

高等学校化学学报 ›› 2024, Vol. 45 ›› Issue (9): 20240126.doi: 10.7503/cjcu20240126
收稿日期:2024-03-18
出版日期:2024-09-10
发布日期:2024-05-15
通讯作者:
詹传郎
E-mail:clzhan@iccas.ac.cn
基金资助:
ZHANG Liyuan, WANG Chasina, HU Jingxiang, ZHAN Chuanlang( )
)
Received:2024-03-18
Online:2024-09-10
Published:2024-05-15
Contact:
ZHAN Chuanlang
E-mail:clzhan@iccas.ac.cn
Supported by:摘要:
铅基卤化物钙钛矿发光材料因具有灵活的晶体结构、 带隙可调性、 缺陷容忍性和高荧光量子产率等优异性能而备受关注. 但金属铅的毒性和钙钛矿的稳定性一直是阻碍其商业应用急需解决的问题. 因此, 需要探索更绿色环保的非铅金属卤化物类钙钛矿发光材料. 近年来, 通过替换铅离子发展起来的非铅双钙钛矿结构成功实现了低毒性和高稳定性, 但由于其是间接带隙或因为宇称禁阻的直接带隙而造成光致发光效率极低. 为了解决此问题, 研究人员开发了离子掺杂策略, 实现了光致发光效率的提升. 本文综合评述了非铅双钙钛矿材料的晶体结构和发光性能, 归纳了主族金属、 稀土金属和过渡金属掺杂对非铅双钙钛矿发光性能的影响及其发光机制. 最后, 对离子掺杂策略的应用和进一步提升非铅双钙钛矿发光材料的性能进行了总结和展望.
中图分类号:
TrendMD:
张丽媛, 王查斯娜, 胡井香, 詹传郎. 离子掺杂调控双钙钛矿量子点发光性能的研究进展. 高等学校化学学报, 2024, 45(9): 20240126.
ZHANG Liyuan, WANG Chasina, HU Jingxiang, ZHAN Chuanlang. Research Progress on Luminescence Performance of Double Perovskite Quantum Dots Regulated by Ion Doping. Chem. J. Chinese Universities, 2024, 45(9): 20240126.
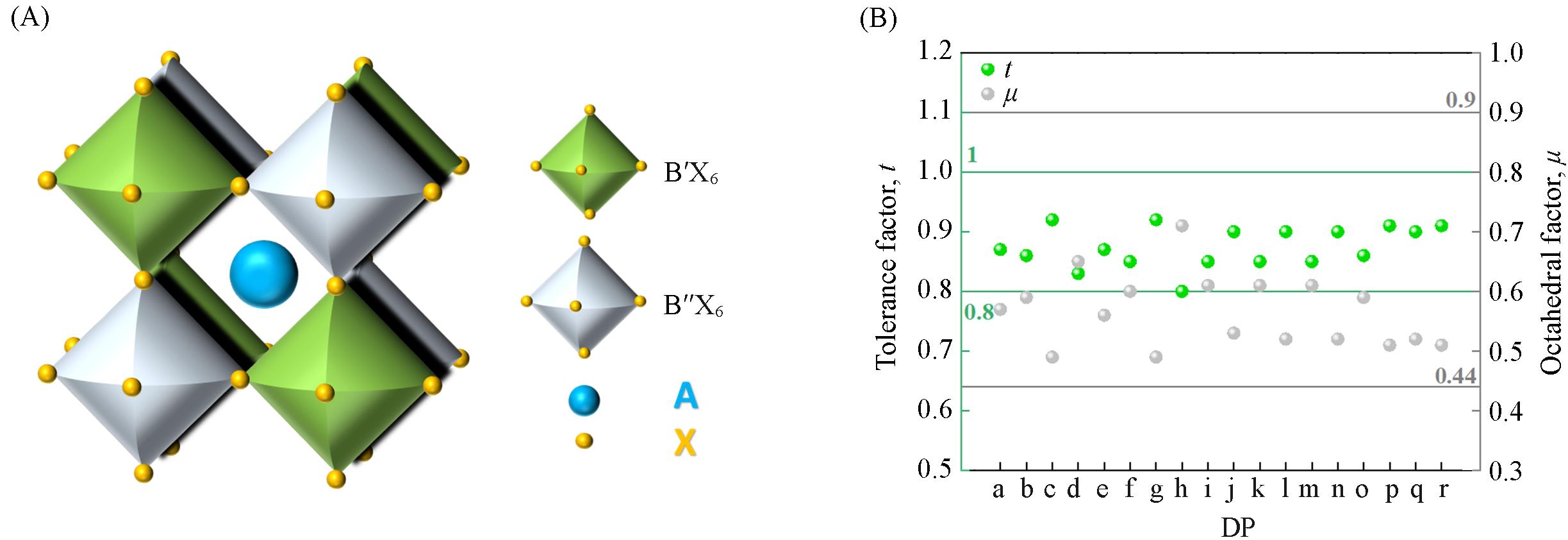
Fig.1 Structure of double perovskites(A), Goldschmidt tolerance factors(t) and octahedral factors(μ) of common double perovskites(B)(B) a. AgIn; b. KIn; c. NaIn; d. AgBi; e. NaBi; f. AgSb; g. NaSc; h. NaY; i. AgSm; j. NaSm; k. AgEu; l. NaEu; m. AgGd; n. NaGd; o. AgEr; p. NaEr; q. NaTb; r. NaHo.
