

高等学校化学学报 ›› 2019, Vol. 40 ›› Issue (10): 2214.doi: 10.7503/cjcu20190117
肖碧源1,邱江源1,覃方红1,万婷1,徐亚群1,农晓慧1,黄在银1,2,*( )
)
收稿日期:2019-02-25
出版日期:2019-10-08
发布日期:2019-10-16
通讯作者:
黄在银
E-mail:huangzaiyin@163.com
基金资助:
XIAO Biyuan1,QIU Jiangyuan1,QIN Fanghong1,WAN Ting1,XU Yaqun1,NONG Xiaohui1,HUANG Zaiyin1,2,*( )
)
Received:2019-02-25
Online:2019-10-08
Published:2019-10-16
Contact:
HUANG Zaiyin
E-mail:huangzaiyin@163.com
Supported by:摘要:
采用不同粒径的单一(100)晶面的立方体纳米Cu2O作为模型材料, 研究了粒径和温度对其吸附动力学和吸附热力学性质的影响规律. 基于已建立的纳米材料吸附热力学和动力学理论, 推导出了单一(100)晶面立方体纳米Cu2O材料的吸附热力学和吸附动力学性质与粒径之间的关系式. 实验结果与理论预测结果一致: 随着纳米Cu2O粒径的减小, 吸附速率常数增大而吸附活化能和吸附指前因子减小; 标准摩尔吸附Gibbs自由能 Δa $G^{\rlap{-}0}_{m}$减小而标准吸附平衡常数ln $K^{\rlap{-}0}$、 标准摩尔吸附焓 Δa $H^{\rlap{-}0}_{m}$和标准摩尔吸附熵 Δa$S^{\rlap{-}0}_{m}$均增大, 且以上参数均与粒度的倒数具有较好的线性关系.
中图分类号:
TrendMD:
肖碧源,邱江源,覃方红,万婷,徐亚群,农晓慧,黄在银. 立方体纳米Cu2O吸附热力学及动力学的粒度效应研究. 高等学校化学学报, 2019, 40(10): 2214.
XIAO Biyuan,QIU Jiangyuan,QIN Fanghong,WAN Ting,XU Yaqun,NONG Xiaohui,HUANG Zaiyin. Study on Particle Size Effect on Adsorption Thermodynamics and Kinetics of Cubic Nano-Cu2O †. Chem. J. Chinese Universities, 2019, 40(10): 2214.
| Sample | l/nm | qe,x/(mg·g-1) | Pseudo-first order | Pseudo-second order | ||||
|---|---|---|---|---|---|---|---|---|
| k1 | qe,c/(mg·g-1) | R2 | k2 | qe,c/(mg·g-1) | R2 | |||
| A | 42 | 41.6844 | 0.03387 | 13.7536 | 0.8482 | 4.5226 | 43.1779 | 0.9994 |
| B | 54 | 35.6667 | 0.02976 | 12.6395 | 0.9197 | 4.1774 | 37.2578 | 0.9995 |
| C | 67 | 28.5160 | 0.04793 | 20.8775 | 0.9911 | 3.7272 | 30.6843 | 0.9986 |
| D | 117 | 23.3437 | 0.03404 | 15.0143 | 0.9923 | 3.2826 | 25.4001 | 0.9994 |
Table 1 Kinetics parameters for adsorption of methyl orange on nano-Cu2O at 298.15 K
| Sample | l/nm | qe,x/(mg·g-1) | Pseudo-first order | Pseudo-second order | ||||
|---|---|---|---|---|---|---|---|---|
| k1 | qe,c/(mg·g-1) | R2 | k2 | qe,c/(mg·g-1) | R2 | |||
| A | 42 | 41.6844 | 0.03387 | 13.7536 | 0.8482 | 4.5226 | 43.1779 | 0.9994 |
