

高等学校化学学报 ›› 2020, Vol. 41 ›› Issue (1): 118.doi: 10.7503/cjcu20190424
收稿日期:2019-07-26
出版日期:2020-01-10
发布日期:2019-12-02
通讯作者:
唐裕才
E-mail:yctang1009@163.com
基金资助:
TANG Yucai( ),QU Huang,ZHANG Wenxi,WANG Feifei,WANG Gang
),QU Huang,ZHANG Wenxi,WANG Feifei,WANG Gang
Received:2019-07-26
Online:2020-01-10
Published:2019-12-02
Contact:
Yucai TANG
E-mail:yctang1009@163.com
Supported by:摘要:
在以单质碘(I2)为催化剂, 叔丁基过氧化氢(TBHP)为氧化剂条件下, 使烯醇硅醚与各种取代的磺酰肼发生自由基磺酰化反应, 经自由基加成和氧化反应, 再水解脱去三甲基碘硅烷(Me3SiI), 在最优条件下, 以22%~72%的收率合成了22种具有不同取代基的α-磺酰基酮衍生物, 采用核磁共振波谱表征了终产物的结构. 实验结果表明, 该方法具有良好的底物普适性, 氟、 氯、 硝基、 三氟甲基、 呋喃和萘等取代基团均能顺利发生转化, 得到相应的目标产物.
中图分类号:
TrendMD:
唐裕才,屈煌,张文熙,王菲菲,王钢. 无金属条件下I2/TBHP体系促进磺酰肼与烯醇硅醚自由基偶联反应合成α-磺酰基酮. 高等学校化学学报, 2020, 41(1): 118.
TANG Yucai,QU Huang,ZHANG Wenxi,WANG Feifei,WANG Gang. Synthesis of α-Sulfonyl Ketones via I2/TBHP Promoted Radical Sulfonylation of Silyl Enol Ethers with Sulfohydrazides under Mild Conditions †. Chem. J. Chinese Universities, 2020, 41(1): 118.
| Compd. | Appearance | Yield(%) | Melting point/℃ | Compd. | Appearance | Yield(%) | Melting point/℃ |
|---|---|---|---|---|---|---|---|
| 3a | White solid | 66 | 99—101 | 3m | White solid | 32 | 99—100 |
| 3b | White solid | 52 | 107—108 | 3n | White solid | 25 | 106—107 |
| 3c | White solid | 55 | 123—124 | 3o | White solid | 22 | 79—80 |
| 3d | White solid | 49 | 132—133 | 3p | White solid | 58 | 80—81 |
| 3e | White solid | 50 | 122—123 | 3q | White solid | 59 | 92—93 |
| 3f | White solid | 50 | 144—145 | 3r | White solid | 55 | 148—149 |
| 3g | Yellow solid | 72 | 140—141 | 3s | White solid | 45 | 130—131 |
| 3h | White solid | 65 | 108—109 | 3t | White solid | 39 | 116—117 |
| 3i | White solid | 34 | 126—127 | 3u | White solid | 63 | 106—107 |
| 3j | White solid | 45 | 102—103 | 3v | Colorless oil | 50 | |
| 3l | White solid | 40 | 139—140 | 3w | White solid | 46 | 130—131 |
Table 1 Appearance, yields and melting point data of target compounds 3a—3w
| Compd. | Appearance | Yield(%) | Melting point/℃ | Compd. | Appearance | Yield(%) | Melting point/℃ |
|---|---|---|---|---|---|---|---|
| 3a | White solid | 66 | 99—101 | 3m | White solid | 32 | 99—100 |
| 3b | White solid | 52 | 107—108 | 3n | White solid | 25 | 106—107 |
| 3c | White solid | 55 | 123—124 | 3o | White solid | 22 | 79—80 |
| 3d | White solid | 49 | 132—133 | 3p | White solid | 58 | 80—81 |