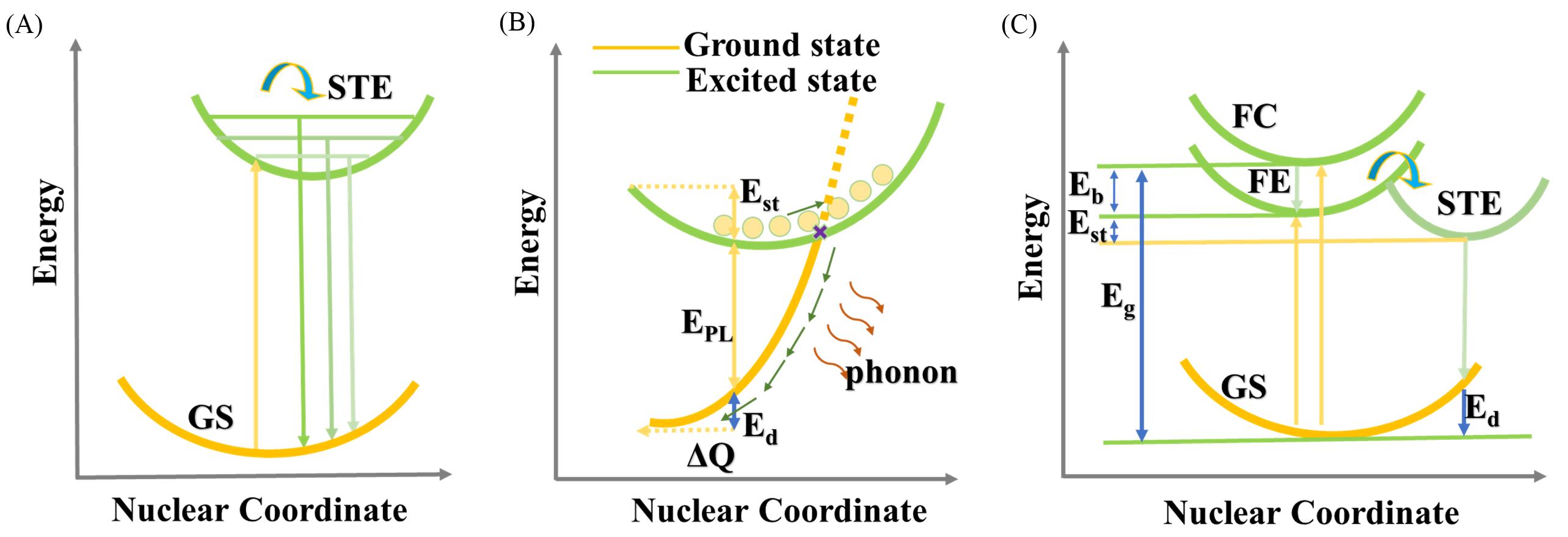
Fig.2 STE emission mechanism diagram(A)[15], schematic diagram of the nonradiative recombination process of STE at larger S(B)[13], schematic diagram of the energy level structure of STE(C)[16](A) Copyright 2018, American Chemical Society; (B) Copyright 2021, Wiley‐VCH; (C) Copyright 2021, American Chemical Society.
| Doped ion and quantum dot | Absorption/nm | Emission/nm | Lifetime/ns | PLQY(%) | Ref. |
|---|---|---|---|---|---|
| Na⁃Cs2AgBiCl6 | 366325 | 605 | 40—120 | — | [ |
| Na K⁃Cs2AgBiCl6 | 300 | 400—581 | — | 8 | [ |
| K⁃Cs2AgBiBr6 | 356 | 590 | 2.08—10.64 | 0.40—6.82 | [ |
| In⁃Cs2AgBiCl6 | 372—270 | 410—395, 570 | — | 0.1—36.6 | [ |
| In⁃Cs2AgBiCl6 | 350 | 570, 605 | <10—774 | 0—34(±4) | [ |
| Bi⁃Cs2AgInCl6 | 270—368 | 470—580 | <10—1633 | 1—11.4 | [ |
| Bi⁃Cs2AgInCl6 | 333, 367 | 426, 650 | <2—1000 | 31.4 | [ |
| Bi⁃Cs2NaScCl6 | 306, 361—337, 395 | 430—590 | 15—693(431), 1198(590) | 38—60 | [ |
| Bi Na⁃Cs2AgInCl6 | 318—365 | 653—827 | — | 0.6—22 | [ |
| Bi Na⁃Cs2AgInCl6 | — | Bi: 573 Na: yellow | t1=1.1 t2=1600 | Bi: 21 Na: 56 | [ |
| Bi Na⁃Cs2AgInCl6 | 378 | 610 | — | 20—100 | [ |
| Bi Er/Bi Yb⁃Cs2AgInCl6 | Bi Er 350—372 Bi Yb 350—372 | NIR Bi Er 1540 Bi Yb 994 | 1.64×107 -2.24×107, 0.52×107 | — | [ |
| Tb Bi⁃Cs2AgInCl6 | 368 | 470—580 | 1371.9 | 1—10.1 | [ |
| Sb⁃Cs2MInCl6(M=Na, K) | — | 445—445(Na) 445—495(K) | — — | 82(Na) 93(K) | [ |
| Sb⁃Cs2MInCl6(M=Na, K) | — | 445 | t1=9×105, 46% t2=1.016×107, 54% | 79 | [ |
| Sb⁃Cs2NaInCl6 | 308 | 442 | — | 75.89 | [ |
| Sb⁃Cs2NaInCl6 | 320, 335 | 427—448 | 1.3—5300 | 50.7 | [ |
| Sb⁃Cs2KInCl6 | 320 | 503—515 | 4700 | 95 | [ |
| Sb⁃Cs2NaYCl6 | 310—335 | 472 | — | 51.8 | [ |
| Sb⁃Cs2NaTbCl6 | 271, 286, 300—378 | 622 | 4.79—5.19×107 | 1.7—46.9 | [ |
| Sb⁃Cs2Ag/NaTbCl6 | 269—269, 360 | 489、 548, 621 | 3.3×107/5.0×107—7.5×107 | 4/12—24 | [ |
Table 1 Luminescence performance of double perovskite quantum dots doping with main group metals
| Doped ion and quantum dot | Absorption/nm | Emission/nm | Lifetime/ns | PLQY(%) | Ref. |
|---|---|---|---|---|---|