| B | 54 | 35.6667 | 0.02976 | 12.6395 | 0.9197 | 4.1774 | 37.2578 | 0.9995 |
| C | 67 | 28.5160 | 0.04793 | 20.8775 | 0.9911 | 3.7272 | 30.6843 | 0.9986 |
| D | 117 | 23.3437 | 0.03404 | 15.0143 | 0.9923 | 3.2826 | 25.4001 | 0.9994 |
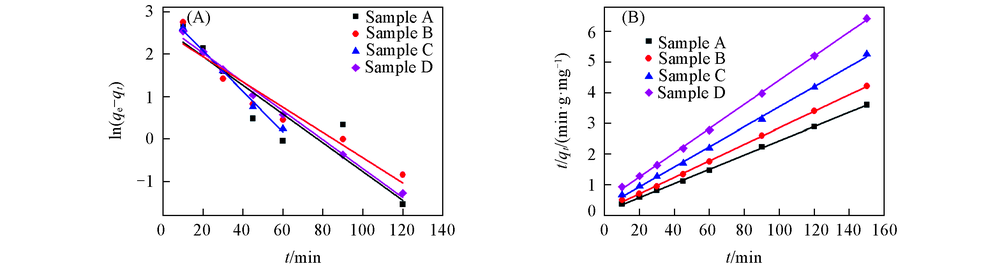
Fig.5 Fit lines of the pseudo-first-order kinetic equation(A) and the pseudo-second-order kinetic equation(B) of adsorption of methyl orange on nano-Cu2O with different sizes in aqueous solution at 298.15 K
| Sample | l/nm | Ea/(kJ·mol-1) | lnA/(g·mg-1·min-1) | Sample | l/nm | Ea/(kJ·mol-1) | lnA/(g·mg-1·min-1) |
|---|---|---|---|---|---|---|---|
| A | 42 | 16.8861 | 8.3560 | C | 67 | 18.0203 | 8.5920 |
| B | 54 | 17.6544 | 8.5462 | D | 117 | 18.9848 | 8.8479 |
Table 2 Activation energy(Ea) and natural logarithm of pre-exponential factor(lnA) of adsorption of methyl orange on nano-Cu2O
| Sample | l/nm | Ea/(kJ·mol-1) | lnA/(g·mg-1·min-1) | Sample | l/nm | Ea/(kJ·mol-1) | lnA/(g·mg-1·min-1) |
|---|---|---|---|---|---|---|---|
| A | 42 | 16.8861 | 8.3560 | C | 67 | 18.0203 | 8.5920 |
| B | 54 | 17.6544 | 8.5462 | D | 117 | 18.9848 | 8.8479 |
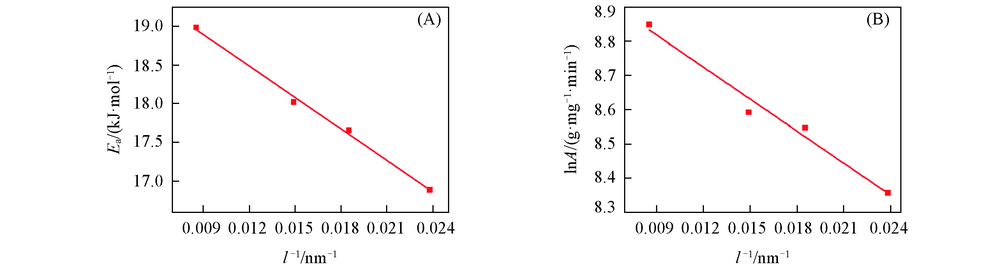
Fig.8 Relationships between the activation energy and the reciprocal of particle size(A) and between the logarithm of pre-exponential factor and the reciprocal of particle size(B) of nano-Cu2O adsorption system
| T/K | lnK 0— | T/K | lnK 0— | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample A | Sample B | Sample C | Sample D | Sample A | Sample B | Sample C | Sample D | ||
| 288.15 | 1.2996 | 0.9165 | 0.5659 | 0.4403 | 318.15 | 2.9303 | 2.2448 | 1.9259 | 1.7246 |
| 298.15 | 1.8977 | 1.5503 | 1.2579 | 0.8801 | 328.15 | 3.4863 | 2.7338 | 2.2453 | 1.9293 |
| 308.15 | 2.4215 | 1.8715 | 1.5852 | 1.4039 | |||||
Table 3 Logarithms of the standard equilibrium constants of the cubic nano-Cu2O with different diameters at different temperatures
