

高等学校化学学报 ›› 2018, Vol. 39 ›› Issue (12): 2700.doi: 10.7503/cjcu20180481
收稿日期:2018-07-05
出版日期:2018-12-03
发布日期:2018-11-05
作者简介:联系人简介: 薛冰纯, 女, 博士, 教授, 主要从事粉体颗粒化理论研究. E-mail:
基金资助:
YAN Xuan, XUE Bingchun*( ), LIU Erbao*(
), LIU Erbao*( )
)
Received:2018-07-05
Online:2018-12-03
Published:2018-11-05
Contact:
XUE Bingchun,LIU Erbao
E-mail:bcxue@sxnu.edu.cn;liueb@sxnu.edu.cn
Supported by:摘要:
通过缓慢蒸发技术合成尿素氯化铵、 尿素溴化铵和尿素氟化铵3种尿素卤化铵共晶. 采用第一性原理方法, 在原尿素氯化铵晶胞结构基础上, 构建同晶系的尿素氟化铵及尿素溴化铵共晶晶胞, 并对3种尿素卤化铵共晶结构的稳定性、 堆积系数和形成能、 卤素离子半径和电负性及离子水合作用进行对比研究. 结果表明, 对于尿素溴化铵共晶, Br-半径较大导致晶胞内部空腔变形, 溴离子水合团簇稳定, 均会阻碍Br-进入尿素孔道内; 对于尿素氟化铵共晶, F-的强电负性使氟离子水合团簇F-(H2O)5稳定性很强, 阻碍裸F-进入尿素空腔中; 对于易于合成的尿素氯化铵共晶, 由于Cl-的电负性及半径大小都适中, 水合离子团簇的稳定性适当, 易于进入尿素空腔中.
中图分类号:
TrendMD:
闫璇, 薛冰纯, 刘二保. 水体系中尿素卤化铵共晶合成及影响因素的理论研究. 高等学校化学学报, 2018, 39(12): 2700.
YAN Xuan,XUE Bingchun,LIU Erbao. Synthesis of Urea Ammonium Halide Cocrystal and Theoretical Study of Its Influencing Factors in Water System†. Chem. J. Chinese Universities, 2018, 39(12): 2700.
| Method | a/nm | b/nm | c/nm | α/(°) | β/(°) | γ/(°) | Volume/nm3 |
|---|---|---|---|---|---|---|---|
| Solid phase honey-like channel method[ | 0.7923 | 1.7121 | 0.8072 | 90 | 90 | 90 | 1.0950 |
| Slow evaporation method | 0.7927 | 1.7152 | 0.8050 | 90 | 90 | 90 | 1.0945 |
Table 1 Comparison of unit cell parameters of urea ammonium chloride prepared by two methods
| Method | a/nm | b/nm | c/nm | α/(°) | β/(°) | γ/(°) | Volume/nm3 |
|---|---|---|---|---|---|---|---|
| Solid phase honey-like channel method[ | 0.7923 | 1.7121 | 0.8072 | 90 | 90 | 90 | 1.0950 |
| Slow evaporation method | 0.7927 | 1.7152 | 0.8050 | 90 | 90 | 90 | 1.0945 |
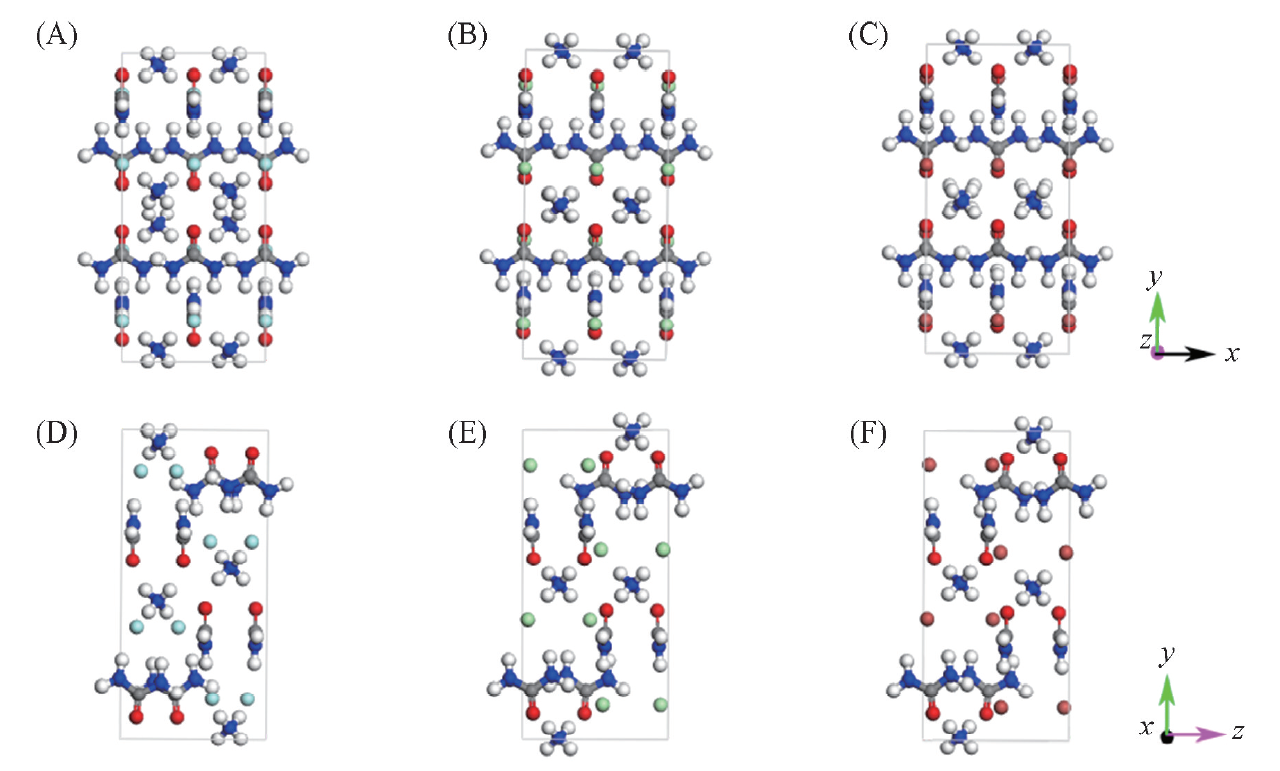
Fig.4 Comparison of three urea ammonium halide cocrystal cell structures(A, D) Urea ammonium fluoride cell structure; (B, E)urea ammonium chloride cell structure;(C, F) urea ammonium bromide cell structure.