| Na⁃Cs2AgBiCl6 | 366325 | 605 | 40—120 | — | [ |
| Na K⁃Cs2AgBiCl6 | 300 | 400—581 | — | 8 | [ |
| K⁃Cs2AgBiBr6 | 356 | 590 | 2.08—10.64 | 0.40—6.82 | [ |
| In⁃Cs2AgBiCl6 | 372—270 | 410—395, 570 | — | 0.1—36.6 | [ |
| In⁃Cs2AgBiCl6 | 350 | 570, 605 | <10—774 | 0—34(±4) | [ |
| Bi⁃Cs2AgInCl6 | 270—368 | 470—580 | <10—1633 | 1—11.4 | [ |
| Bi⁃Cs2AgInCl6 | 333, 367 | 426, 650 | <2—1000 | 31.4 | [ |
| Bi⁃Cs2NaScCl6 | 306, 361—337, 395 | 430—590 | 15—693(431), 1198(590) | 38—60 | [ |
| Bi Na⁃Cs2AgInCl6 | 318—365 | 653—827 | — | 0.6—22 | [ |
| Bi Na⁃Cs2AgInCl6 | — | Bi: 573 Na: yellow | t1=1.1 t2=1600 | Bi: 21 Na: 56 | [ |
| Bi Na⁃Cs2AgInCl6 | 378 | 610 | — | 20—100 | [ |
| Bi Er/Bi Yb⁃Cs2AgInCl6 | Bi Er 350—372 Bi Yb 350—372 | NIR Bi Er 1540 Bi Yb 994 | 1.64×107 -2.24×107, 0.52×107 | — | [ |
| Tb Bi⁃Cs2AgInCl6 | 368 | 470—580 | 1371.9 | 1—10.1 | [ |
| Sb⁃Cs2MInCl6(M=Na, K) | — | 445—445(Na) 445—495(K) | — — | 82(Na) 93(K) | [ |
| Sb⁃Cs2MInCl6(M=Na, K) | — | 445 | t1=9×105, 46% t2=1.016×107, 54% | 79 | [ |
| Sb⁃Cs2NaInCl6 | 308 | 442 | — | 75.89 | [ |
| Sb⁃Cs2NaInCl6 | 320, 335 | 427—448 | 1.3—5300 | 50.7 | [ |
| Sb⁃Cs2KInCl6 | 320 | 503—515 | 4700 | 95 | [ |
| Sb⁃Cs2NaYCl6 | 310—335 | 472 | — | 51.8 | [ |
| Sb⁃Cs2NaTbCl6 | 271, 286, 300—378 | 622 | 4.79—5.19×107 | 1.7—46.9 | [ |
| Sb⁃Cs2Ag/NaTbCl6 | 269—269, 360 | 489、 548, 621 | 3.3×107/5.0×107—7.5×107 | 4/12—24 | [ |
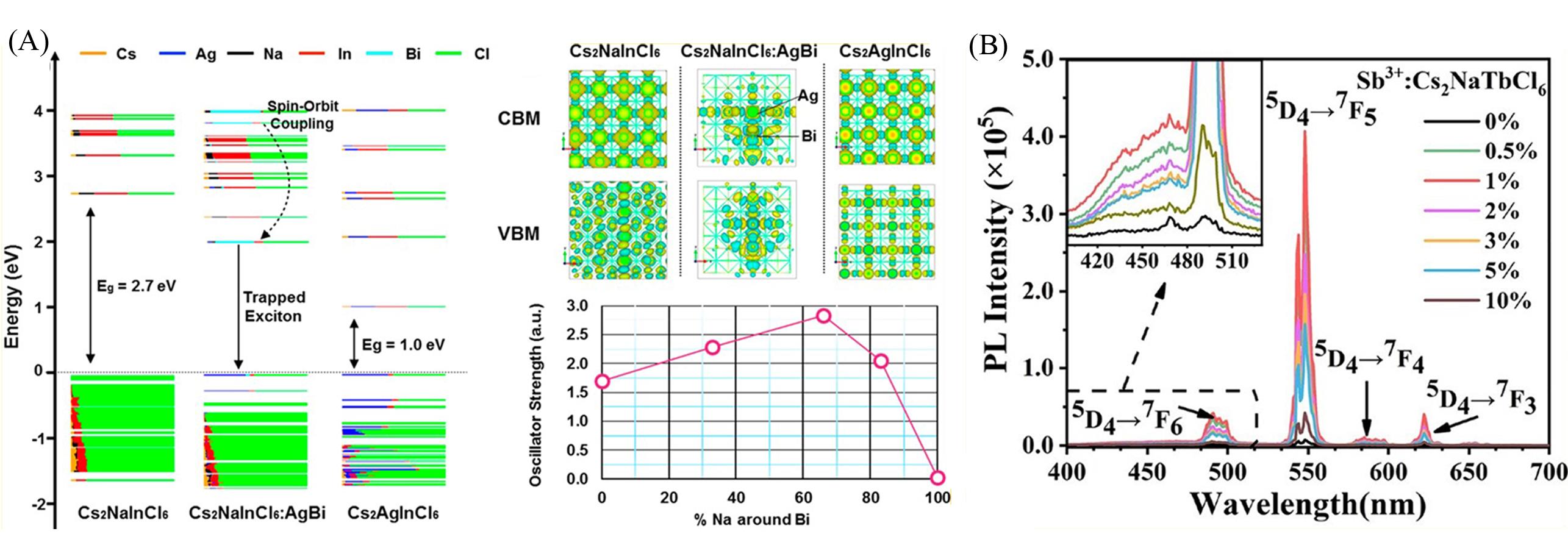
Fig.3 Electronic structures of Cs2NaInCl6, Cs2NaInCl6 doped with Ag+ and Bi3+ ions, as well as Cs2AgInCl6 calculated under the DFT/PBE framework(A)[30], PL spectra, energy level structures, and luminescence mechanisms of Cs2NaTbCl6 doped with different Sb3+ ions(B)[42](A) The orbital representation of CBM and VBM in real space shows the delocalized wave functions of electrons and holes before doping, while the localized wave functions are displayed after doping. The relationship between the oscillator intensity trend of BiCl6→AgCl6 transition and the percentage of Na ions around Bi ions.(A) Copyright 2019, American Chemical Society; (B) Copyright 2023, American Chemical Society.