| 3e | White solid | 50 | 122—123 | 3q | White solid | 59 | 92—93 |
| 3f | White solid | 50 | 144—145 | 3r | White solid | 55 | 148—149 |
| 3g | Yellow solid | 72 | 140—141 | 3s | White solid | 45 | 130—131 |
| 3h | White solid | 65 | 108—109 | 3t | White solid | 39 | 116—117 |
| 3i | White solid | 34 | 126—127 | 3u | White solid | 63 | 106—107 |
| 3j | White solid | 45 | 102—103 | 3v | Colorless oil | 50 | |
| 3l | White solid | 40 | 139—140 | 3w | White solid | 46 | 130—131 |
| Compd. | 1H NMR(500 MHz), δ | 13C NMR(125 MHz), δ |
|---|---|---|
| 3a | 7.97(d,J=7.4 Hz, 2H), 7.77(d, J=8.2 Hz, 2H), 7.62(t, J=7.4 Hz, 1H), 7.46(t, J=7.8 Hz, 2H), 7.33(d, J=8.2 Hz, 2H), 4.72(s, 2H), 2.44(s, 3H) | 188.2, 145.4, 135.8, 135.8, 134.3, 129.8, 129.3, 128.8, 128.6, 63.6, 21.6 |
| 3b | 7.86(d,J=8.3 Hz, 2H), 7.75(d, J=8.3 Hz, 2H), 7.33(d, J=8.0 Hz, 2H), 7.27(d, J=9.4 Hz, 2H), 4.69(s, 2H), 2.44(s, 3H), 2.42(s, 3H) | 187.6, 145.6, 145.3, 135.9, 133.4, 129.8, 129.5, 129.5, 128.6, 63.6, 21.8, 21.6 |
| 3c | 8.03—7.98(m, 2H), 7.75(d, J=8.3 Hz, 2H), 7.34(d, J=8.1 Hz, 2H), 7.19—7.13(m, 2H), 4.69(s, 2H), 2.45(s, 3H) | 186.6, 166.5(d, J=256.3 Hz ), 145.5, 135.7, 132.2(d, J=2.5 Hz ), 132.2, 129.9, 128.6, 116.1(d, J=22.5 Hz ), 63.8, 21.6 |
| 3d | 7.90(d,J=8.6 Hz, 2H), 7.74(d, J=8.2 Hz, 2H), 7.45(d, J=8.6 Hz, 2H), 7.34(d, J=8.1 Hz, 2H), 4.69(s, 2H), 2.44(s, 3H) | 187.0, 145.5, 141.0, 135.7, 134.1, 130.8, 129.9, 129.2, 128.5, 63.7, 21.6 |
| 3e | 7.84—7.81(m, 2H), 7.76—7.71(m, 2H), 7.66—7.61(m, 2H), 7.35(d, J=7.9 Hz, 2H), 4.67(s, 2H), 2.46(s, 3H) | 187.3, 145.6, 134.6, 132.6, 132.3, 130.9, 129.9, 129.0, 128.6, 63.8, 21.8 |
| 3f | 8.07(d, J=8.2 Hz, 2H), 7.74(d, J=8.2 Hz, 4H), 7.34(d, J=8.0 Hz, 2H), 4.75(s, 2H), 2.45(s, 3H) | 187.5, 145.7, 138.4, 135.6, 135.4(q,J=32.5 Hz), 129.9, 129.8, 128.6, 125.9(q, J=3.8 Hz), 123.4(q, J=271.3 Hz), 63.9, 21.6 |
| 3g | 8.34(d, J=8.8 Hz, 2H), 8.16(d, J=8.8 Hz, 2H), 7.75(d, J=8.3 Hz, 2H), 7.37(d, J=8.1 Hz, 2H), 4.75(s, 2H), 2.47(s, 3H) | 186.9, 150.9, 145.9, 139.9, 135.4, 130.5, 130.0, 128.5, 123.9, 64.2, 21.6 |
| 3h | 7.77—7.74(m, 2H), 7.74—7.68(m, 2H), 7.40(d, J=7.6 Hz, 1H), 7.33(dd, J=17.5, 7.8 Hz, 3H), 4.71(s, 2H), 2.42(s, 3H), 2.37(s, 3H) | 188.3, 145.3, 138.7, 135.8, 135.8, 135.1, 129.8, 129.6, 128.7, 128.6, 126.6, 63.5, 21.7, 21.3 |
