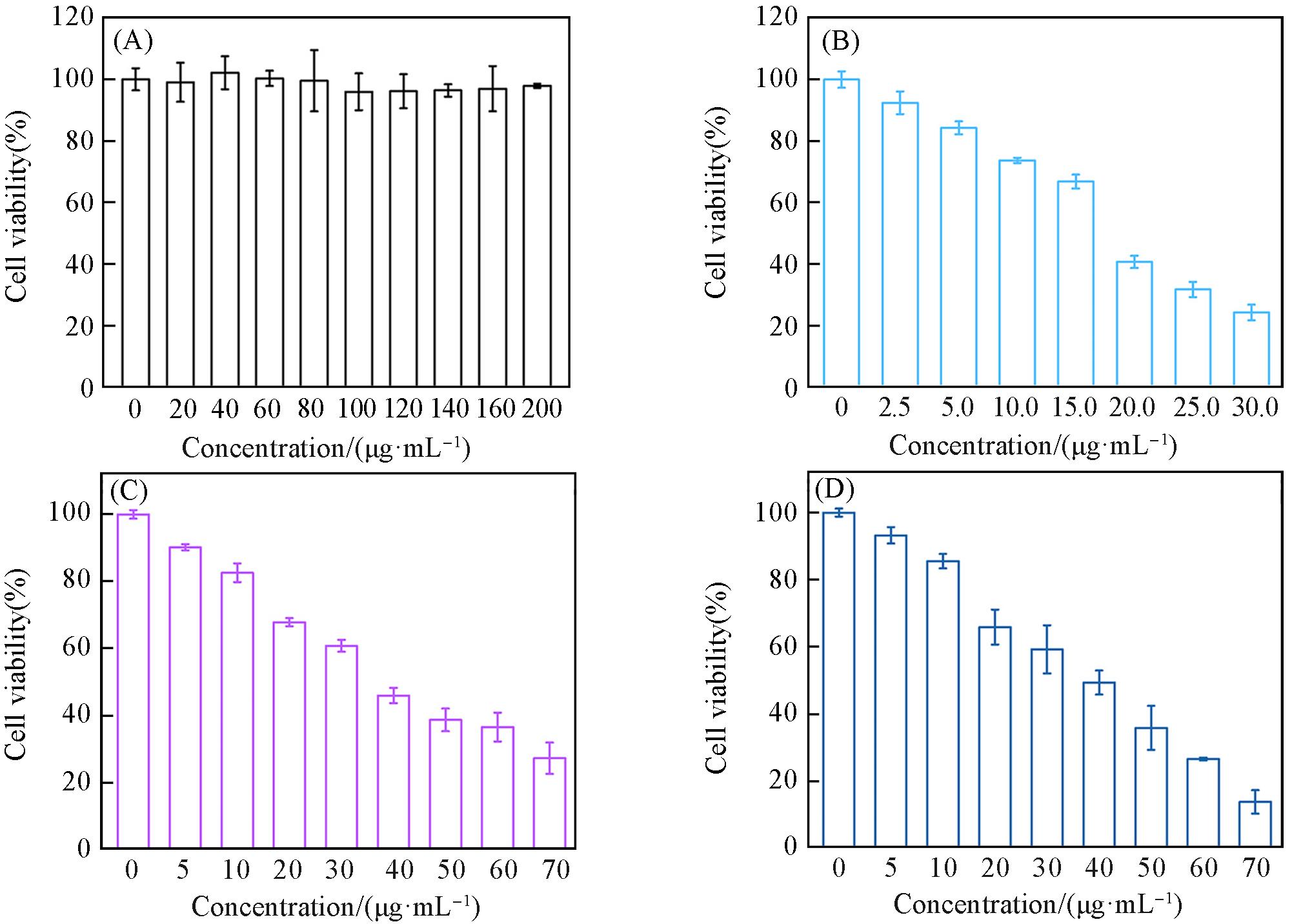
Fig.3 Relative cell viability of B16⁃OVA cells after 24 h incubation with different concentrations of OVA(A), Fe/Shik(B), OVA@Fe/Shik(C) and OVA/R837@Fe/Shik(D)Data are shown as mean±SD. n represents the number of biologically independent samples. Statistical significance was calculated by Student’s t⁃test and designated as *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
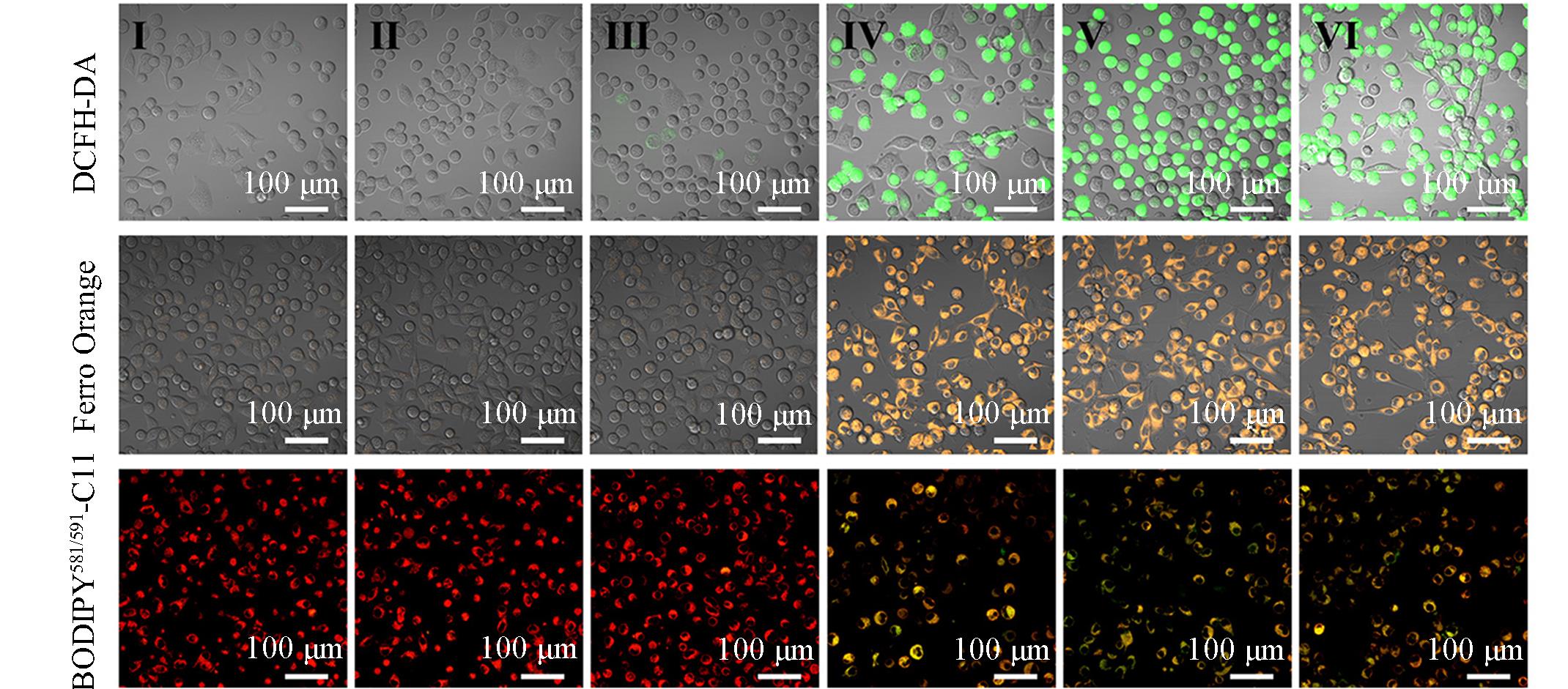
Fig.4 CLSM images of ROS, Fe2+ and LPO levels in B16⁃OVA cells after different treatmentsGroup: I(control), II(OVA), III(Shik), IV(Fe/Shik), V(OVA@Fe/Shik), VI(OVA/R837@Fe/Shik).
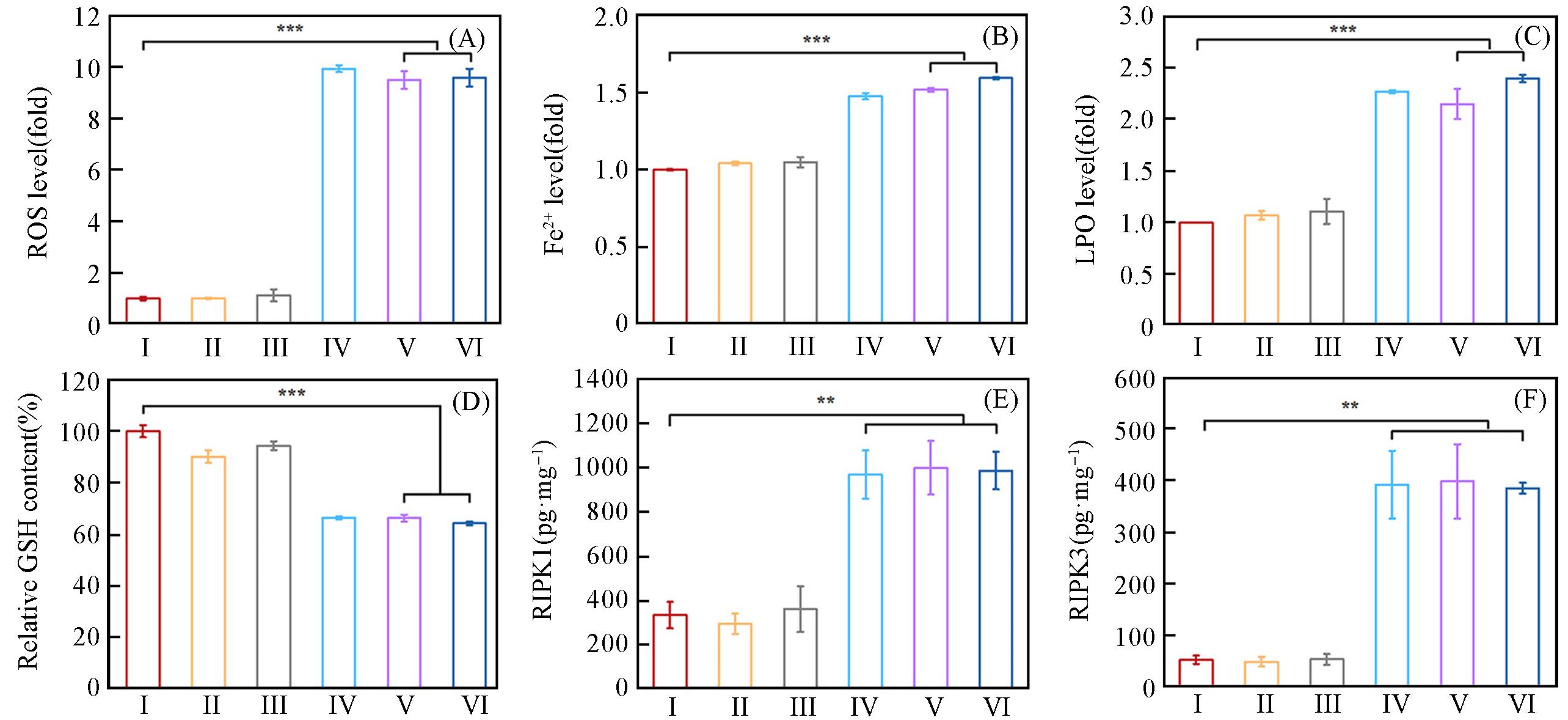
Fig.5 Quantification of ROS(A), Fe2+(B) and LPO(C) levels by flow cytometry in B16⁃OVA cells after different treatments, GSH content of B16⁃OVA cells after different treatments by GSH assay kid(D), the expression of RIPK1(E) and RIPK3(F) analyzed by ELISA assay kit after different treatmentsGroup: I(OVA), II(OVA), III(Shik), IV(Fe/Shik), V(OVA@Fe/Shik), VI(OVA/R837@Fe/Shik). Data are shown as mean±SD. n represents the number of