| Doped ion and quantum dot | Absorption/nm | Emission/nm | Lifetime/ns | PLQY(%) | Ref. |
|---|---|---|---|---|---|
| Yb/Er/YbEr⁃Cs2AgInCl6 | 240 | 395—996/1573 | — | 0.5—3.6/0.05 | [ |
| Yb/Mn⁃Cs2AgBiCl6 | 366 | 680—1000 | Vis 20.0—26.0 NIR 0.97—1.44×107 | — | [ |
| Nd⁃Cs2AgIn0.99Bi0.01Cl6 | 365 | 630, 890, 1074 | STE 10.05—280.26 | NIR 0—56.7 | [ |
| Cs2NaScCl6 | 313 | 445 | 1059 | 1.6—29.05 | [ |
| Er⁃Cs2NaScCl6 | — | NIR1540 | — | NIR 28.3 | [ |
| Cs2NaHoCl6 | 321, 362, 402 | 452, 540, 646 | — | 82.3 | [ |
| Cs2NaLnCl6 | 307, 363 | 438 | 7.16 | 68.5 | [ |
| Cs2AgLnCl6 | 350 | Sm: 450 Gd: 435 Eu: 615 Er: 440, 555 | 3.0 3.3 4.7×106 1.8, 2.6 | 1.17 3.09 1.16 7.37 | [ |
| Na ErYb⁃Cs2AgBiCl6 | — | NIR 995/1540 | 1190 1170 | 19 4.3 | [ |
| Na Bi Nd⁃Cs2AgInCl6 | 320—370 | STE 610 NIR 890 | STE 0.19—2030 NIR 2.25—2.99×107 | NIR 0.16—30.3 | [ |
| Sb Yb⁃Cs2AgInCl6 | — | 600—660 | — | NIR 50 | [ |
| Cr Er⁃Cs2AgInCl6 | 350—350, 560, 800 | 1010, 1540 | — | NIR 29 | [ |
| Cr Ln(Er/Tm)⁃Cs2NaScCl6 | — | 457—970 1540/1220 | 13×107 5.5×107 | NIR 26 NIR 56 | [ |
| Tb Ho⁃Cs2AgInCl6 | 350 | 530 | 6.65—4.85×107 | — | [ |
Table 2 Luminescence performance of double perovskite quantum dots doped with rare earth metals
| Doped ion and quantum dot | Absorption/nm | Emission/nm | Lifetime/ns | PLQY(%) | Ref. |
|---|---|---|---|---|---|
| Yb/Er/YbEr⁃Cs2AgInCl6 | 240 | 395—996/1573 | — | 0.5—3.6/0.05 | [ |
| Yb/Mn⁃Cs2AgBiCl6 | 366 | 680—1000 | Vis 20.0—26.0 NIR 0.97—1.44×107 | — | [ |
| Nd⁃Cs2AgIn0.99Bi0.01Cl6 | 365 | 630, 890, 1074 | STE 10.05—280.26 | NIR 0—56.7 | [ |
| Cs2NaScCl6 | 313 | 445 | 1059 | 1.6—29.05 | [ |
| Er⁃Cs2NaScCl6 | — | NIR1540 | — | NIR 28.3 | [ |