| Species | a/nm | b/nm | c/nm | d(H1—H2)/nm | d(N1—N2)/nm | d(X1—H4)/nm | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Urea-NH4F | 0.7533 | 1.6318 | 0.8069 | 0.3433 | 0.3179 | 0.1709 | |||||
| Urea-NH4Cl | 0.8082 | 1.7301 | 0.8580 | 0.3998 | 0.3417 | 0.2253 | |||||
| Urea-NH4Br | 0.8250 | 1.7844 | 0.8875 | 0.4171 | 0.3438 | 0.2425 | |||||
| Species | d(X1—X4)/nm | d(X2—X3)/nm | d(C2—C3)/nm | d(O1—O2)/nm | ÐH3—N3—H4/(°) | ÐH4—N3—H5/(°) | |||||
| Urea-NH4F | 0.4690 | 0.4370 | 0.4343 | 0.4506 | 110.976 | 111.331 | |||||
| Urea-NH4Cl | 0.5315 | 0.5075 | 0.4937 | 0.4830 | 108.549 | 110.592 | |||||
| Urea-NH4Br | 0.5476 | 0.5220 | 0.5025 | 0.4892 | 108.162 | 110.392 | |||||
Table 2 Unit cell parameters of three units and structural parameters between halogen ions and the main atoms around them
| Species | a/nm | b/nm | c/nm | d(H1—H2)/nm | d(N1—N2)/nm | d(X1—H4)/nm | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Urea-NH4F | 0.7533 | 1.6318 | 0.8069 | 0.3433 | 0.3179 | 0.1709 | |||||
| Urea-NH4Cl | 0.8082 | 1.7301 | 0.8580 | 0.3998 | 0.3417 | 0.2253 | |||||
| Urea-NH4Br | 0.8250 | 1.7844 | 0.8875 | 0.4171 | 0.3438 | 0.2425 | |||||
| Species | d(X1—X4)/nm | d(X2—X3)/nm | d(C2—C3)/nm | d(O1—O2)/nm | ÐH3—N3—H4/(°) | ÐH4—N3—H5/(°) | |||||
| Urea-NH4F | 0.4690 | 0.4370 | 0.4343 | 0.4506 | 110.976 | 111.331 | |||||
| Urea-NH4Cl | 0.5315 | 0.5075 | 0.4937 | 0.4830 | 108.549 | 110.592 | |||||
| Urea-NH4Br | 0.5476 | 0.5220 | 0.5025 | 0.4892 | 108.162 | 110.392 | |||||
| Atom | Urea-NH4F | Urea-NH4Cl | Urea-NH4Br | ||||||
|---|---|---|---|---|---|---|---|---|---|
| x/nm | y/nm | z/nm | x/nm | y/nm | z/nm | x/nm | y/nm | z/nm | |
| H1 | 0.0272 | 0.0661 | 0.0436 | 0.0253 | 0.0667 | 0.0437 | 0.0247 | 0.0667 | 0.0432 |
| H2 | 0.0728 | 0.0661 | 0.0436 | 0.0747 | 0.0667 | 0.0437 | 0.0753 | 0.0667 | 0.0432 |
| N1 | 0.0153 | 0.0691 | 0.0425 | 0.0143 | 0.0695 | 0.0422 | 0.0140 | 0.0694 | 0.0415 |
| N2 | 0.0347 | 0.0691 | 0.0075 | 0.0357 | 0.0695 | 0.0078 | 0.0360 | 0.0694 | 0.0086 |