| T/K | lnK 0— | T/K | lnK 0— | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample A | Sample B | Sample C | Sample D | Sample A | Sample B | Sample C | Sample D | ||
| 288.15 | 1.2996 | 0.9165 | 0.5659 | 0.4403 | 318.15 | 2.9303 | 2.2448 | 1.9259 | 1.7246 |
| 298.15 | 1.8977 | 1.5503 | 1.2579 | 0.8801 | 328.15 | 3.4863 | 2.7338 | 2.2453 | 1.9293 |
| 308.15 | 2.4215 | 1.8715 | 1.5852 | 1.4039 | |||||
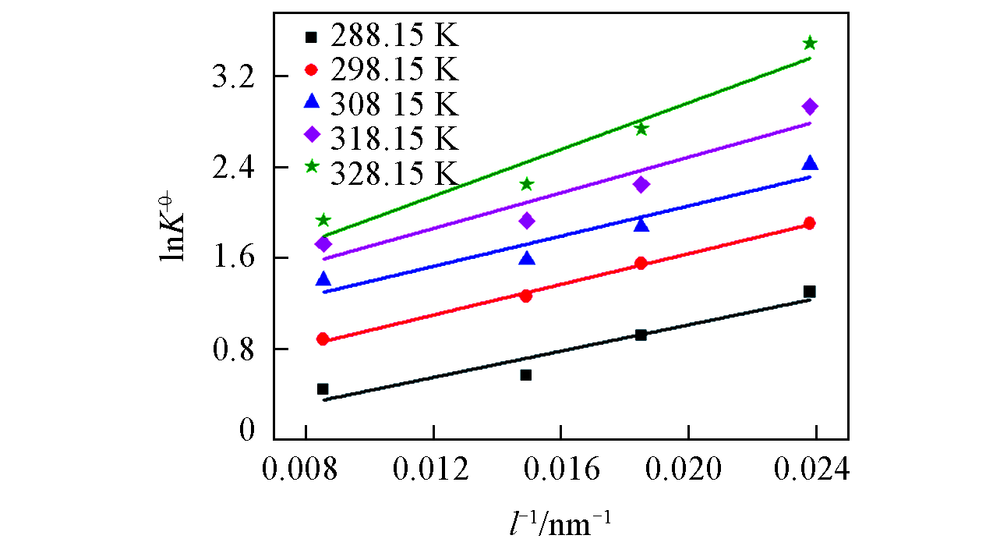
Fig.9 Relationships between the logarithm of the standard and the reciprocal of the diameter of nano-Cu2O equilibrium constants at different temperatures
| T/K | ΔaG 0— m/(kJ·mol-1) | T/K | ΔaG 0— m/(kJ·mol-1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample A | Sample B | Sample C | Sample D | Sample A | Sample B | Sample C | Sample D | ||
| 288.15 | -3.1134 | -2.1956 | -1.3556 | -1.0547 | 318.15 | -7.7509 | -5.9378 | -5.0943 | -4.5616 |
| 298.15 | -4.7041 | -3.8428 | -3.1180 | -2.1816 | 328.15 | -9.5114 | -7.4585 | -6.1256 | -5.2635 |
| 308.15 | -6.2038 | -4.7947 | -4.0612 | -3.5967 | |||||
Table 4 Change in standard Gibbs free energy of the adsorption of the nano-Cu2O with various sizes at different temperatures
| T/K | ΔaG 0— m/(kJ·mol-1) | T/K | ΔaG 0— m/(kJ·mol-1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample A | Sample B | Sample C | Sample D | Sample A | Sample B | Sample C | Sample D | ||
| 288.15 | -3.1134 | -2.1956 | -1.3556 | -1.0547 | 318.15 | -7.7509 | -5.9378 | -5.0943 | -4.5616 |
| 298.15 | -4.7041 | -3.8428 | -3.1180 | -2.1816 | 328.15 | -9.5114 | -7.4585 | -6.1256 | -5.2635 |
| 308.15 | -6.2038 | -4.7947 | -4.0612 | -3.5967 | |||||
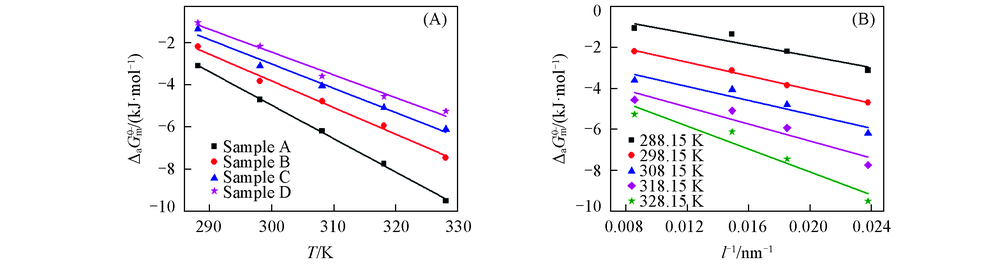
Fig.10 Relationships between the standard molar adsorption Gibbs free energy and temperature of nano-Cu2O(A), and between the standard molar adsorption Gibbs free energy and reciprocal grain size of nano-Cu2O(B)
| Sample | l/nm | ΔaH 0— m/(kJ·mol-1) | ΔaS 0— m/(J·mol-1·K-1) | Sample | l/nm | ΔaH 0— m/(kJ·mol-1) | ΔaS 0— m/(J·mol-1·K-1) |