| Cs2NaHoCl6 | 321, 362, 402 | 452, 540, 646 | — | 82.3 | [ |
| Cs2NaLnCl6 | 307, 363 | 438 | 7.16 | 68.5 | [ |
| Cs2AgLnCl6 | 350 | Sm: 450 Gd: 435 Eu: 615 Er: 440, 555 | 3.0 3.3 4.7×106 1.8, 2.6 | 1.17 3.09 1.16 7.37 | [ |
| Na ErYb⁃Cs2AgBiCl6 | — | NIR 995/1540 | 1190 1170 | 19 4.3 | [ |
| Na Bi Nd⁃Cs2AgInCl6 | 320—370 | STE 610 NIR 890 | STE 0.19—2030 NIR 2.25—2.99×107 | NIR 0.16—30.3 | [ |
| Sb Yb⁃Cs2AgInCl6 | — | 600—660 | — | NIR 50 | [ |
| Cr Er⁃Cs2AgInCl6 | 350—350, 560, 800 | 1010, 1540 | — | NIR 29 | [ |
| Cr Ln(Er/Tm)⁃Cs2NaScCl6 | — | 457—970 1540/1220 | 13×107 5.5×107 | NIR 26 NIR 56 | [ |
| Tb Ho⁃Cs2AgInCl6 | 350 | 530 | 6.65—4.85×107 | — | [ |
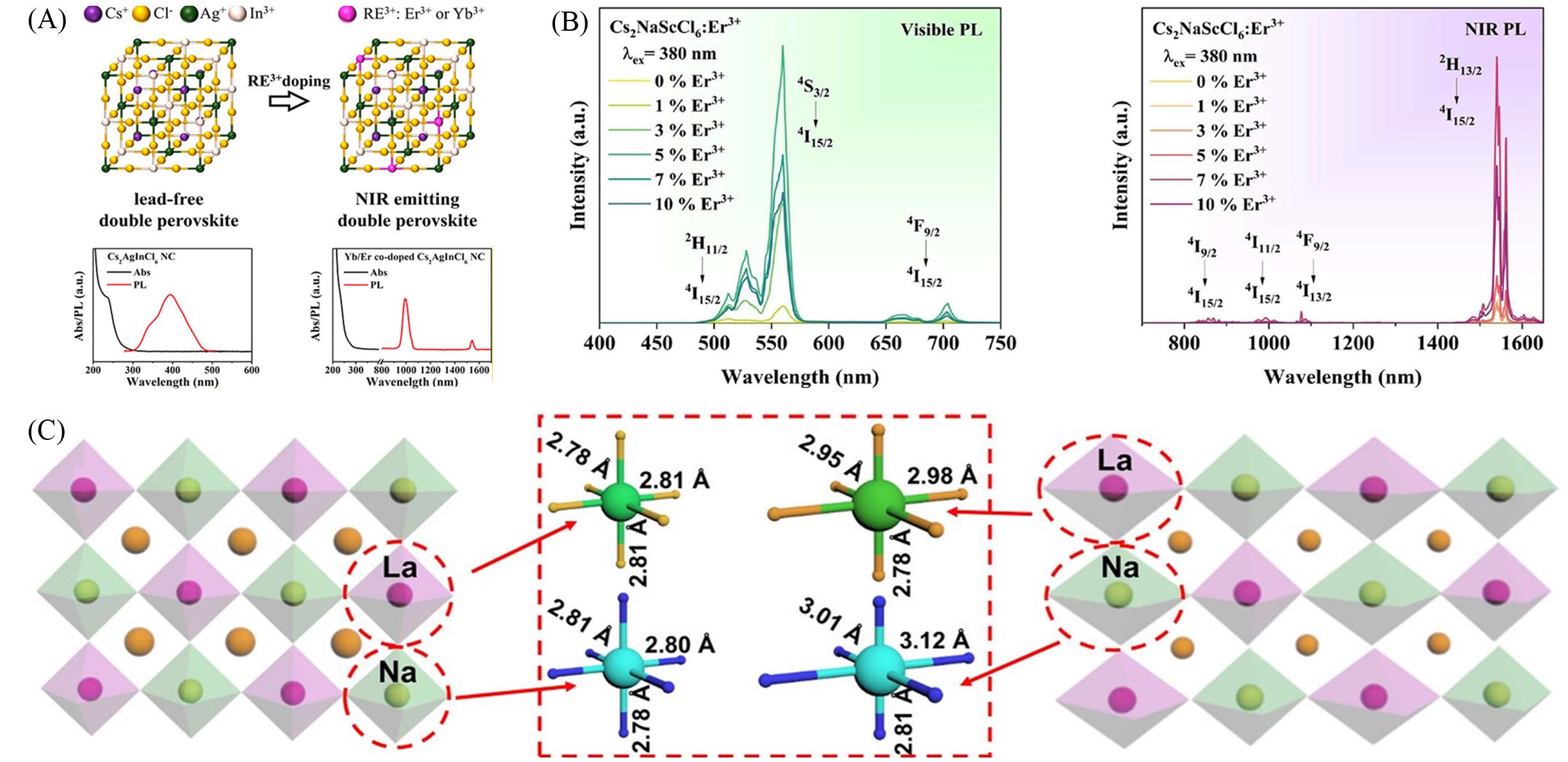
Fig.4 UV⁃Vis absorption and PL spectra of undoped and Er3+ or Yb3+ ion doped Cs2AgInCl6 quantum dots(A)[43], Vis PL and NIR PL spectra of Er3+ doped Cs2NaScCl6(B)[47], schematic diagram of sublattice distortion caused by Jahn⁃Teller distortion of [NaCl6]5- and [LnCl6]3- octahedral(C)[49](A) Copyright 2019, American Chemical Society; (B) Copyright 2023, American Chemical Society; (C) Copyright 2023, Wiley‐VCH.
| Doped ion and quantum dot | Absorption/nm | Emission/nm | Lifetime/ns | PLQY(%) | Ref. |
|---|---|---|---|---|---|
| Mn⁃Cs2AgInCl6 | 290 | 560—620 | 107 | (1.6±1)—(16±4) | [ |
| Mn⁃Cs2NaBiCl6 | — | 345—590 | — | 15 | [ |
| Mn⁃Cs2NaIn x Bi1-x Cl6 | 326 | 583—614 | 3—9×107 | 38—44.6 | [ |
| Mn⁃Cs2NaScCl6 | — | 445—635 | — | 29.05 | [ |
| Mn⁃Cs2NaTbCl6 | 278 | 548—622 | 5.10—13.28×107 | 52.6 | [ |
| Ag⁃Cs2NaInCl6 | 269 | 535 | — | 31.1 | [ |
| Ag/Mn⁃Cs2NaBiCl6 | 324—356 | 375—613/585 | 4.74×105—1.058×106, 385×106 | 1.7—20/3 | [ |