biologically independent samples. Statistical significance was calculated by Student's t⁃test and designated as *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
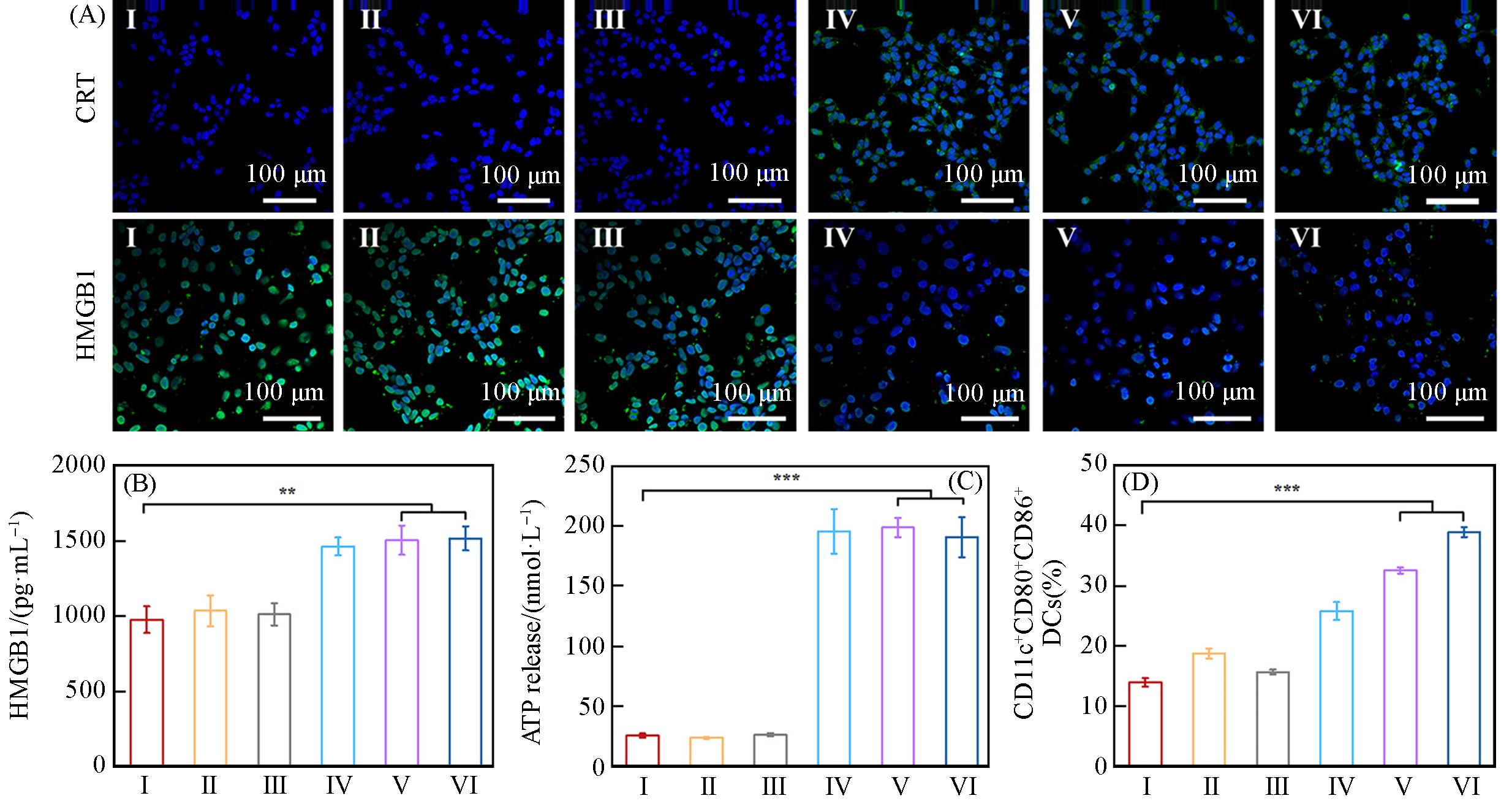
Fig.6 CLSM images of CRT and HMGB1 expression in B16⁃OVA cells after different treatments(A), the expression of HMGB1(B) and ATP(C) analyzed by ELISA assay kit after different treatments, corresponding quantification DCs maturation(CD11c+CD80+CD86+) by flow cytometry(D)Group: I(control), II(OVA), III(Shik), IV(Fe/Shik), V(OVA@Fe/Shik), VI(OVA/R837@Fe/Shik). Data are shown as mean±SD. n represents the number of biologically independent samples. Statistical significance was calculated by Student's t⁃test and designated as *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
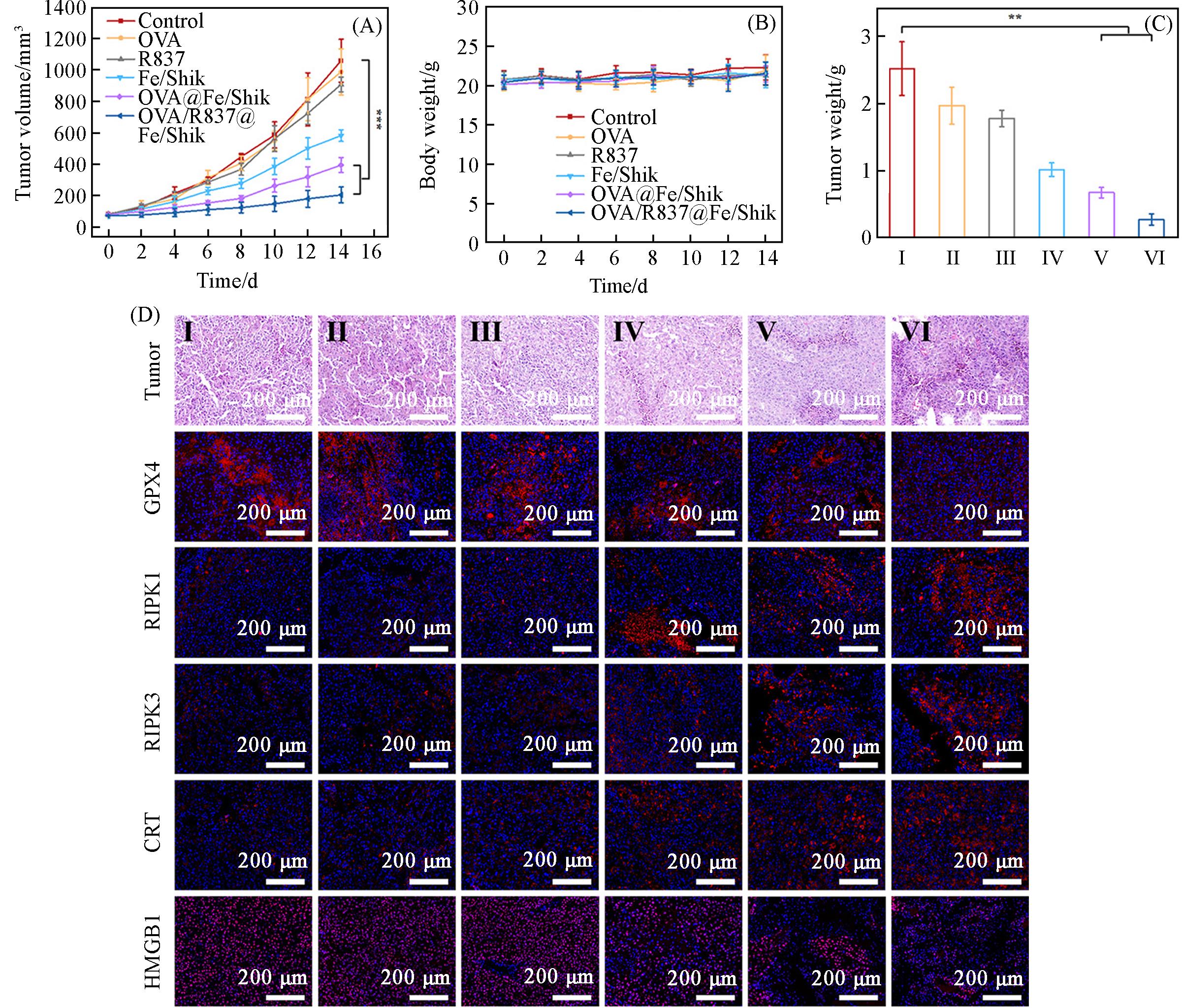
Fig.7 Average tumor growth curves(A), average body weight of mice(B) and average tumor weight(C) with various treatments, H&E⁃stained images of tumor and immunofluorescence staining images(GPX4, RIPK1, RIPK3, CRT and HMGB1) of tumor tissue slices after different treatments(D)Group: I(control), II(OVA), III(Shik), IV(Fe/Shik), V(OVA@Fe/Shik), VI(OVA/R837@Fe/Shik). Data are shown as mean±SD. n represents the number of biologically independent samples. Statistical significance was calculated by Student’s t⁃test and designated as *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
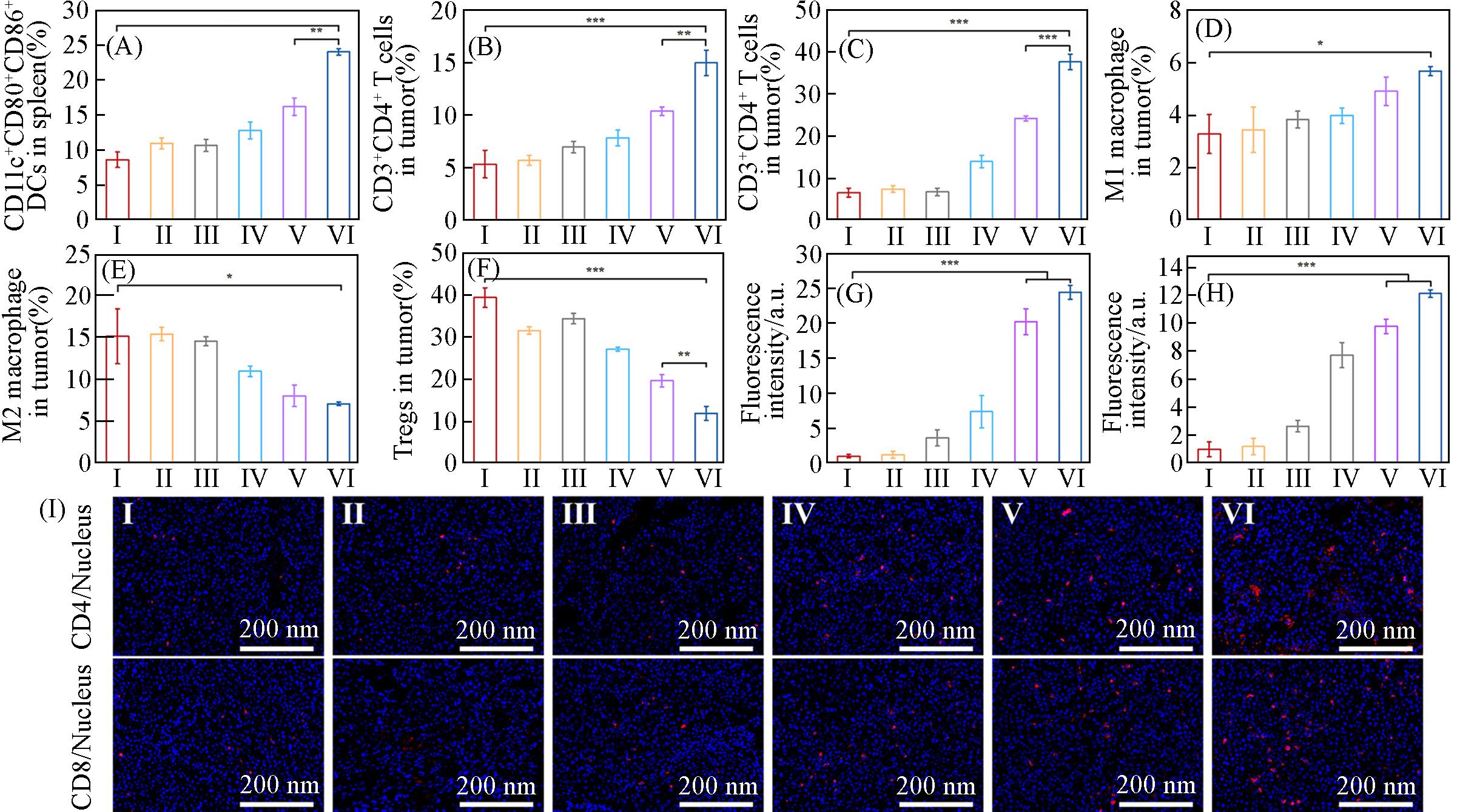
Fig.8 Corresponding quantification DCs maturation(CD11c+CD80+CD86+) by flow cytometry in spleen(A), flow cytometric statistical analysis of the percentages of T helper cells(CD3+CD4+)(B), cytotoxic T lymphocytes(CD3+CD8+)(C), M1 macrophage(D), M2 macrophage(E) and Tregs(F) in tumors after different treatments, fluorescence intensity measured by Image J(G, H) and corresponding immunofluorescence staining images(I) of CD4+ and CD8+ T cells in tumor tissue slices after different treatmentsGroup: I(control), II(OVA), III(Shik), IV(Fe/Shik), V(OVA@Fe/Shik), VI(OVA/R837@Fe/Shik). Data are shown as mean±SD. n represents the number of biologically independent samples. Statistical significance was calculated by Student’s t⁃test and designated as *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