| 3i | 8.01(t,J=1.8 Hz, 1H), 7.89(dt, J=7.8, 1.3 Hz, 1H), 7.77—7.71(m, 3H), 7.40—7.32(m, 3H), 4.68(s, 2H), 2.45(s, 3H) | 186.9, 145.6, 137.4, 137.1, 135.6, 132.1, 130.4, 129.9, 128.6, 127.9, 123.2, 63.7, 21.6 |
| 3j | 7.75(dd, J=12.7, 8.4 Hz, 3H), 7.42(td, J=7.5, 1.1 Hz, 1H), 7.32(d, J=8.0 Hz, 2H), 7.30—7.23(m, 2H), 4.69(s, 2H), 2.44(s, 3H), 2.43(s, 3H) | 190.6, 145.2, 140.0, 136.1, 135.8, 132.7, 132.3, 130.4, 129.8, 128.5, 125.9, 65.6, 21.7, 21.5 |
| 3l | 8.42(s, 1H), 8.00—7.90(m, 2H), 7.91—7.83(m, 2H), 7.77(d, J=8.2 Hz, 2H), 7.67—7.51(m, 2H), 7.28(d, J=7.9 Hz, 2H), 4.84(s, 2H), 2.38(s, 3H) | 188.0, 145.4, 135.9, 135.7, 133.1, 132.2, 132.1, 129.9, 129.8, 129.3, 128.8, 128.6, 127.8, 127.1, 123.9, 63.7, 21.6 |
| 3m | 7.82(dd,J=3.9, 1.0 Hz, 1H), 7.79—7.73(m, 3H), 7.34(d, J=8.0 Hz, 2H), 7.17(dd, J=4.9, 3.9 Hz, 1H), 4.61(s, 2H), 2.44(s, 3H) | 180.3, 145.5, 143.3, 136.3, 135.2, 129.9, 129.7, 128.7, 128.6, 64.8, 21.6 |
| 3n | 7.79—7.74(m, 2H), 7.61(dd, J=1.6, 0.7 Hz, 1H), 7.36—7.31(m, 3H), 6.58(dd, J=3.7, 1.7 Hz, 1H), 4.57(s, 2H), 2.44(s, 3H) | 175.9, 151.9, 148.1, 145.4, 135.7, 129.8, 128.5, 120.4, 113.2, 63.6, 21.7 |
| 3o | 8.01—7.96(m, 2H), 7.66(d, J=8.3 Hz, 2H), 7.63—7.57(m, 1H), 7.52—7.45(m, 2H), 7.31(d, J=8.0 Hz, 2H), 5.16(q, J=6.9 Hz, 1H), 2.43(s, 3H), 1.56(d, J=6.9 Hz, 3H) | 192.6, 145.3, 136.3, 133.9, 129.8, 129.5, 129.2, 128.7, 125.5, 65.0, 21.7, 13.2 |
| 3p | 7.94(ddd, J=17.6, 8.4, 1.1 Hz, 4H), 7.64(dt, J=22.5, 7.4 Hz, 2H), 7.54(t, J=7.8 Hz, 2H), 7.47(t, J=7.8 Hz, 2H), 4.75(s, 2H) | 187.9, 138.8, 135.7, 134.4, 134.2, 129.3, 129.2, 128.9, 128.6, 63.4 |
| 3q | 7.96(dd, J=8.4, 1.1 Hz, 2H), 7.83—7.78(m, 2H), 7.65—7.59(m, 1H), 7.51—7.46(m, 2H), 7.01—6.96(m, 2H), 4.71(s, 2H), 3.88(s, 3H) | 187.8, 163.7, 135.4, 133.9, 130.5, 129.8, 128.98, 128.5, 114.1, 63.6, 55.6 |
| 3r | 8.00—7.87(m, 4H), 7.63(t, J=6.8 Hz, 1H), 7.49(q, J=6.7 Hz, 2H), 7.21(t, J=7.7 Hz, 2H), 4.75(s, 2H) | 187.7, 165.5(d, J=256 Hz), 135.4, 134.3, 134.3, 131.7(d, J=10 Hz), 129.0, 128.7, 116.3(d, J=23 Hz), 63.3 |
| 3s | 7.94(dt, J=8.5, 1.5 Hz, 2H), 7.86—7.81(m, 2H), 7.67—7.61(m, 1H), 7.55—7.47(m, 4H), 4.75(s, 2H) | 187.9, 141.2, 137.1, 135.6, 134.5, 130.2, 129.5, 129.2, 128.9, 63.3 |
| 3t | 7.95(d, J=7.4 Hz, 2H), 7.75(d, J=8.6 Hz, 2H), 7.68(d, J=8.5 Hz, 2H), 7.63(t, J=7.4 Hz, 1H), 7.48(t, J=7.8 Hz, 2H), 4.75(s, 2H) | 187.9, 137.8, 135.6, 134.5, 132.5, 130.2, 129.7, 129.2, 128.9, 63.3 |
