

高等学校化学学报 ›› 2019, Vol. 40 ›› Issue (11): 2396.doi: 10.7503/cjcu20190371
收稿日期:2019-07-03
出版日期:2019-11-10
发布日期:2019-10-15
通讯作者:
张志明,于良民
E-mail:zzmcyj@ouc.edu.cn;yuyan@ouc.edu.cn
基金资助:
ZHANG Jiayi,JIA Mengyang,JIANG Xiaohui,ZHANG Zhiming( ),YU Liangmin(
),YU Liangmin( ),WANG Xuan
),WANG Xuan
Received:2019-07-03
Online:2019-11-10
Published:2019-10-15
Contact:
ZHANG Zhiming,YU Liangmin
E-mail:zzmcyj@ouc.edu.cn;yuyan@ouc.edu.cn
Supported by:摘要:
采用化学氧化聚合法合成了一系列十二烷基苯磺酸掺杂的聚吡咯(PPy-DBSA), 并研究了其电化学防污性能. 循环伏安(CV)曲线表明, PPy-DBSA在天然海水中具有良好的电化学活性和稳定性. 采用循环伏安扫描方法实现阳极极化和阴极极化交替进行, 并对极化后的PPy-DBSA电极进行了抑菌性能研究, 发现PPy-DBSA在循环伏安阳极-阴极交替(-1.0~2.0 V vs. SCE)极化下, 可成功抑制微生物(大肠杆菌)的附着, 其中在-0.6~0.8 V范围内循环伏安阳极-阴极交替极化20 min时防污效果最佳, 抑菌率可达99.8%, 明显优于恒电位阳极极化和恒电位阴极极化的结果.
中图分类号:
TrendMD:
张珈漪,贾梦洋,姜晓辉,张志明,于良民,王璇. 十二烷基苯磺酸掺杂聚吡咯在阳极-阴极交替极化下的防污性能. 高等学校化学学报, 2019, 40(11): 2396.
ZHANG Jiayi,JIA Mengyang,JIANG Xiaohui,ZHANG Zhiming,YU Liangmin,WANG Xuan. Antifouling Properties of Dodecyl Benzene Sulfonic Acid Doped Polypyrrole Under Alternating Anodic-cathodic Polarization †. Chem. J. Chinese Universities, 2019, 40(11): 2396.
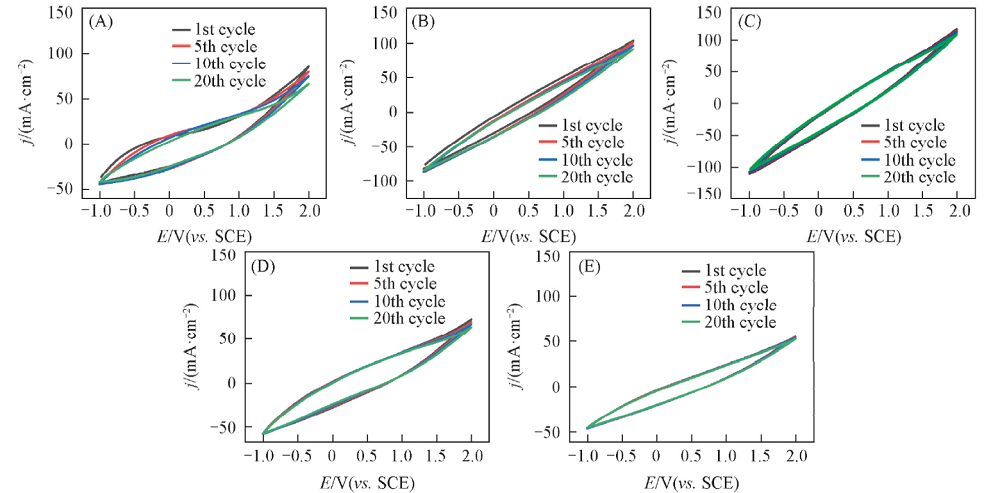
Fig.4 CV curves of different PPy-DBSA samples (A) PPy-DBSA-0.1; (B) PPy-DBSA-0.3; (C) PPy-DBSA-0.5; (D) PPy-DBSA-1.0; (E) PPy-DBSA-2.0. Scan rate: 100 mV/s.