|---|---|---|---|---|---|---|---|
| A | 42 | 42.56 | 158.43 | C | 67 | 31.54 | 115.16 |
| B | 54 | 34.04 | 126.21 | D | 117 | 29.94 | 107.98 |
Table 5 Standard molar adsorption enthalpies and standard molar adsorption entropies of cubic nano-Cu2O with different diameters
| Sample | l/nm | ΔaH 0— m/(kJ·mol-1) | ΔaS 0— m/(J·mol-1·K-1) | Sample | l/nm | ΔaH 0— m/(kJ·mol-1) | ΔaS 0— m/(J·mol-1·K-1) |
|---|---|---|---|---|---|---|---|
| A | 42 | 42.56 | 158.43 | C | 67 | 31.54 | 115.16 |
| B | 54 | 34.04 | 126.21 | D | 117 | 29.94 | 107.98 |
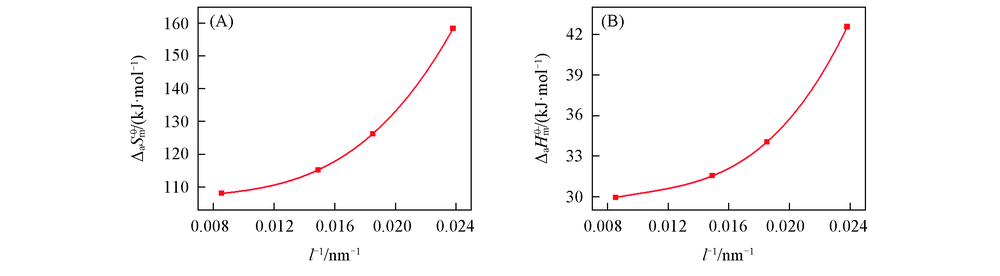
Fig.11 Relationships between the standard molar adsorption entropies and the reciprocals of particle size(A) and between the standard molar adsorption enthalpies and the reciprocals of particle size(B) of nano-Cu2O adsorption system
| [1] | Yean S., Cong L., Yavuz C. T., Mayo J. T., Yu W. W., Kan A. T., Colvin V. L., Tomson M. B., Journal of Materials Research, 2005,20(12), 10 |
| [2] | Zhou X. C., Xu W. L., Liu G. K., Panda D., Chen P., Journal of the American Chemical Society, 2010,132(1), 138— 146 |
| [3] | Han B. C., Miranda C. R., Ceder G., Physical Review B, 2008,77(7), 075410 |
| [4] | Wen Y. Z., Xue Y. Q., Cui Z. X., Wang Y., The Journal of Chemical Thermodynamics, 2015,80, 112— 118 |
| [5] | Wang N ., Sun W. J., Hou Y. L., Ge B. H., Hu L., Nie J. Q., Qian W. Z., Wei F., Journal of Catalysis, 2018,360, 89— 96 |
| [6] | Wang H. L., Jin B. F., Wang H. B., Ma N. N., Liu W., Weng D., Wu X. D., Liu S., Applied Catalysis B: Environmental, 2018,237, 251— 262 |
| [7] | Vittadini A., Selloni A., Rotzinger F P., Grätzel M., Physical Review Letter, 1998,81(14), 2954— 2957 |
| [8] | Hardcastle T. P., Brydson R. M. D., Livi K. J. T., Seabourne C. R., Scott A., [J]. Journal of Physics Conference Series, 2012,371(1), 012059 |
| [9] | Cui Z. X., Zhang J., Xue Y. Q., Duan H. J., Langmuir, 2018,34(10), 3197— 3206 |