| 3u | 8.06(d, J=8.2 Hz, 2H), 7.92(dd, J=8.3, 1.1 Hz, 2H), 7.81(d, J=8.3 Hz, 2H), 7.67—7.61(m, 1H), 7.49(t, J=7.9 Hz, 2H), 4.80(s, 2H) | 187.5, 141.8, 135.7, 135.3(d,J=15.7 Hz), 134.4, 129.2, 129.0, 128.8, 126.9(q, J=3 Hz), 123.1(q, J=272 Hz), 62.9 |
| 3v | 8.08—8.02(m, 1H), 7.97—7.91(m, 2H), 7.64—7.55(m, 3H), 7.51—7.41(m, 3H), 5.06(s, 2H) | 187.6, 136.2, 135.5, 134.9, 134.3, 132.5, 131.8, 131.6, 128.9, 128.7, 127.3, 60.9 |
| 3w | 8.48(s, 1H), 8.01—7.91(m, 5H), 7.87(dd, J=8.6, 1.9 Hz, 1H), 7.73—7.65(m, 1H), 7.65—7.55(m, 2H), 7.45(t, J=7.8 Hz, 2H), 4.81(s, 2H) | 187.9, 135.5, 135.4, 135.3, 134.1, 131.8, 130.4, 129.4, 129.3, 129.1, 128.6,128.0, 127.8, 127.5, 122.7, 63.6 |
Table 2 1H NMR and 13C NMR data of target compounds 3a—3w
| Compd. | 1H NMR(500 MHz), δ | 13C NMR(125 MHz), δ |
|---|---|---|
| 3a | 7.97(d,J=7.4 Hz, 2H), 7.77(d, J=8.2 Hz, 2H), 7.62(t, J=7.4 Hz, 1H), 7.46(t, J=7.8 Hz, 2H), 7.33(d, J=8.2 Hz, 2H), 4.72(s, 2H), 2.44(s, 3H) | 188.2, 145.4, 135.8, 135.8, 134.3, 129.8, 129.3, 128.8, 128.6, 63.6, 21.6 |
| 3b | 7.86(d,J=8.3 Hz, 2H), 7.75(d, J=8.3 Hz, 2H), 7.33(d, J=8.0 Hz, 2H), 7.27(d, J=9.4 Hz, 2H), 4.69(s, 2H), 2.44(s, 3H), 2.42(s, 3H) | 187.6, 145.6, 145.3, 135.9, 133.4, 129.8, 129.5, 129.5, 128.6, 63.6, 21.8, 21.6 |
| 3c | 8.03—7.98(m, 2H), 7.75(d, J=8.3 Hz, 2H), 7.34(d, J=8.1 Hz, 2H), 7.19—7.13(m, 2H), 4.69(s, 2H), 2.45(s, 3H) | 186.6, 166.5(d, J=256.3 Hz ), 145.5, 135.7, 132.2(d, J=2.5 Hz ), 132.2, 129.9, 128.6, 116.1(d, J=22.5 Hz ), 63.8, 21.6 |
| 3d | 7.90(d,J=8.6 Hz, 2H), 7.74(d, J=8.2 Hz, 2H), 7.45(d, J=8.6 Hz, 2H), 7.34(d, J=8.1 Hz, 2H), 4.69(s, 2H), 2.44(s, 3H) | 187.0, 145.5, 141.0, 135.7, 134.1, 130.8, 129.9, 129.2, 128.5, 63.7, 21.6 |
| 3e | 7.84—7.81(m, 2H), 7.76—7.71(m, 2H), 7.66—7.61(m, 2H), 7.35(d, J=7.9 Hz, 2H), 4.67(s, 2H), 2.46(s, 3H) | 187.3, 145.6, 134.6, 132.6, 132.3, 130.9, 129.9, 129.0, 128.6, 63.8, 21.8 |
| 3f | 8.07(d, J=8.2 Hz, 2H), 7.74(d, J=8.2 Hz, 4H), 7.34(d, J=8.0 Hz, 2H), 4.75(s, 2H), 2.45(s, 3H) | 187.5, 145.7, 138.4, 135.6, 135.4(q,J=32.5 Hz), 129.9, 129.8, 128.6, 125.9(q, J=3.8 Hz), 123.4(q, J=271.3 Hz), 63.9, 21.6 |
| 3g | 8.34(d, J=8.8 Hz, 2H), 8.16(d, J=8.8 Hz, 2H), 7.75(d, J=8.3 Hz, 2H), 7.37(d, J=8.1 Hz, 2H), 4.75(s, 2H), 2.47(s, 3H) | 186.9, 150.9, 145.9, 139.9, 135.4, 130.5, 130.0, 128.5, 123.9, 64.2, 21.6 |