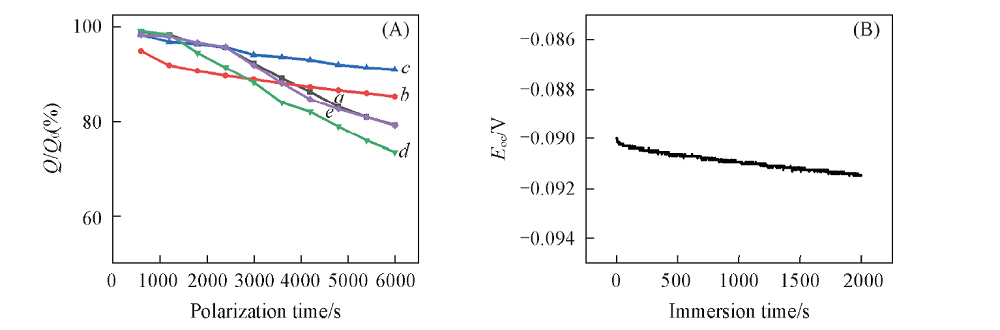
Fig.5 Current capacity percentage of different PPy-DBSA in natural seawater after cyclic voltammetry scan(-1.0—2.0 V) and the open circuit potentials(B) of PPy-DBSA-0.5 electrode in natural seawater(pH=8.0) a. PPy-DBSA-0.1; b. PPy-DBSA-0.3; c. PPy-DBSA-0.5; d. PPy-DBSA-1.0; e. PPy-DBSA-2.0.
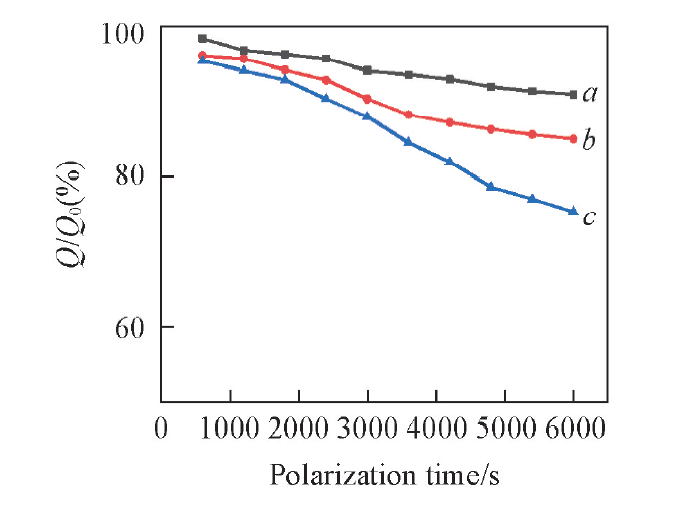
Fig.6 Current capacity percentage of PPy-DBSA-0.5 in natural seawater after cyclic voltammetry scan with different scan range a. -1.0—2.0 V; b. -1.0—-0.091 V; c. -0.091—2.0 V.
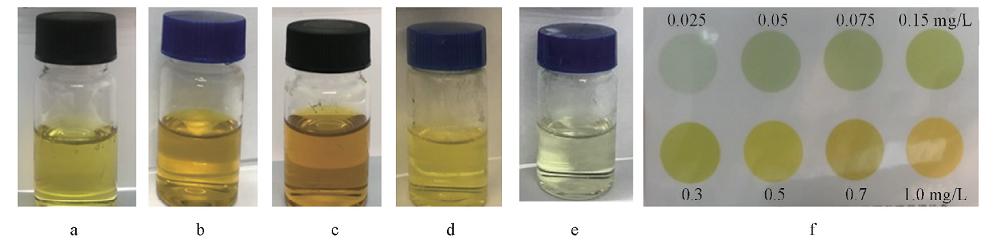
Fig.7 Photographs of polarized seawater tested with residual chlorine test kit after CV scan within -1.0—2.0 V(vs. SCE) for 30 min using differen PPy-DBSA samples Scan rate: 100 mV/s. a. PPy-DBSA-0.1; b. PPy-DBSA-0.3; c. PPy-DBSA-0.5; d. PPy-DBSA-1; e. PPy-DBSA-2. f. standard colour card of residual chlorine concentration.