| X1 | 0.0500 | 0.0632 | 0.0577 | 0.0500 | 0.0617 | 0.0549 | 0.0500 | 0.0616 | 0.0547 |
| H4 | 0.0337 | 0.0579 | 0.0678 | 0.0320 | 0.0540 | 0.0676 | 0.0315 | 0.0532 | 0.0676 |
| X2 | 0.0500 | 0.0862 | 0.0113 | 0.0500 | 0.0881 | 0.0071 | 0.0500 | 0.0883 | 0.0070 |
| X3 | 0 | 0.0862 | 0.0387 | 0 | 0.0881 | 0.0429 | 0 | 0.0883 | 0.0430 |
| C2 | 0.0500 | 0.0849 | 0.0616 | 0.0500 | 0.0843 | 0.0585 | 0.0500 | 0.0843 | 0.0588 |
| C3 | 0 | 0.0849 | 0.0884 | 0 | 0.0843 | 0.0915 | 0 | 0.0843 | 0.0912 |
| X4 | 0 | 0.0632 | 0.0923 | 0 | 0.0617 | 0.0951 | 0 | 0.0616 | 0.0953 |
| O1 | 0.0500 | 0.05670 | 0.0097 | 0.0500 | 0.0581 | 0.0096 | 0.0500 | 0.0584 | 0.0102 |
| O2 | 0 | 0.0570 | 0.0403 | 0 | 0.0581 | 0.0404 | 0 | 0.0584 | 0.0398 |
Table 3 Atomic coordinates of the main numbered atoms
| Atom | Urea-NH4F | Urea-NH4Cl | Urea-NH4Br | ||||||
|---|---|---|---|---|---|---|---|---|---|
| x/nm | y/nm | z/nm | x/nm | y/nm | z/nm | x/nm | y/nm | z/nm | |
| H1 | 0.0272 | 0.0661 | 0.0436 | 0.0253 | 0.0667 | 0.0437 | 0.0247 | 0.0667 | 0.0432 |
| H2 | 0.0728 | 0.0661 | 0.0436 | 0.0747 | 0.0667 | 0.0437 | 0.0753 | 0.0667 | 0.0432 |
| N1 | 0.0153 | 0.0691 | 0.0425 | 0.0143 | 0.0695 | 0.0422 | 0.0140 | 0.0694 | 0.0415 |
| N2 | 0.0347 | 0.0691 | 0.0075 | 0.0357 | 0.0695 | 0.0078 | 0.0360 | 0.0694 | 0.0086 |
| X1 | 0.0500 | 0.0632 | 0.0577 | 0.0500 | 0.0617 | 0.0549 | 0.0500 | 0.0616 | 0.0547 |
| H4 | 0.0337 | 0.0579 | 0.0678 | 0.0320 | 0.0540 | 0.0676 | 0.0315 | 0.0532 | 0.0676 |
| X2 | 0.0500 | 0.0862 | 0.0113 | 0.0500 | 0.0881 | 0.0071 | 0.0500 | 0.0883 | 0.0070 |
| X3 | 0 | 0.0862 | 0.0387 | 0 | 0.0881 | 0.0429 | 0 | 0.0883 | 0.0430 |
| C2 | 0.0500 | 0.0849 | 0.0616 | 0.0500 | 0.0843 | 0.0585 | 0.0500 | 0.0843 | 0.0588 |
| C3 | 0 | 0.0849 | 0.0884 | 0 | 0.0843 | 0.0915 | 0 | 0.0843 | 0.0912 |
| X4 | 0 | 0.0632 | 0.0923 | 0 | 0.0617 | 0.0951 | 0 | 0.0616 | 0.0953 |
| O1 | 0.0500 | 0.05670 | 0.0097 | 0.0500 | 0.0581 | 0.0096 | 0.0500 | 0.0584 | 0.0102 |