| 3h | 7.77—7.74(m, 2H), 7.74—7.68(m, 2H), 7.40(d, J=7.6 Hz, 1H), 7.33(dd, J=17.5, 7.8 Hz, 3H), 4.71(s, 2H), 2.42(s, 3H), 2.37(s, 3H) | 188.3, 145.3, 138.7, 135.8, 135.8, 135.1, 129.8, 129.6, 128.7, 128.6, 126.6, 63.5, 21.7, 21.3 |
| 3i | 8.01(t,J=1.8 Hz, 1H), 7.89(dt, J=7.8, 1.3 Hz, 1H), 7.77—7.71(m, 3H), 7.40—7.32(m, 3H), 4.68(s, 2H), 2.45(s, 3H) | 186.9, 145.6, 137.4, 137.1, 135.6, 132.1, 130.4, 129.9, 128.6, 127.9, 123.2, 63.7, 21.6 |
| 3j | 7.75(dd, J=12.7, 8.4 Hz, 3H), 7.42(td, J=7.5, 1.1 Hz, 1H), 7.32(d, J=8.0 Hz, 2H), 7.30—7.23(m, 2H), 4.69(s, 2H), 2.44(s, 3H), 2.43(s, 3H) | 190.6, 145.2, 140.0, 136.1, 135.8, 132.7, 132.3, 130.4, 129.8, 128.5, 125.9, 65.6, 21.7, 21.5 |
| 3l | 8.42(s, 1H), 8.00—7.90(m, 2H), 7.91—7.83(m, 2H), 7.77(d, J=8.2 Hz, 2H), 7.67—7.51(m, 2H), 7.28(d, J=7.9 Hz, 2H), 4.84(s, 2H), 2.38(s, 3H) | 188.0, 145.4, 135.9, 135.7, 133.1, 132.2, 132.1, 129.9, 129.8, 129.3, 128.8, 128.6, 127.8, 127.1, 123.9, 63.7, 21.6 |
| 3m | 7.82(dd,J=3.9, 1.0 Hz, 1H), 7.79—7.73(m, 3H), 7.34(d, J=8.0 Hz, 2H), 7.17(dd, J=4.9, 3.9 Hz, 1H), 4.61(s, 2H), 2.44(s, 3H) | 180.3, 145.5, 143.3, 136.3, 135.2, 129.9, 129.7, 128.7, 128.6, 64.8, 21.6 |
| 3n | 7.79—7.74(m, 2H), 7.61(dd, J=1.6, 0.7 Hz, 1H), 7.36—7.31(m, 3H), 6.58(dd, J=3.7, 1.7 Hz, 1H), 4.57(s, 2H), 2.44(s, 3H) | 175.9, 151.9, 148.1, 145.4, 135.7, 129.8, 128.5, 120.4, 113.2, 63.6, 21.7 |
| 3o | 8.01—7.96(m, 2H), 7.66(d, J=8.3 Hz, 2H), 7.63—7.57(m, 1H), 7.52—7.45(m, 2H), 7.31(d, J=8.0 Hz, 2H), 5.16(q, J=6.9 Hz, 1H), 2.43(s, 3H), 1.56(d, J=6.9 Hz, 3H) | 192.6, 145.3, 136.3, 133.9, 129.8, 129.5, 129.2, 128.7, 125.5, 65.0, 21.7, 13.2 |
| 3p | 7.94(ddd, J=17.6, 8.4, 1.1 Hz, 4H), 7.64(dt, J=22.5, 7.4 Hz, 2H), 7.54(t, J=7.8 Hz, 2H), 7.47(t, J=7.8 Hz, 2H), 4.75(s, 2H) | 187.9, 138.8, 135.7, 134.4, 134.2, 129.3, 129.2, 128.9, 128.6, 63.4 |
| 3q | 7.96(dd, J=8.4, 1.1 Hz, 2H), 7.83—7.78(m, 2H), 7.65—7.59(m, 1H), 7.51—7.46(m, 2H), 7.01—6.96(m, 2H), 4.71(s, 2H), 3.88(s, 3H) | 187.8, 163.7, 135.4, 133.9, 130.5, 129.8, 128.98, 128.5, 114.1, 63.6, 55.6 |
| 3r | 8.00—7.87(m, 4H), 7.63(t, J=6.8 Hz, 1H), 7.49(q, J=6.7 Hz, 2H), 7.21(t, J=7.7 Hz, 2H), 4.75(s, 2H) | 187.7, 165.5(d, J=256 Hz), 135.4, 134.3, 134.3, 131.7(d, J=10 Hz), 129.0, 128.7, 116.3(d, J=23 Hz), 63.3 |