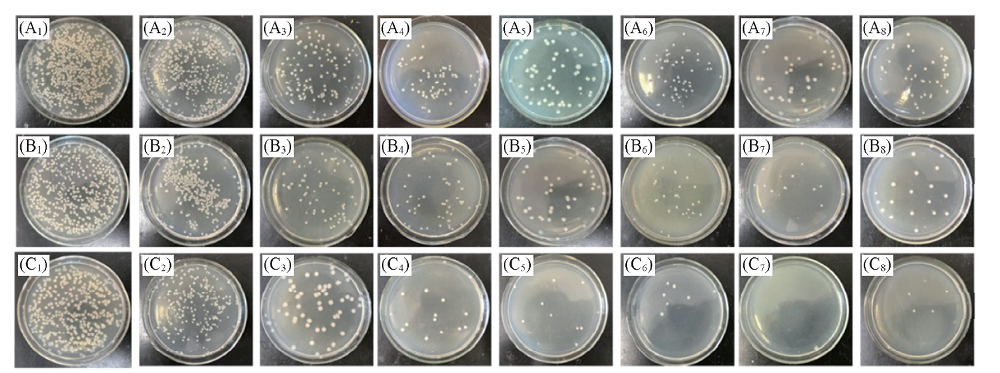
Fig.8 Photographs of E.coli incubated on agar plates from dilute solution collected from PPy-DBSA electrode after polarization under different potentials (A1—C1) Blank; (A2—C2) 0 V; (A3—C3) cathodic polarization(-0.6 V); (A4—C4) anodic polarization(+0.8 V); (A5—C5) CV(-0.6—0.6 V); (A6—C6) CV(-0.6—0.7 V); (A7—C7) -CV(-0.6—0.8 V); (A8—C8) CV(-0.6—1.0 V). (A1—A8) 10 min; (B1—B8) 15 min; (C1—C8) 20 min.
| E/V(vs. SCE) | t/min | E/V(vs. SCE) | t/min | ||||
|---|---|---|---|---|---|---|---|
| 10 | 15 | 20 | 10 | 15 | 20 | ||
| Blank | 0 | 0 | 0 | -0.6~0.6 | 90.1% | 91.9% | 97.5% |
| 0 | 31.2% | 41.6% | 43.2% | -0.6~0.7 | 92.3% | 94.1% | 98.6% |
| -0.6 | 88.8% | 90.9% | 91.9% | -0.6~0.8 | 94.7% | 96.7% | 99.8% |
| +0.6 | 82.5% | 87.5% | 87.6% | -0.6~1.0 | 94.1% | 96.3% | 99.0% |
| +0.8 | 89.8% | 91.5% | 96.4% | ||||
Table 1 Antimicrobial efficiency of PPy-DBSA-0.5 electrode
| E/V(vs. SCE) | t/min | E/V(vs. SCE) | t/min | ||||
|---|---|---|---|---|---|---|---|
| 10 | 15 | 20 | 10 | 15 | 20 | ||
| Blank | 0 | 0 | 0 | -0.6~0.6 | 90.1% | 91.9% | 97.5% |
| 0 | 31.2% | 41.6% | 43.2% | -0.6~0.7 | 92.3% | 94.1% | 98.6% |
| -0.6 | 88.8% | 90.9% | 91.9% | -0.6~0.8 | 94.7% | 96.7% | 99.8% |
| +0.6 | 82.5% | 87.5% | 87.6% | -0.6~1.0 | 94.1% | 96.3% | 99.0% |
| +0.8 | 89.8% | 91.5% | 96.4% | ||||
| [1] | Callow M. E., Callow J. A., Biologist, 2002,49(1), 1— 5 |
| [2] | Gaw S. L., Sarkar S., Nir S., Schnell Y., Mandler D., Xu Z. J., Lee P. S., Reches M., ACS Applied Materials & Interfaces, 2017,9(31), 26503— 26509 |
| [3] | Zhang B., Nagle A. R., Wallace G. G., Hanks T. W., Molino P. J., Biofouling, 2015,31(6), 493— 502 |
| [4] | Baldissera A. F., Miranda K. L., Bressy C., Martin C., Margaillan A., Ferreira C. A., Materials Research, 2015,18(6), 1129— 1139 |
| [5] | Jia M. Y., Zhang Z. M., Yu L. M., Wang J., Zheng T. T., Colloids and Surfaces B: Biointerfaces, 2018,164, 247— 254 |
| [6] | Qiu R., Wang P., Zhang D., Wang W., Advanced Materials Research, 2011,189/193, 786— 789 |
| [7] | Alzieu C., Thibaud Y., Heral M., Boutier B ., Review Travel Institution, 1980,44(4), 305— 348 |
| [8] | Wezel A. P., Vlaardinger P., Aquatic Toxicology, 2004,66(4), 427— 444 |
| [9] | Evans S. M., Evans P. M., Leksono T., Marine Pollution Bulletin, 1996,32(3), 263— 269 |
| [10] | Guerreiro A. S., Rola R. C., Rovani M. T., Costa S. R., Sandrini J. Z., Aquatic Toxicology, 2017,189, 194— 199 |