| O2 | 0 | 0.0570 | 0.0403 | 0 | 0.0581 | 0.0404 | 0 | 0.0584 | 0.0398 |
| Urea cocrystal | Packing coefficient | Eform/(kJ·mol-1) | ||
|---|---|---|---|---|
| Urea | Cocrystal | NH4X | ||
| Urea-NH4F | 0.8574 | 0.9541 | 0.5487 | -6.2549 |
| Urea-NH4Cl | 0.8574 | 0.9390 | 1.0000 | -40.7346 |
| Urea-NH4Br | 0.8574 | 0.9034 | 1.0000 | -34.9912 |
Table 4 Formation energy and crystal stacking coefficient of three urea ammonium halide cocrystals
| Urea cocrystal | Packing coefficient | Eform/(kJ·mol-1) | ||
|---|---|---|---|---|
| Urea | Cocrystal | NH4X | ||
| Urea-NH4F | 0.8574 | 0.9541 | 0.5487 | -6.2549 |
| Urea-NH4Cl | 0.8574 | 0.9390 | 1.0000 | -40.7346 |
| Urea-NH4Br | 0.8574 | 0.9034 | 1.0000 | -34.9912 |
| X-(H2O)n | RX—H/nm | DE/(kJ·mol-1) |
|---|---|---|
| F-(H2O)5 | 0.1669 | -365.6760 |
| Cl-(H2O)6 | 0.2287 | -240.5609 |
| Br-(H2O)6 | 0.2456 | -249.2675 |
Table 5 Average distance of hydrogen bonds O—H…X in the first hydrated layer and binding energy(DE) of three hydrated ion clusters
| X-(H2O)n | RX—H/nm | DE/(kJ·mol-1) |
|---|---|---|
| F-(H2O)5 | 0.1669 | -365.6760 |
| Cl-(H2O)6 | 0.2287 | -240.5609 |
| Br-(H2O)6 | 0.2456 | -249.2675 |
| [1] | Lara O.F., Espinosa P.G., Supramol. Chem.,2007, 19(8), 553—557 |
| [2] | Shan N., Zaworotko M.J., Drug Discov. Today,2008, 13, 440—446 |
| [3] | Zhao G.Z., Fan R.R., Yan X.L., Yang W., Tang M.F., Jia J.F., Wu H.S., Chem. J. Chinese Universities,2018, 39(2), 292—298 |
| (赵国政, 范荣荣, 颜熹琳, 唐维, 唐明峰, 贾建峰, 武海顺. 高等学校化学学报, 2018, 39(2), 292—298) | |
| [4] | Luo Y.H., Sun B.W., Spectrochim. Acta A,2014, 120, 228—236 |
| [5] | Ghosh S., Bag P.P., Reddy C.M., Cryst. Growth Des., 2011, 11(8), 3489—3503 |
| [6] | Ross S.A., Lamprou D.A., Douroumis D., Chem. Commun.,2016, 52, 8772—8786 |
| [7] | Hebbar H.U., Rastogi N.K., Subramanian R., Int. J. Food Prop.,2008, 11(4), 804—819 |
| [8] | Rusa C.C., Tonelli A.E., Macromolecules,2000, 33, 1813—1818 |
| [9] | Tian H., Zhang M.L., Wang L.S., Tong B.H., Zhao Z., Chem. J. Chinese Universities,2018, 39(6), 1191—1196 |