| Cu⁃Cs2(Ag/Na)InCl6 | 380—410 | 605 | 6400—3900 | 19.0—62.6 | [ |
| Cu⁃Cs2AgInCl6 | 344—427 | 450 | — | — | [ |
| Cu⁃Cs2AgSbCl6 | 477—1240 | — | — | — | [ |
| Cu⁃Cs2AgInCl6 | 344—561 | — | — | — | [ |
| Fe⁃Cs2NaBiCl6 | — | 585 | 38 | — | [ |
Table 3 Luminescence performance of double perovskite quantum dots doped with transition metals
| Doped ion and quantum dot | Absorption/nm | Emission/nm | Lifetime/ns | PLQY(%) | Ref. |
|---|---|---|---|---|---|
| Mn⁃Cs2AgInCl6 | 290 | 560—620 | 107 | (1.6±1)—(16±4) | [ |
| Mn⁃Cs2NaBiCl6 | — | 345—590 | — | 15 | [ |
| Mn⁃Cs2NaIn x Bi1-x Cl6 | 326 | 583—614 | 3—9×107 | 38—44.6 | [ |
| Mn⁃Cs2NaScCl6 | — | 445—635 | — | 29.05 | [ |
| Mn⁃Cs2NaTbCl6 | 278 | 548—622 | 5.10—13.28×107 | 52.6 | [ |
| Ag⁃Cs2NaInCl6 | 269 | 535 | — | 31.1 | [ |
| Ag/Mn⁃Cs2NaBiCl6 | 324—356 | 375—613/585 | 4.74×105—1.058×106, 385×106 | 1.7—20/3 | [ |
| Cu⁃Cs2(Ag/Na)InCl6 | 380—410 | 605 | 6400—3900 | 19.0—62.6 | [ |
| Cu⁃Cs2AgInCl6 | 344—427 | 450 | — | — | [ |
| Cu⁃Cs2AgSbCl6 | 477—1240 | — | — | — | [ |
| Cu⁃Cs2AgInCl6 | 344—561 | — | — | — | [ |
| Fe⁃Cs2NaBiCl6 | — | 585 | 38 | — | [ |
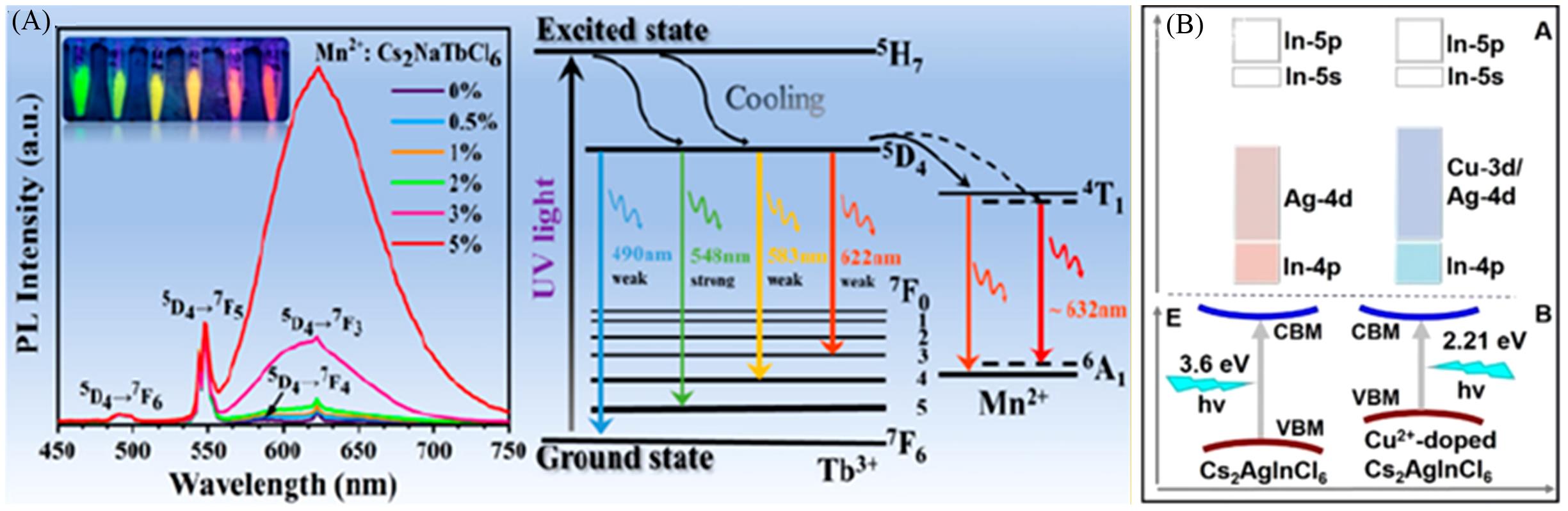
Fig.5 Normalized PL spectra and luminescence mechanism diagrams of Cs2NaTbCl6 with different Mn2+ doping concentrations(A)[60], energy band diagrams of undoped and Cu2+ doped Cs2AgInCl6(B)[66](A) Copyright 2022, American Chemical Society; (B) Copyright 2020, American Chemical Society.
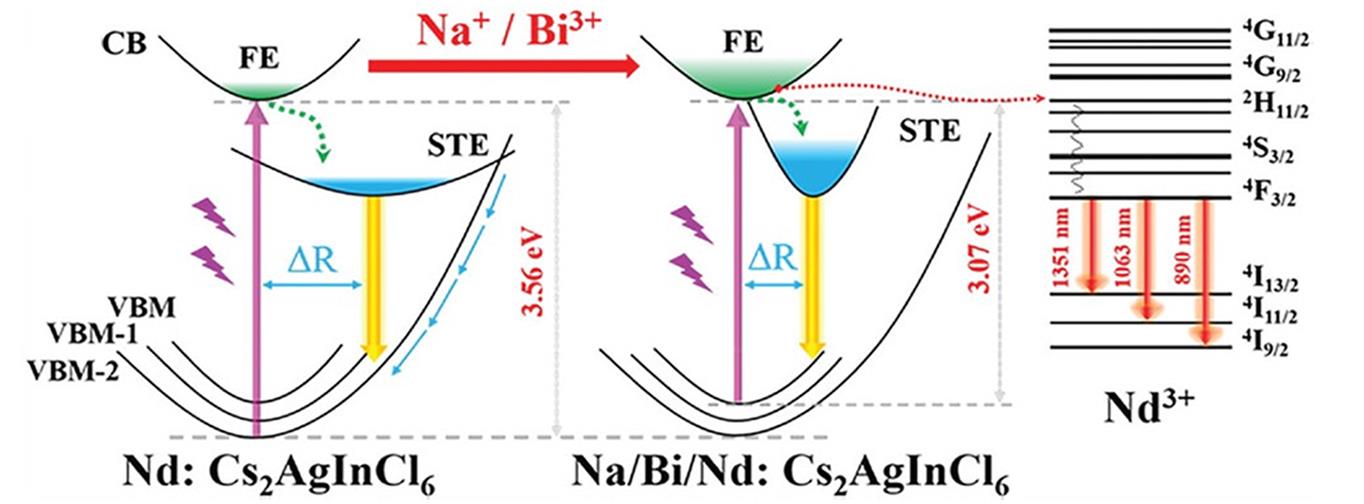
Fig.7 Nd3+ 4f⁃4f transition process sensitized by free excitons in Cs2InAgCl6 doped with Nd3+ and Na+/Bi3+/Nd3+ triple doping[52]Copyright 2023, Wiley‐VCH.