| [11] | Garayzar A. B. S., Bahamonde P. A., Martyniuk C. J., Betancourt M., Munkittrick K. R., Comparative Biochemistry and Physiology Part D: Genomics and Proteomics, 2016,19, 102— 111 |
| [12] | Hudspith M., Reichelt B. A., Harrison P. L., Aquatic Toxicology, 2017,184, 1— 13 |
| [13] | Chiang C., Fincher C., Park Y., Heeger A., Shirakawa H., Louis E., Gau S ., Physical Review Letters, 1977,39(17), 1098— 1101 |
| [14] | Tsumura A., Koezuka H., Ando T ., Applied Physics Letters, 1986,49(18), 1210— 1212 |
| [15] | Novak P., Muller K., Santhanam K. S. V., Haas O., Chemical Reviews, 1997,97, 207— 281 |
| [16] | Janata J., Josowicz M ., Nature Materials, 2003,2(1), 19— 24 |
| [17] | Michalska A., Ocypa M., Maksymiuk K ., Analytical and Bioanalytical Chemistry, 2006,385(1), 203— 207 |
| [18] | Deberry D. W., Journal of the Electrochemical Society, 1985,132(5), 1022— 1026 |
| [19] | Khan M. I., Chaudhry A. U., Hashim S., Zahoor M. K., Iqbal M. Z., Chemical Engineering Research Bulletin, 2010,14, 73— 86 |
| [20] | Hideo Kobayashi, Masao Kobayashi, , Antifouling Material in Water JPS 62-249906, 1987 -10-30 |
| [21] | Wang X. H., Li J., Zhang J. Y., Sun Z. C., Yu L., Jing X. B., Wang F. S., Sun Z. X., Ye Z. J., Synthetic Metals, 1999,102(1), 1377— 1380 |
| [22] | Van D. V., Theo G. M., Journal of Colloid and Interface Science, 1989,143, 593 |
| [23] | Shim S., Hong S. H., Tak Y., Yoon J., Biofouling, 2011,27(2), 217— 224 |
| [24] | Charpentier T. V., Neville A., Baudin S., Smith M. J., Euvrard M., Bell A., Wang C., Barker R., Journal of Colloid & Interface Science, 2015,444, 81— 86 |
| [25] | Jia M. Y., Zhang Z. M., Yu L. M., Wang J., Electrochemistry Communications, 2017,84, 57— 60 |
| [26] | Omae I ., Chemical Reviews, 2003,103(9), 3431— 3448 |
| [27] | Omae I ., Applied Organometallic Chemistry, 2003,17(2), 81— 105 |
| [28] | Chen S. K., Zhu J. F., Zhou T. G., He B., Huang W. Z., Wang B. C., International Journal of Electrochemical Science, 2012,7(9), 8170— 8184 |
| [29] | Zhang L. L., Xu J., Tang Y. Y., Hou J. W., Yu L. M., Gao C. J., Journal of Materials Chemistry A, 2016,4(26), 10352— 10362 |
| [30] | Xu L., Zhang G. Q., Yuan G. E., Liu H. Y., Yang F. L., RSC Advances, 2015,5(29), 22533— 22543 |
| [31] | Song M. K., Kim Y. T., Kim B. S., Kim J., Char K., Rhee H. W., Synthetic Metals, 2004,141(3), 315— 319 |
| [32] | Bose S., Mishra A. K., Kuila T., Kim N. H., Park O. K., Lee J. H., Chemical Engineering Journal, 2012,187, 334— 340 |