| (田欢, 张梦龙, 王莉莎, 童碧海, 赵卓. 高等学校化学学报, 2018, 39(6), 1191—1196) | |
| [10] | Yang Z.W., Zhang Y.L., Li H.Z., Chinese J. Energ. Mater.,2012, 20(6), 674—679 |
| (杨宗伟, 张艳丽, 李洪珍. 含能材料, 2012, 20(6), 674—679) | |
| [11] | Thottempudi V., Shreeve J.M., J. Am.Chem. Soc.,2011, 49, 19982—19992 |
| [12] | Liu Z.Y., Xue Q.H., Bulletin Chinese Ceram. Soc.,2010, 29(5), 1041—1044 |
| (刘振英, 薛群虎. 硅酸盐通报, 2010, 29(5), 1041—1044) | |
| [13] | Maulny A. P.E., Beckett S.T., Mackenzi G., J. Food Sci.,2005, 70(9), 567—572 |
| [14] | Xue D.F., Kitamura K.J., Wang J.Y., Opt. Mater.,2003, 23, 399—402 |
| [15] | Skoepová E., Hušák M., Čejka J., Zámostný P., Kratochvíl B., J. Cryst. Growth,2014, 399, 19—26 |
| [16] | Vinothkumar P., Rajeswari K., Kumar R.M., Bhaskaran A., Spectrochim. Acta A,2015, 145(1), 33—39 |
| [17] | Yang J.Q., Li S.X., Zhao H.W., Song B., Zhang G.X., Zhang J.B., Zhu Y.M., Han J.G., J. Phys. Chem. A,2014, 118, 10927—10933 |
| [18] | Buanz A. B.M., Parkinson G.N., Gaisford S., Cryst. Growth Des., 2011, 11(4), 1177—1181 |
| [19] | Lashua A.F., Smith T.M., Hu H.G., Wei L.H., Allis D.G., Sponsler M.B., Hudson B.S., Cryst. Growth Des., 2013, 13(9), 3852—3855 |
| [20] | Rightmire N.R., Hanusa T.P., Dalton Trans.,2016, 45, 2352—2362 |
| [21] | Xue B.C., Mao M.L., Liu Y.H., Guo J.Y., Li J., Liu E.B., J. Cryst. Growth,2016, 442, 110—113 |
| [22] | Segall M., Lindan P.J., Probert M., Pickard C., Hasnip P., Clark S., Payne M., J. Phys. Condens. Mat.,2002, 14, 2717—2744 |
| [23] | Frisch M, Trucks G. W., Schlegel H. B., Scuseria G. E., Robb M. A., Cheeseman J. R., Scalmani G., Barone V., Mennucci B., Petersson G. A., Nakatsuji H., Caricato M., Li X., Hratchian H. P., Izmaylov A. F., Bloino J., Zheng G., Sonnenberg J. L., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Vreven T., Montgomery J. A. Jr., Peralta J. E., Ogliaro F., Bearpark M., Heyd J. J., Brothers E., Kudin K. N., Staroverov V. N., Keith T., Kobayashi