| 1 | Bhalla S., Melnekoff D. T., Aleman A., Leshchenko V., Restrepo P., Keats J., Onel K., Sawyer J. R., Madduri D., Richter J., Sci. Adv., 2021, 7(47), eabg9551 |
| 2 | Stranks S. D., Eperon G. E., Grancini G., Menelaou C., Alcocer M. J., Leijtens T., Herz L. M., Petrozza A., Snaith H. J., Science, 2013, 342(6156), 341—344 |
| 3 | Green M. A., Ho⁃Baillie A., Snaith H. J., Nature Photonics, 2014, 8(7), 506—514 |
| 4 | Wu H., Ge Y., Niu G., Tang J., Matter, 2021, 4(1), 144—163 |
| 5 | Zhou Y., Chen J., Bakr O. M., Mohammed O. F., ACS Energy Lett., 2021, 6(2), 739—768 |
| 6 | Kojima A., Teshima K., Shirai Y., Miyasaka T., J. Am. Chem. Soc., 2009, 131(17), 6050—6051 |
| 7 | Liang Z., Zhang Y., Xu H., Chen W., Liu B., Zhang J., Zhang H., Wang Z., Kang D. H., Zeng J., Nature, 2023, 624(7992), 557—563 |
| 8 | Jiang J., Chu Z., Yin Z., Li J., Yang Y., Chen J., Wu J., You J., Zhang X., Adv. Mater., 2022, 34(36), 2204460 |
| 9 | Jiang Y., Sun C., Xu J., Li S., Cui M., Fu X., Liu Y., Liu Y., Wan H., Wei K., Nature, 2022, 612(7941), 679—684 |
| 10 | Kim J. S., Heo J. M., Park G. S., Woo S. J., Cho C., Yun H. J., Kim D. H., Park J., Lee S. C., Park S. H., Nature, 2022, 611(7937), 688—694 |
| 11 | Chen K., Schünemann S., Song S., Tüysüz H., Chem. Soc. Rev., 2018, 47(18), 7045—7077 |
| 12 | Chung I., Lee B., He J., Chang R. P., Kanatzidis M. G., Nature, 2012, 485(7399), 486—489 |
| 13 | Tang H., Xu Y., Hu X., Hu Q., Chen T., Jiang W., Wang L., Jiang W., Adv. Sci., 2021, 8(7), 2004118 |
| 14 | Wei Y., Cheng Z., Lin J., Chem. Soc. Rev., 2019, 48(1), 310—350 |
| 15 | Smith M. D., Karunadasa H. I., Acc. Chem. Res., 2018, 51(3), 619—627 |
| 16 | Yao S., Wang J., Yang J., Yao H., Acc. of Chem. Res., 2021, 54(2), 441—451 |
| 17 | Yang X., Zhang X., Deng J., Chu Z., Jiang Q., Meng J., Wang P., Zhang L., Yin Z., You J., Nature Commun., 2018, 9(1), 570 |
| 18 | Veldhuis S. A., Boix P. P., Yantara N., Li M., Sum T. C., Mathews N., Mhaisalkar S. G., Adv. Mater., 2016, 28(32), 6804—6834 |
| 19 | Yang B., Chen J., Yang S., Hong F., Sun L., Han P., Pullerits T., Deng W., Han K., Angew. Chem., 2018, 130(19), 5457—5461 |
| 20 | Ahmad R., Zdrazil L., Kalytchuk S., Naldoni A., Rogach A. L., Schmuki P., Zboril R., Kment S., ACS Appl. Mater. Interfaces, 2021, 13(40), 47845—47859 |
| 21 | Luo J., Wang X., Li S., Liu J., Guo Y., Niu G., Yao L., Fu Y., Gao L., Dong Q., Nature, 2018, 563(7732), 541—545 |
| 22 | Lamba R. S., Basera P., Bhattacharya S., Sapra S., J. Phys. Chem. Lett., 2019, 10(17), 5173—5181 |
| 23 | Vashishtha P., Griffith B. E., Fang Y., Jaiswal A., Nutan G. V., Bartók A. P., White T., Hanna J. V., J. Mater. Chem. A, 2022, 10(7), 3562—3578 |
| 24 | Sun R., Jiang W., Wang S., Cui W., Qi L., Pan K., ACS Appl. Nano Mater., 2023, 6(16), 15247—15254 |
| 25 | Yang B., Mao X., Hong F., Meng W., Tang Y., Xia X., Yang S., Deng W., Han K., J. Am. Chem. Soc., 2018, 140(49), 17001—17006 |
| 26 | Karmakar A., Bernard G. M., Meldrum A., Oliynyk A. O., Michaelis V. K., J. Am. Chem. Soc., 2020, 142(24), 10780—10793 |
| 27 | Liu Y., Jing Y., Zhao J., Liu Q., Xia Z., Chem. Mater., 2019, 31(9), 3333—3339 |
| 28 | Zhang Y., Zhang Z., Yu W., He Y., Chen Z., Xiao L., Shi J. J., Guo X., Wang S., Qu B., Adv. Sci., 2022, 9(2), 2102895 |
| 29 | Zeng Z., Sun M., Zhang S., Zhang H., Shi X., Ye S., Huang B., Du Y., Yan C. H., Adv. Funct. Mater., 2022, 32(32), 2204780 |
| 30 | Locardi F., Sartori E., Buha J., Zito J., Prato M., Pinchetti V., Zaffalon M. L., Ferretti M., Brovelli S., Infante I., ACS Energy Lett., 2019, 4(8), 1976—1982 |
| 31 | Zheng W., Sun R., Liu Y., Wang X., Liu N., Ji Y., Wang L., Liu H., Zhang Y., ACS Appl. Mater. Interfaces, 2021, 13(5), 6404—6410 |
| 32 | Jin S., Li R., Zhu J., Pang T., Wu T., Zhan H., Zheng Y., Huang F., Chen X., Chen D., Mater. Horizons, 2023, 10(4), 1406—1415 |
| 33 | Arfin H., Kaur J., Sheikh T., Chakraborty S., Nag A., Angew. Chem. Inter. Ed., 2020, 59(28), 11307—11311 |
| 34 | Liu Y., Rong X., Li M., Molokeev M. S., Zhao J., Xia Z., Angew. Chem. Inter. Ed., 2020, 59(28), 11634—11640 |
| 35 | Noculak A., Morad V., McCall K. M., Yakunin S., Shynkarenko Y., Wörle M., Kovalenko M. V., Chem. Mater., 2020, 32(12), 5118—5124 |
| 36 | Gray M. B., Hariyani S., Strom T. A., Majher J. D., Brgoch J., Woodward P. M., J. Mater. Chem. C, 2020, 8(20), 6797—6803 |