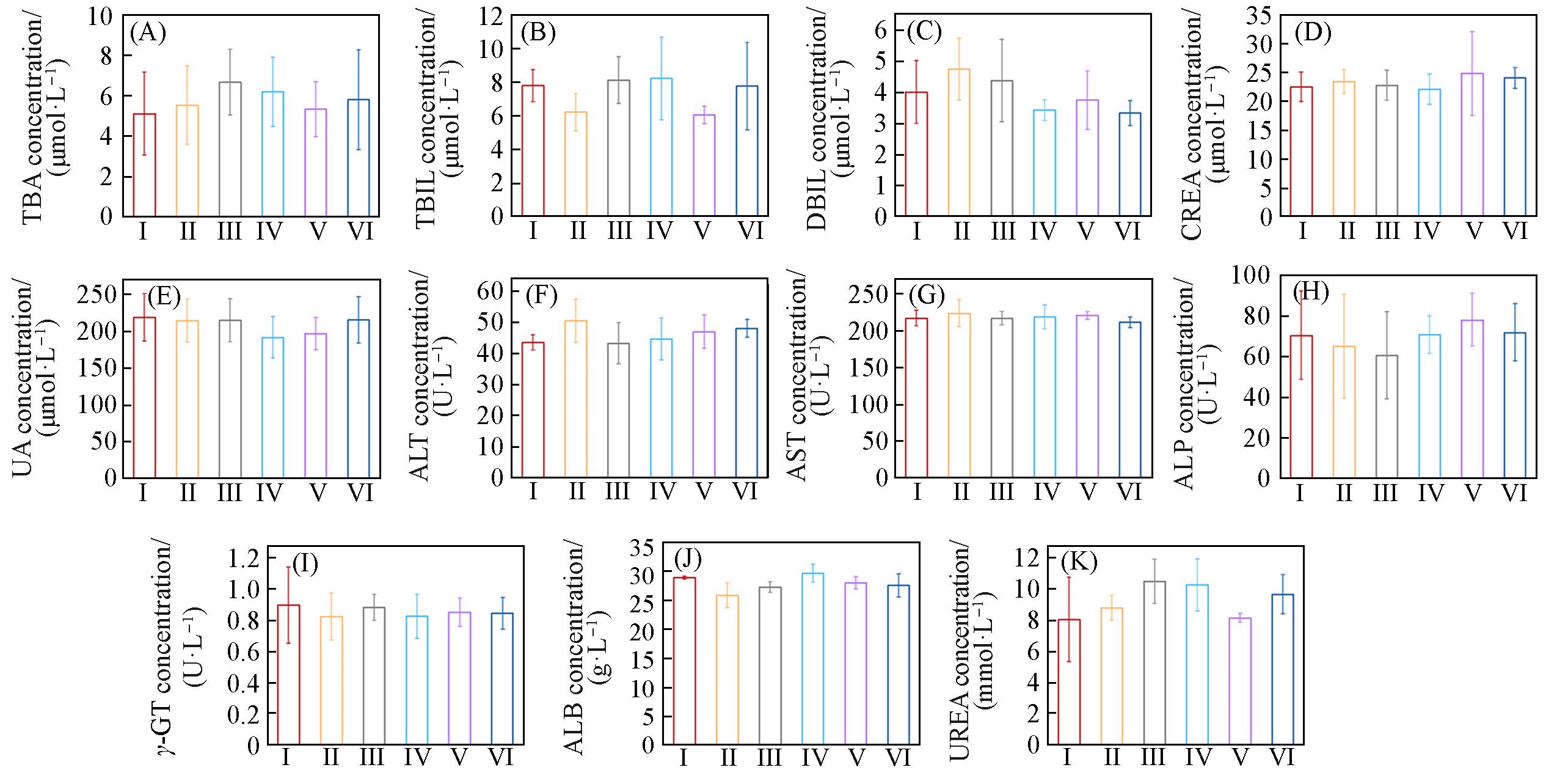
Fig.10 Main indexes of liver and renal functions tests of mice in each group(A) Total bile acid(TBA); (B) total bilirubin(TBIL); (C) direct bilirubin(DBIL); (D) creatinine(CREA); (E) uric acid(UA); (F) alanine aminotransferase(ALT); (G) aspartate transaminase(AST); (H) alkaline phosphatase(ALP); (I) γ⁃glutamyl transpeptidase; (J) albumin(ALB); (K) urea. Group: I(OVA), II(OVA), III(Shik), IV(Fe/Shik), V(OVA@Fe/Shik), VI(OVA/R837@Fe/Shik). Data are shown as mean±SD. n represents the number of biologically independent samples. Statistical significance was calculated by Student’s t⁃test and designated as *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
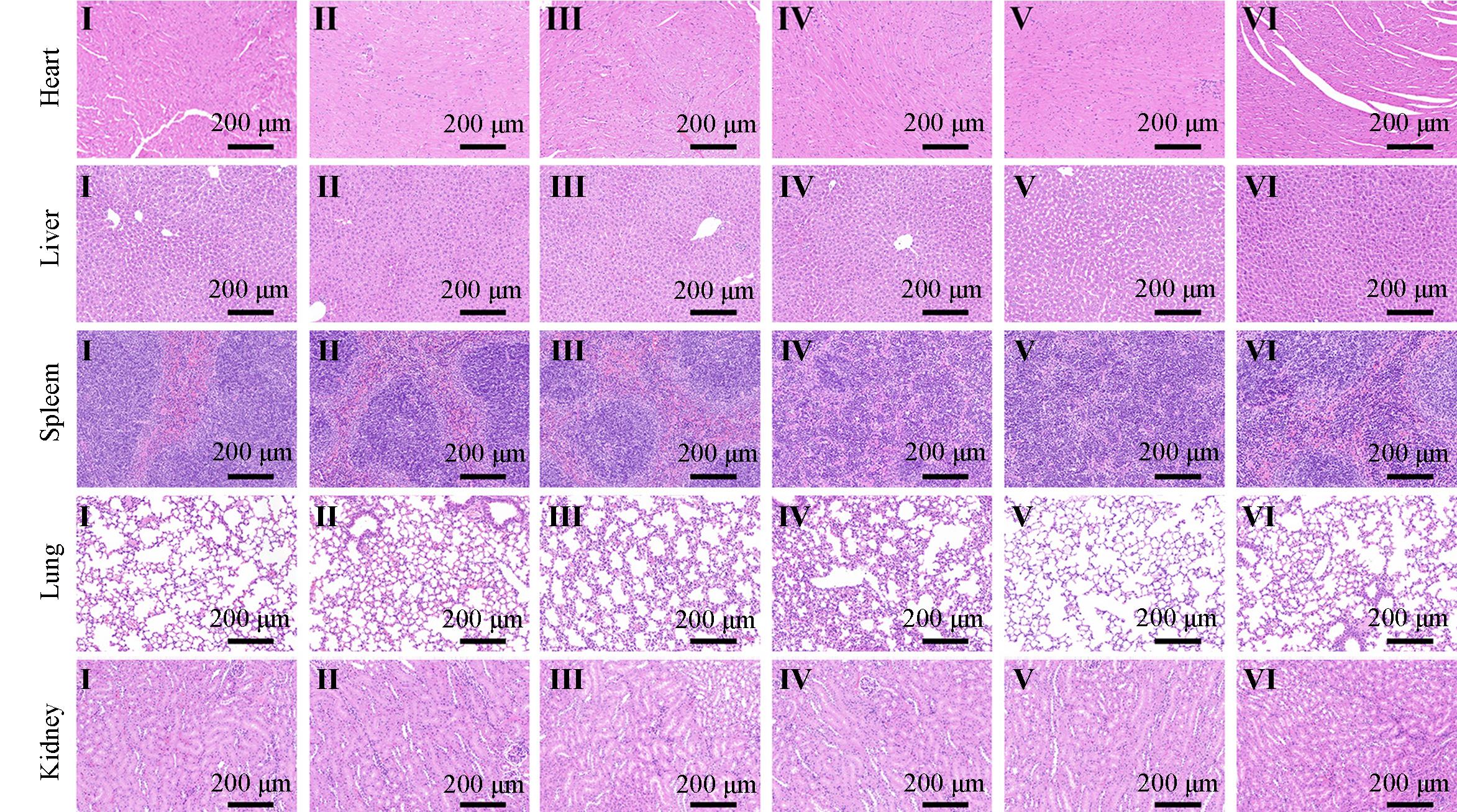
Fig.11 H&E⁃stained splanchnic slices of heart, liver, spleen, lungs and kidneys of mice after different treatmentsGroup: I(OVA), II(OVA), III(Shik), IV(Fe/Shik), V(OVA@Fe/Shik), VI(OVA/R837@Fe/Shik).
| 1 | Miyata Y., Murakami N., Honma Y., Mori T., Yoshimoto S., Kashihara T., Takemori M., Nakayama Y., Itami J., Ogo E., Igaki H., J. Radiat. Res., 2022, 63(6), 879—883 |