| [10] | Wang S. T., Wen Y. Z., Cui Z. X., Xue Y. Q., Journal of Nanoparticle Research, 2016,18(1), 1— 9 |
| [11] | Wen Y. Z., Particle Size Effect of Adsorption Thermodynamics and Kinetics of Nanomaterials, Taiyuan University of Technology, Taiyuan, 2015 |
| ( 温艳珍 . 纳米材料吸附热力学和动力学的粒度效应, 太原: 太原理工大学, 2015) | |
| [12] | Susman M. D., Feldman Y., Vaskevich A., Rubinstein I., ACS Nano, 2014,8(1), 162— 174 |
| [13] | Tang H. F., Huang Z. Y., Xiao M. , Acta Physico-chimica. Sinica, 2016,32(11), 2678— 2684 |
| ( 汤焕丰, 黄在银, 肖明 . 物理化学学报, 2016,32(11), 2678— 2684) | |
| [14] | Fan G. C., Sun L., Huang Z. Y., Jiang J. Y., Li Y. F., Materials Letters, 2011,65, 2783— 2785 |
| [15] | Fan G. C., Huang Z.. Y., Wang T. H., Solid State Sciences, 2013,16, 121— 124 |
| [16] | Xue Y. Q., Wang X.. P., Cui Z. X., Progress in Reaction Kinetics and Mechanism, 2011,36, 329— 341 |
| [17] | Gao H. F. , Study on Adsorption Thermodynamics and Kinetics of Nano-sized MgO by Particle Size and Morphology, Taiyuan University of Technology, Taiyuan, 2018 |
| ( 高慧芳 . 粒度和形貌对纳米MgO吸附热力学与动力学的研究, 太原: 太原理工大学, 2018) | |
| [18] | Pan L., Zou J. J., Zhang T. R., Wang S. B., Li Z., Wang L., Zhang X. W., The Journal of Physical Chemistry C, 2013,118(30), 16335— 16343 |
| [19] | Li C., Li Y., Delaunay J J., ACS Applied Materials & Interfaces, 2013,6(1), 480— 486 |
| [20] | Wu L. L., Tsui L. K., Swami N., Zangari G., The Journal of Physical Chemistry C, 2010,114(26), 11551— 11556 |
| [21] | Solache-Carranco H., Juárez-Díaz G., Esparza-García A., Briseño-García M., Galván-Arellano M., Martínez-Juárez J., Romero-Paredes G., Peña-Sierra R ., Journal of Luminescence, 2009,129(12), 1483— 1487 |
| [22] | Sun D., Yin P. G., Guo L ., Acta Physico-chimica Sinica, 2011,27(6), 1543— 1550 |
| ( 孙都, 殷鹏刚, 郭林 . 物理化学学报, 2011,27(6), 1543— 1550) | |
| [23] | Parker J. C., Siegel R. W., Applied Physics Letters, 1990,57(9), 943— 945 |