| 37 | Zeng R., Zhang L., Xue Y., Ke B., Zhao Z., Huang D., Wei Q., Zhou W., Zou B., J. Phy. Chem. Lett., 2020, 11(6), 2053—2061 |
| 38 | Zhu D., Zaffalon M. L., Zito J., Cova F., Meinardi F., de Trizio L., Infante I., Brovelli S., Manna L., ACS Energy Lett., 2021, 6(6), 2283—2292 |
| 39 | Cong M., Zhang Q., Yang B., Chen J., Xiao J., Zheng D., Zheng T., Zhang R., Qing G., Zhang C., Nano Lett., 2021, 21(20), 8671—8678 |
| 40 | Wang Z., Zhang R., Mao X., Zheng D., Liu S., Liu F., Han K., Yang B., J. Phy. Chem. Lett., 2022, 13(36), 8613—8619 |
| 41 | Chen Y., Wu J., Zhang S., Zhu X., Zou B., Zeng R., J. Phy. Chem. Lett., 2023, 14(31), 7108—7117 |
| 42 | Zhou W., Li C., Wu T., Liu R., Ding Z., Zhang R., Yu Y., Han P., Lu R., J. Phy. Chem. Lett., 2023, 14(38), 8577—8583 |
| 43 | Lee W., Hong S., Kim S., J. Phy. Chem. C, 2019, 123(4), 2665—2672 |
| 44 | Chen N., Cai T., Li W., Hills⁃Kimball K., Yang H., Que M., Nagaoka Y., Liu Z., Yang D., Dong A., ACS Appl. Mater. Interfaces, 2019, 11(18), 16855—16863 |
| 45 | Zhao J., Pan G., Zhu Y., Liu K., You W., Chen X., Song H., Mao Y., ACS Appl. Mater. Interfaces, 2022, 14(37), 42215—42222 |
| 46 | Zhang R., Wang Z., Xu X., Mao X., Xiong J., Yang Y., Han K., Adv. Opt. Mater., 2021, 9(19), 2100689 |
| 47 | Zhao C., Gao Y., Song T., Wang J., Qiu J., J. Phys. Chem. Lett., 2023, 14(40), 9011—9018 |
| 48 | Sun R., Jia M., Chen X., Zhang F., Ma Z., Liu Y., Zhang J., Lian L., Han Y., Li M., Laser Photon. Rev., 2023, 2301028 |
| 49 | Sun L., Dong B., Sun J., Wang Y., Sun R., Hu S., Zhou B., Xu W., Bai X., Xu L., Laser Photon. Rev., 2023, 17(8), 2300045 |
| 50 | Feng J., Cao Q., Xue J., Lu H., Inorg. Chem., 2024, 63(4), 2241—2246 |
| 51 | Pei Y., Tu D., Li C., Han S., Xie Z., Wen F., Wang L., Chen X., Angew. Chem. Inter. Ed., 2022, 61(30), e202205276 |
| 52 | Jin S., Yuan H., Pang T., Zhang M., He Y., Zhuang B., Wu T., Zheng Y., Chen D., Adv. Funct. Mater., 2023, 33(50), 2304577 |
| 53 | Cao L., Jia X., Gan W., Ma C. G., Zhang J., Lou B., Wang J., Adv. Funct. Mater., 2023, 33(13), 2212135 |
| 54 | Gan W., Cao L., Gu S., Lian H., Xia Z., Wang J., Chem. Mater., 2023, 35(14), 5291—5299 |
| 55 | Huang W., Peng H., Huang J., Yang Y., Wei Q., Ke B., Khan M. S., Zhao J., Zou B., EcoMat, 2024, e12437 |
| 56 | Wu Y., Lin L., Lu P., Ma W., Li Z., Zhang M., Yang Y., Ju N., Zhang Y., Liao H., ACS Appl. Opt. Mater., 2023, 1(10), 1697—1705 |
| 57 | Locardi F., Cirignano M., Baranov D., Dang Z., Prato M., Drago F., Ferretti M., Pinchetti V., Fanciulli M., Brovelli S., J. Am. Chem. Soc., 2018, 140(40), 12989—12995 |
| 58 | Majher J. D., Gray M. B., Strom T. A., Woodward P. M., Chem. Mater., 2019, 31(5), 1738—1744 |
| 59 | Han P., Zhang X., Luo C., Zhou W., Yang S., Zhao J., Deng W., Han K., ACS Central Science, 2020, 6(4), 566—572 |
| 60 | Chen Y., Zeng R., Wei Q., Zhang S., Luo B., Chen C., Zhu X., Cao S., Zou B., Zhang J. Z., J. Phys. Chem. Lett., 2022, 13(36), 8529—8536 |
| 61 | Han P., Mao X., Yang S., Zhang F., Yang B., Wei D., Deng W., Han K., Angew. Chem., 2019, 131(48), 17391—17395 |
| 62 | Yao M. M., Wang L., Yao J. S., Wang K. H., Chen C., Zhu B. S., Yang J. N., Wang J. J., Xu W. P., Zhang Q., Adv. Opt. Mater., 2020, 8(8), 1901919 |
| 63 | Cheng X., Xie Z., Zheng W., Li R., Deng Z., Tu D., Shang X., Xu J., Gong Z., Li X., Adv. Sci., 2022, 9(7), 2103724 |
| 64 | Li Y., Li J., Ye S., Liu Y., Meng L., Yao H., Chen Q., Phys. Chem. Chem. Phys., 2024, 26(8), 6984—6990 |
| 65 | Karmakar A., Dodd M. S., Agnihotri S., Ravera E., Michaelis V. K., Chem. Mater., 2018, 30(22), 8280—8290 |
| 66 | Liao Q., Chen J., Zhou L., Wei T., Zhang L., Chen D., Huang F., Pang Q., Zhang J. Z., J. Phys. Chem. Lett., 2020, 11(19), 8392—8398 |
| 67 | Udavant R., Thawarkar S., Rondiya S., Shelke A., Aher R., Ajithkumar T. G., Cross R. W., Dzade N. Y., Jadkar S., Inorg. Chem., 2023, 62(12), 4861—4871 |
| 68 | Lei Z., Yang X., Bai C., Dai K., Cheng S., Small, 2018, 14(11), 1703762 |
| [1] | 王辉, 裴彦博, 胡绍争, 马文涛, 石朔宇. 能级可调的钾离子掺杂石墨相氮化碳的可控制备及“双渠道”光催化合成过氧化氢性能[J]. 高等学校化学学报, 2018, 39(7): 1503. |
| [2] | 张冬, 李亭亭, 邱海龙, 魏英进, 王春忠, 陈岗, 岳惠娟. 氮掺杂硅酸亚铁锂正极材料的制备及电化学性能[J]. 高等学校化学学报, 2017, 38(9): 1633. |
| [3] | 佟倜, 赵洋洋, 付义林, 单桂晔. 以Au/Cu纳米棒为基底的肺腺癌组织的特征表面增强拉曼光谱研究[J]. 高等学校化学学报, 2017, 38(9): 1536. |
| [4] | 杨穆, 王晓娜, 徐金梧, 王戈. 双掺杂介孔催化剂Fe-Ti-MCM-41的制备及催化性能[J]. 高等学校化学学报, 2012, 33(07): 1559. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||