| 2 | Guo Y. Y., Gao F. Y., Ahmed A., Rafiq M., Yu B., Cong H. L., Shen Y. Q., J. Mater. Chem. B, 2023, 11(36), 8586—8604 |
| 3 | Kubecek O., Trojanova P., Molnarova V., Kipecky J., Med. Hypotheses, 2016, 93, 74—76 |
| 4 | Huang W., Xu R., Zhou B., Lin C., Guo Y. K., Xu H. Y., Guo X., Front. Cardiovasc. Med., 2022, 9, 912329 |
| 5 | Nagaraju G. P., Malla R. R., Basha R., Motofei I. G., Semin. Cancer Biol., 2022, 86, 616—621 |
| 6 | Hu Y., Li Y., Yao Z. C., Huang F. L., Cai H. Z., Liu H. Y., Zhang X. Y., Zhang J. Y., Cancers, 2023, 15(3), 563 |
| 7 | Tan S. Z., Li D. P., Zhu X., Biomed. Pharmacother., 2020, 124, 109821 |
| 8 | Zhao H., Xu J., Li Y., Guan X. X., Han X., Xu Y. Y., Zhou H. T., Peng R., Wang J., Liu Z., ACS Nano, 2019, 13(11), 13127—13135 |
| 9 | Li F., Ding X. H., Lv Z. Y., Li J., Yang D. Y., Nano Today, 2024, 54, 102061 |
| 10 | Lu Z. X., Zhang Y. W, Wang Y., Tan G. H., Huang F. Y., Cao R., He N. Y, Zhang L. M., J. Controlled Release, 2021, 332, 245—259 |
| 11 | Choi I. K., Wang Z., Ke Q., Hong M., Paul Jr D. W., Fernandes S. M., Hu Z., Stenvens J., Guleria I., Kim H. J., Cantor H., |