| [33] | Scott J. C., Pfluger P., Krounbim T., Street G. B., Physical Review B, 1983,28(4), 2140— 2145 |
| [34] | Bredas J. L., Street G. B., Accounts of Chemical Research, 1985,18(10), 309— 315 |
| [35] | Lannoy C. F., J assby D., Gloe K., Gordon A. D., Wiesner M. R., Environmental Science & Technology, 2013,47(6), 2760— 2768 |
| [36] | Huang J. R., Lin W. T., Huang R., Lin C. Y., Wu J. K., Journal of Coatings Technology and Research, 2010,7(1), 111— 117 |
| [37] | Wake H., Takimoto T., Takayanagi H., Ozawa K., Kadoi H., Mukai S., Matsunaga T ., Journal of Chemical Engineering of Japan, 2010,43(7), 608— 611 |
| [38] | Yoo D., Kim J., Kim J. H., Nano Research, 2014,7, 717— 730 |
| [39] | March J. G., Gual M., Talanta, 2002,58(5), 995— 1001 |
| [40] |
Ma X. D., Zhang Z. M., Yu L. M., . Chem. J. Chinese Universities, 2016,37(2), 373— 380
doi: 10.7503/cjcu20150627 |
|
( 马晓丹, 张志明, 于良民 . 高等学校化学学报, 2016,37(2), 373— 380
doi: 10.7503/cjcu20150627 |
|
| [41] | Qi K., Qiu Y. B., Chen Z. Y., Guo X. P., Corrosion Science, 2013,69, 376— 388 |
| [1] | 蔡延超,牛鹏飞,沈振陆,李美超. 伯胺在电催化媒介ABNO作用下的自氧化偶联反应[J]. 高等学校化学学报, 2019, 40(11): 2308. |
| [2] | 孙杰, 明庭云, 钱慧璇, 张曼珂, 谭勇. BMIMPF6离子液体中铜沉积的电化学行为[J]. 高等学校化学学报, 2018, 39(7): 1497. |
| [3] | 赵邦屯, 陶晶晶, 陈小纪, 付慧敏, 朱卫民. 含噻吩和吡啶基的插烯式四硫富瓦烯衍生物的合成、 结构和电化学性质[J]. 高等学校化学学报, 2018, 39(7): 1449. |
| [4] | 赵长江, 刘欣, 田利, 赵仑. 具有穿插结构的金属钴配合物的合成、 结构及电化学性质[J]. 高等学校化学学报, 2018, 39(5): 861. |
| [5] | 赵邦屯, 马书修, 陶晶晶, 朱卫民. 含吡啶基四硫富瓦烯衍生物的合成、 结构和电化学性质[J]. 高等学校化学学报, 2017, 38(2): 193. |
| [6] | 高丽娟, 王莉, 王圣燕, 井淑波. 溶剂对镍(Ⅱ)金属有机骨架结构的影响[J]. 高等学校化学学报, 2016, 37(9): 1589. |
| [7] | 张雪娜, 钟新文, 钟岩, 陆海彦. 甲基苯丙胺电化学检测条件的优化[J]. 高等学校化学学报, 2016, 37(10): 1799. |
| [8] | 吕江维, 曲有鹏, 冯玉杰, 刘峻峰. 硼掺杂金刚石薄膜电极上二氯酚的电化学阻抗谱研究[J]. 高等学校化学学报, 2016, 37(1): 142. |
| [9] | 吴冉冉, 田晓春, 吴慎剑, 刘源岗, 姜艳霞, 赵峰. 趋磁细菌的电化学活性研究[J]. 高等学校化学学报, 2015, 36(9): 1730. |
| [10] | 景蔚萱, 周帆, 陈路加, 齐含, 蒋庄德, 王兵, 牛玲玲. 基于ZnO纳米线的螺旋线形跨尺度葡萄糖传感器[J]. 高等学校化学学报, 2014, 35(3): 493. |
| [11] | 梁方圆, 吴冉冉, 曹昌丽, 郑越, 杨朝晖, 赵峰. 氧化亚铁硫杆菌的胞外电子传递研究[J]. 高等学校化学学报, 2014, 35(2): 372. |
| [12] | 玉山江·哈斯木, 刘瑞泉, 米红宇. 离子液体[BMIM]PF6中铬的电沉积行为[J]. 高等学校化学学报, 2014, 35(1): 140. |
| [13] | 李恒东, 苏小龙, 陈平华, 谢莉莉, 袁耀锋. 5-二茂铁基吡唑啉衍生物的合成及性质[J]. 高等学校化学学报, 2013, 34(7): 1653. |
| [14] | 徐莉, 林瑞, 丁蕾, 戴先峰, 乔锦丽. 高温处理对碳载吡啶钴催化氧还原性能的影响[J]. 高等学校化学学报, 2012, 33(07): 1534. |
| [15] | 陈丽军, 张密林, 韩伟, 颜永得, 曹鹏. Mn(Ⅱ)在LiCl-KCl-MgCl2-MnCl2熔盐体系中的电化学行为[J]. 高等学校化学学报, 2012, 33(02): 327. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||