| 3s | 7.94(dt, J=8.5, 1.5 Hz, 2H), 7.86—7.81(m, 2H), 7.67—7.61(m, 1H), 7.55—7.47(m, 4H), 4.75(s, 2H) | 187.9, 141.2, 137.1, 135.6, 134.5, 130.2, 129.5, 129.2, 128.9, 63.3 |
| 3t | 7.95(d, J=7.4 Hz, 2H), 7.75(d, J=8.6 Hz, 2H), 7.68(d, J=8.5 Hz, 2H), 7.63(t, J=7.4 Hz, 1H), 7.48(t, J=7.8 Hz, 2H), 4.75(s, 2H) | 187.9, 137.8, 135.6, 134.5, 132.5, 130.2, 129.7, 129.2, 128.9, 63.3 |
| 3u | 8.06(d, J=8.2 Hz, 2H), 7.92(dd, J=8.3, 1.1 Hz, 2H), 7.81(d, J=8.3 Hz, 2H), 7.67—7.61(m, 1H), 7.49(t, J=7.9 Hz, 2H), 4.80(s, 2H) | 187.5, 141.8, 135.7, 135.3(d,J=15.7 Hz), 134.4, 129.2, 129.0, 128.8, 126.9(q, J=3 Hz), 123.1(q, J=272 Hz), 62.9 |
| 3v | 8.08—8.02(m, 1H), 7.97—7.91(m, 2H), 7.64—7.55(m, 3H), 7.51—7.41(m, 3H), 5.06(s, 2H) | 187.6, 136.2, 135.5, 134.9, 134.3, 132.5, 131.8, 131.6, 128.9, 128.7, 127.3, 60.9 |
| 3w | 8.48(s, 1H), 8.01—7.91(m, 5H), 7.87(dd, J=8.6, 1.9 Hz, 1H), 7.73—7.65(m, 1H), 7.65—7.55(m, 2H), 7.45(t, J=7.8 Hz, 2H), 4.81(s, 2H) | 187.9, 135.5, 135.4, 135.3, 134.1, 131.8, 130.4, 129.4, 129.3, 129.1, 128.6,128.0, 127.8, 127.5, 122.7, 63.6 |
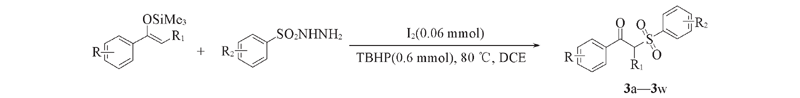
Scheme 1 Synthesis of compounds 3a—3w with I2 as catalyst and TBHP as oxidant R=CH3, F, Cl, Br, CF3, NO2, naphthyl, thienyl, furyl; R1=H, CH3; R2=H, CH3, CH3O, F, Cl, CF3, naphthyl.
| Entry | Catalyst | Oxidant | Solvent | Yieldb(%) | Entry | Catalyst | Oxidant | Solvent | Yieldb(%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | KI | TBHP | CH3CN | 25 | 10 | I2 | TBHP | DCE | 66 |
| 2 | I2 | TBHP | CH3CN | 43 | 11 | I2 | TBHP | Toluene | Trace |
| 3 | NaI | TBHP | CH3CN | 20 | 12 | | I2O5 | DCE | Trace |
| 4 | TBAI | TBHP | CH3CN | 32 | 13 | I2 | K2S2O8 | DCE | Trace |
| 5 | I2 | TBHP | CH3CN | 55c | 14 | I2 | DTBP | DCE | Trace |
| 6 | I2 | TBHP | CH3CN | 34d | 15 | I2 | TBHP | DCE | 44 |
| 7 | I2 | TBHP | THF | Trace | 16 | I2 | TBHP | DCE | 64e |
| 8 | I2 | TBHP | 1,4-Dioxane | 35 | 17 | I2 | TBHP | DCE | 62f |
| 9 | I2 | TBHP | DMF | Trace |
Table 3 Optimization of reaction conditionsa
| Entry | Catalyst | Oxidant | Solvent | Yieldb(%) | Entry | Catalyst | Oxidant | Solvent | Yieldb(%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | KI | TBHP | CH3CN | 25 | 10 | I2 | TBHP | DCE | 66 |
| 2 | I2 | TBHP | CH3CN | 43 | 11 | I2 | TBHP | Toluene | Trace |
| 3 | NaI | TBHP | CH3CN | 20 | 12 | | I2O5 | DCE | Trace |
| 4 | TBAI | TBHP | CH3CN | 32 | 13 | I2 | K2S2O8 | DCE | Trace |