| [24] | Parker J. C., Siegel R. W., Journal of Materials Research, 1990,5(6), 1246— 1252 |
| [25] | Giammar D. E., Maus C.. J., Xie L. Y., Environment Engineering Science, 2007,24, 85— 95 |
| [26] | Xu Z. H., Meng X. G., Journal of Hazardous Materials, 2009,168, 747— 752 |
| [27] | Zhang H. Z., Penn R. L., Hamers R. J., Banfield J. F., Journal Physical Chemistry B, 1999,103, 4656— 4662 |
| [28] | Zhang W., Rittmann B., Chen Y S., Environment Science and Technology, 2011,45, 2172— 2178 |
| [29] | Shen Y. F., Tang J., Nie Z. H., Wang Y. D., Ren Y., Zuo L., Separation Purification Technology, 2009,68, 312— 319 |
| [30] | Xue Y. Q., Du J. P., Wang P. D., Wang Z. Z., Acta Physico-chimica Sinica, 2005,21(7), 758— 762 |
| [31] | Xue Y. Q., Zhao H., Du J. P., Chinese Inorganic Chemistry, 2006,22, 1952— 1956 |
| [32] | Zhang L., Zhu Y., Li H. M., Liu N., Liu X. Y., Guo X. J., Rare Metals, 2010,29, 16— 20 |
| [33] | Boparai H. K., Joseph M., O’Carroll D. M., Journal of Hazardous Materials, 2011,186, 458— 465 |
| [1] | 李志刚, 张艺璇, 张青松, 马友伟, 胡涛, 白海会, 刘鹏飞, 王珂, 张小勇. 半互穿海藻酸钠/聚丙烯酰胺凝胶吸附结晶紫动力学/热力学行为和吸/脱附机理[J]. 高等学校化学学报, 2017, 38(11): 2118. |
| [2] | 刘克峰, 任丹妮, 孙辉, 沈本贤, 刘纪昌. ZIF-8的合成、 表征及正己烷吸附性能[J]. 高等学校化学学报, 2016, 37(10): 1856. |
| [3] | 刘小霞, 邓浩, 王妍媖, 鲁志伟, 曾宪垠, 王显祥, 邹平, 饶含兵. BSA在不同形貌氧化锌材料上的吸附热力学[J]. 高等学校化学学报, 2014, 35(10): 2156. |
| [4] | 胡佶, 王江涛. 四环素在海洋沉积物上的吸附[J]. 高等学校化学学报, 2010, 31(2): 320. |
| [5] | 陈宝梁, 毛洁菲, 吕少芳. 阳离子表面活性剂在膨润土上的有序化作用及吸附热力学[J]. 高等学校化学学报, 2009, 30(9): 1830. |
| [6] | 郭卓,袁悦 . 介孔碳CMK-3对苯酚的吸附动力学和热力学研究[J]. 高等学校化学学报, 2007, 28(2): 289. |
| [7] | 李倩, 岳钦艳, 高宝玉, 刘莉莉. 阳离子膨润土对分散染料的吸附动力学研究[J]. 高等学校化学学报, 2006, 27(6): 1113. |
| [8] | 王春红, 施荣富, 温珍珍, 史作清, 何炳林. 吸附质大小对吸附树脂粒内扩散行为的影响(Ⅰ)——大孔聚苯乙烯型吸附树脂粒内扩散机制的考察[J]. 高等学校化学学报, 2001, 22(S1): 240. |
| [9] | 马万红, 蔡汝秀, 林智信. 流动注射分析在线研究Cr(Ⅵ)在纳米TiO2表面上的吸附动力学性质[J]. 高等学校化学学报, 1998, 19(10): 1566. |
| [10] | 陈禹银, 耿信笃. 液-固吸附体系中计量置换吸附模型的热力学研究[J]. 高等学校化学学报, 1993, 14(10): 1432. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||