| Wucherpfennig K. W., Brown J. R., Ritz J., Zhang B. C., Nature, 2021, 590(7844), 157—162 | |
| 12 | de Gruijl T. D., van den Eertwegh A. J. M., Pinedo H. M., Scheper R. J., Cancer Immunol. Immun., 2008, 57(10), 1569—1577 |
| 13 | Tan J., Ding B. B., Teng B., Ma P. A., Lin J., Adv. Funct. Mater., 2022, 32(16), 2111670 |
| 14 | Zhou B. Q., Liu J. X., Lin M. A., Zhu J. Y., Chen W. R., Coord. Chem. Rev., 2021, 442, 214009 |
| 15 | Chen D. S., Mellman I., Immunity, 2013, 39(1), 1—10 |
| 16 | Hubbell J. A., Thomas S. N., Swartz M. A., Nature, 2009, 462(7272), 449—460 |
| 17 | Kuai R., Ochyl L. J., Bahjat K. S., Schwendeman A., Moon J. J., Nat. Mater., 2017, 16(4), 489—496 |
| 18 | Zheng Y. R., Zhong Z. Y., J. Controlled Release, 2022, 347, 308—313 |
| 19 | Aikins M. E., Xu C., Moon J. J., Acc. Chem. Res., 2020, 53(10), 2094—2105 |
| 20 | Yu L., Yu M., Chen W., Sun S. J., Huang W. X., Wang T. Q., Peng Z. W., Luo Z. W., Fang Y. X., Li Y. J., Deng Y., Wu M. Y., Tao W., J. Am. Chem. Soc., 2023, 145, 8375—8388 |