| 5 | I2 | TBHP | CH3CN | 55c | 14 | I2 | DTBP | DCE | Trace |
| 6 | I2 | TBHP | CH3CN | 34d | 15 | I2 | TBHP | DCE | 44 |
| 7 | I2 | TBHP | THF | Trace | 16 | I2 | TBHP | DCE | 64e |
| 8 | I2 | TBHP | 1,4-Dioxane | 35 | 17 | I2 | TBHP | DCE | 62f |
| 9 | I2 | TBHP | DMF | Trace |
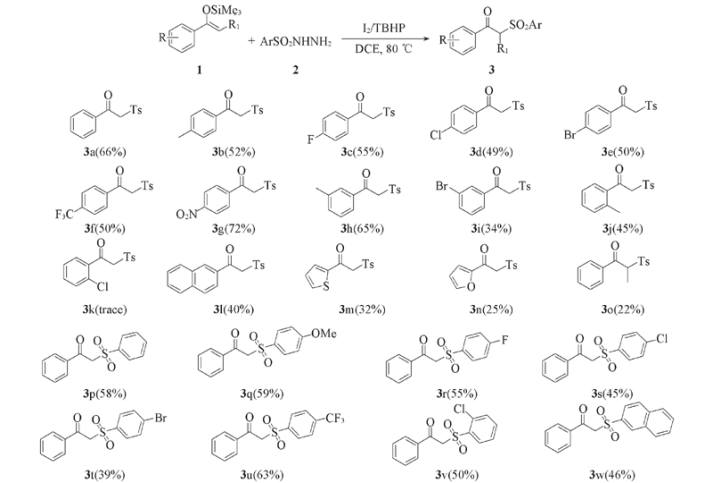
Scheme 2 Substrate scope of enoxysilanes and sulfonylhydrazides Reaction conditions: 0.3 mmol of compound 1, 0.6 mmol of compound 2, 0.06 mmol of I2, 0.6 mmol of TBHP and 2.0 mL of DCE at 80 ℃ for 8 h; data of isolated yield is based on compound 1.
| [1] |
Ma M. L ., Cheng Y. Y.,Xu Z. H.,Xu P.,Qu H. O.,Fang Y. J.,Xu T. W.,Wen L. P., Eur.J. Med. Chem., 2007,42, 93— 98
doi: 10.1016/j.ejmech.2006.07.015 URL pmid: 17095123 |
| [2] | Lythgoe B., Waterhouse I., Tetrahedron Lett ., 1978,19, 2625— 2628 |
| [3] |
Bartlett P. A ., Green F. R.,Rose E. H., J. Am. Chem. Soc., 1978,100, 4852— 4858
doi: 10.1021/ja00483a035 URL |
| [4] |
Ihara M., Suzuki S., Taniguchi T., Tokunaga Y., Fukumoto K ., Tetrahedron, 1995,51, 9873— 9890
doi: 10.1016/0040-4020(95)00565-P URL |
| [5] |
Sengupta S., Sarma D. S ., Mondal S.,. Tetrahedron: Asymmetry, 1998,9, 2311— 2316
doi: 10.1016/S0957-4166(98)00235-3 URL |
| [6] |
Marco J. L ., Fernandez I.,Khiar N.,Fernandez P.,Romero A., J. Org. Chem., 1995,60, 6678— 6679
doi: 10.1021/jo00126a014 URL |
| [7] |
Gotor V., Rebolledo F., Liz R ., Tetrahedron: Asymmetry, 2001,12, 513— 515
doi: 10.1016/S0957-4166(01)00048-9 URL |
| [8] |
Wolf W. M ., J. Mol. Struct., 1999,474, 113— 124
doi: 10.1016/S0022-2860(98)00565-1 URL |
| [9] | Vennstra G. E ., Zwaneburg B.,. Synthesis, 1975, 519— 520 |
| [10] |
Xie Y. Y ., Chen Z. C. Synth. Commun., 2001,31, 3145— 3149
doi: 10.1081/SCC-100105890 URL |
| [11] | Jiang H., Chen Y. Z ., Zhang Y.,Yu S. Y., Eur.J. Org. Chem., 2013, 5485— 5492 |
| [12] |
Tang X. D ., Huang L. B.,Xu Y. L.,Yang J. D.,Wu W. Q.,Jiang H. F., Angew. Chem. Int. Ed., 2014,53, 4205— 4208
doi: 10.1002/anie.201311217 URL pmid: 24677481 |
| [13] |
Tang Y. C ., Zhang Y.,Wang K. F.,Li X. Q.,Xu X. S.,Du X. H., Org. Biomol. Chem., 2015,13, 7084— 7090
doi: 10.1039/c5ob00742a URL pmid: 26052796 |
| [14] |
Wang H. M., Wang G. Y., Lu Q. Q., Chiang C. W., Peng P., Zhou J. F., Lei A. W., Chem. Eur. J., 2016,22, 14489— 14493