| 21 | Oh J. M., Venters C. C., Di C., Pinto A. M., Wan L. L., Younis I., Cai Z. Q., Arai C., So B. R., Duan J. Q., Dreyfuss G., Nat. Commun., 2020, 11(1), 6299 |
| 22 | Kroemer G., Galluzzi L., Kepp O., Zitvogel L., Annu. Rev. Immunol., 2013, 31, 51—72 |
| 23 | Hu H., Wu G. X., Shu Z. Q., Yu D. D., Nan N., Yuan F. Y., Liu X. Y., Wang H. Y., Front. Cell Dev. Biol., 2020, 8, 595253 |
| 24 | Lee S. Y., Ju M. K., Jeon H. M., Jeong E. K., Lee Y. J., Kim C. H., Park H. G., Han S. I., Kang H. S., Oxid. Med. Cell. Longevity, 2018, 2018, 3537471 |
| 25 | Hou S. Q., Zhang J., Jiang X., Yang Y. X., Shan B., Zhang M. M., Liu C., Yuan J. Y., Xu D. C., Mol. Cell, 2024, 84(5), 938—954 |
| 26 | Wang R. S., Liu C. Z., Lyu C. G., Sun J. H., Kang C. Z., Ma Y., Wan X. F., Guo J., Shi L. Y., Wang J. Y., Huang L. Q., Wang S., Guo L. P., Front. Plant Sci., 2023, 14, 1160571 |
| 27 | Wen Q. R., Liu J., Kang R., Zhou B. R., Tang D. L., Biochem. Biophys. Res. Commun., 2019, 510(2), 278—283 |
| 28 | Liao P., Wang W. M., Wang W. C., Kryczek I., Li X., Bian Y. J., Sell A., Wei S., Grove S., Johnson J. K., Kennedy P. D., Gijón M., Shan Y. M., Zou W. P., Cancer Cell, 2022, 40(4), 365—378 |