doi: 10.1002/chem.201603041 URL pmid: 27500979 |
| [15] |
Tang Y. C ., Fan Y. Y., Gao H. J., Li X. Q., Xu X. S., Tetrahedron Lett., 2015,56, 5616— 5618
doi: 10.1016/j.tetlet.2015.08.055 URL |
| [16] |
Yadav V. K ., Srivastava V. P.,Yadav L. D. S., Synlett., 2016,27, 427— 431
doi: 10.1055/s-00000083 URL |
| [17] |
Li L., Chen Q. Y ., Guo Y., J. Org. Chem., 2014,79, 5145— 5152
doi: 10.1021/jo500713f URL pmid: 24836973 |
| [18] |
Shen H., Li J. Q ., Liu Q.,Pan J.,Huang R. F.,Xiong Y., J. Org. Chem., 2015,80, 7212— 7218
doi: 10.1021/acs.joc.5b01102 URL pmid: 26067204 |
| [19] |
Zhang X. X ., Huang H. M., Org. Lett., 2018,20, 4998— 5001
doi: 10.1021/acs.orglett.8b02154 URL pmid: 30074810 |
| [20] | Kamigata N., Udodaira K., Shimizu T ., J. Chem. Soc., Perkin Trans. 1, 1997, 783— 786 |
| [21] |
Tang Y. C ., Chen Y.,Liu H.,Guo M., Tetrahedron Lett., 2018,59, 3703— 3705
doi: 10.1016/j.tetlet.2018.09.005 URL |
| [22] |
Tang Y. C., Ran S. T.,Wang P.,Chen P., Chin. J. Org. Chem., 2019,39(4), 1116— 1121
doi: 10.6023/cjoc201810002 URL |
|
( 唐裕才, 冉书童, 王萍, 陈飘. 有机化学, 2019,39(4), 1116— 1121)
doi: 10.6023/cjoc201810002 URL |
|
| [23] | Tang Y. C., Wang P.,Ran S. T.,Chen P., Chin. J. Appl. Chem., 2019,36(6), 664— 670 |
| ( 唐裕才, 王萍, 冉书童, 陈飘. 应用化学, 2019,36(6), 664— 670) | |
| [24] |
Li X. Q ., Xu X. S.,Shi X. H., Tetrahedron Lett., 2013,54, 3071— 3074
doi: 10.1016/j.tetlet.2013.03.117 URL |
| [25] |
Zhan Z. Z ., Ma H. J.,Wei D. D.,Pu J. H.,Zhang Y. X.,Huang G. S., Tetrahedron Lett., 2018,59, 1446— 1450
doi: 10.1016/j.tetlet.2018.02.078 URL |
| [26] |
Zheng L., Zhou Z. Z ., He Y. T.,Li L. H.,Ma J. W.,Qiu Y. F.,Zhou P. X.,Liu X. Y.,Xu P. F.,Liang Y. M., J. Org. Chem., 2016,81, 66— 76
doi: 10.1021/acs.joc.5b02161 URL pmid: 26642246 |
| [27] |
Yu H. Y., Pi C.,Wang Y.,Cui X. L.,Wu Y. J., Chin. J. Org. Chem., 2018,38(1), 124— 130
doi: 10.6023/cjoc201709054 URL |
|
( 余海洋, 皮超, 王勇, 崔秀灵, 吴养洁. 有机化学, 2018,38(1), 124— 130
doi: 10.6023/cjoc201709054 URL |
| [1] | 余益民, 郭兴华, 刘子阳, 刘淑莹, 傅芳信, 钱明星. C60与n-Bu3SnH的自由基反应研究[J]. 高等学校化学学报, 1996, 17(2): 303. |
| [2] | 李良助, 赵秀敏, 赵亚军, 袁晋芳. 一种新型的芳基重排反应(Ⅱ)──苯并环己酮中β-芳基的重排[J]. 高等学校化学学报, 1994, 15(2): 216. |
| [3] | 曾百肇, 郑玉霞, 周性尧. 氢醌对胆红素氧化过程的促进作用及其分析意义[J]. 高等学校化学学报, 1993, 14(2): 167. |
| [4] | 李良助, 芮元金. 黄烷酮烯醇硅醚氧化重排成异黄酮[J]. 高等学校化学学报, 1991, 12(6): 777. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||