| 29 | Dixon S. J., Lemberg K. M., Lamprecht M. R., Skouta R., Zaitsev E. M., Gleason C. E., Patel D. N., Bauer A. J., Cantley A. M., |
| Yang W. S., Morrison B., Stockwell B. R., Cell, 2012, 149(5), 1060—1072 | |
| 30 | Stockwell B. R., Angeli J. P. F., Bayir H., Bush A. I., Conrad M., Dixon S. J., Fulda S., Gascón S., Hatzios S. K., Kagan V. E., |
| Noel K., Jiang X. J., Linkermann A., Murphy M. E., Overholtzer M., Oyagi A., Pagnussat G. C., Park J., Ran Q. T., Rosenfeld C. S., Zhang D. D., Cell, 2017, 171(2), 273—285 | |
| 31 | Feng W. J., Shi W. R., Cui Y. Q., Xu J. J., Liu S. W., Gao H., Zhu S. J., Liu Y., Zhang H., Theranostics, 2023, 13(15), 5266—5289 |
| [1] | 马爽, 吕明杨, 张赐童, 刘轶. 化疗-光热治疗-自促进饥饿治疗纳米治疗平台的构筑及对乳腺癌的治疗效果[J]. 高等学校化学学报, 2025, 46(1): 20240467. |
| [2] | 彭海月, 汪婷, 李国瑞, 黄静. 黑色素的合成及小分子对其功能的调控[J]. 高等学校化学学报, 2021, 42(11): 3357. |
| [3] | 王欣欣, 吕侠, 葛广波, 冯磊, 辛红, 李耀光, 曹云峰, 韩冠英, 郭斌. β-葡萄糖醛酸苷酶荧光底物试卤灵葡萄糖醛酸苷的高效制备[J]. 高等学校化学学报, 2015, 36(7): 1321. |
| [4] | 赵文静, 牛凤兰. 3,4,5-三羟基苯甲酸通过线粒体途径诱导人肝癌细胞SMMC-7721的凋亡[J]. 高等学校化学学报, 2009, 30(2): 320. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||