R., Normand J., Raghavachari K., Rendell A., Burant J. C., Iyengar S. S., Tomasi J., Cossi M., Rega N., Millam J. M., Klene M., Knox J. E., Cross J. B., Bakken V., Adamo C., Jaramillo J., Gomperts R., Stratmann R. E., Yazyev O., Austin A. J., Cammi R., Pomelli C., Ochterski J. W., Martin R. L., Morokuma K., Zakrzewski V. G., Voth G. A., Salvador P., Dannenberg J. J., Dapprich S., Daniels A. D., Farkas O., Foresman J. B., Ortiz J. V., Cioslowski J., Fox D. J., Gaussian 09, Revision B.01, Gaussian Inc., Wallingford CT, 2010 |
| [24] | Zhang A.B., Cao Y.F., Ma Y., Zhu Y.Q., Zhang C.Y., Chinese J. Energ. Mater.,2015, 23(9), 848—857 |
| (张安帮, 曹耀峰, 马宇, 朱元强, 张朝阳. 含能材料, 2015, 23(9), 848—857 | |
| [25] | Wang J., Xia S.W., Yu L.M., Acta Chim. Sinica,2013, 71, 1307—1312 |
| (王娟, 夏树伟, 于良民. 化学学报, 2013, 71, 1307—1312 | |
| [26] | Krekeler C., Hess B., Site L.D., J. Chem. Phys.,2006, 125, 054305 |
| [1] | 周颖, 贺培楠, 丰海松, 张欣. 双原子位点M-N-C电催化剂在CO2还原反应中活性位点的最佳分布[J]. 高等学校化学学报, 2022, 43(2): 20210640. |
| [2] | 吴芳玲, 储艳秋, 陈新, 魏王慧, 丁传凡. 电喷雾电离质谱法研究影响五肽间非共价作用的主要因素[J]. 高等学校化学学报, 2018, 39(9): 1927. |
| [3] | 王璇, 陈启元. 生物膜电极法降解水中2-萘酚的影响因素[J]. 高等学校化学学报, 2017, 38(5): 855. |
| [4] | 董德明, 孙家倩, 赵天宇, 李明, 花修艺, 梁大鹏, 郭志勇. 自然水体生物膜体系中过氧化氢浓度的影响因素[J]. 高等学校化学学报, 2015, 36(7): 1337. |
| [5] | 蒋举兴, 王家俊, 段焰青, 刘亚, 王文元, 吴少华. 水催化2个酯分子相互转化反应的理论研究[J]. 高等学校化学学报, 2014, 35(9): 1919. |
| [6] | 刘清宇, 何圣贵. 原子团簇上一氧化碳的氧化[J]. 高等学校化学学报, 2014, 35(4): 665. |
| [7] | 吕浩挺, 刘婷婷, 张明涛, 刘洁, 孙怀林. η6-[三(三甲基硅基)苯基硅烷]三羰基铬类化合物的合成及其分子内硅硅键与金属铬之间相互作用的理论研究[J]. 高等学校化学学报, 2014, 35(11): 2341. |
| [8] | 李恒东, 苏小龙, 陈平华, 谢莉莉, 袁耀锋. 5-二茂铁基吡唑啉衍生物的合成及性质[J]. 高等学校化学学报, 2013, 34(7): 1653. |
| [9] | 于奡, 王会凯, 薛小松, 蔡余, 王永健, 何家骐. 咪唑类化合物在乙腈溶液中负氢解离焓的理论研究[J]. 高等学校化学学报, 2012, 33(02): 276. |
| [10] | 王锋, 李稳宏, 李冬, 延绥红, 徐抗震, 孙晓红. 2-氨基-4-羰基噻吩的Knoevenagel反应[J]. 高等学校化学学报, 2011, 32(4): 903. |
| [11] | 季长春, 许正江, 李静, 刘光祥, 郑和根. 四种新型柔性吡啶基配合物的合成、结构及配体构象与配合物结构的关系[J]. 高等学校化学学报, 2010, 31(5): 867. |
| [12] | 于擎, 曹洁, 张成根. 三元非对映异构体复合物的直接实验证据及其在对映体手性分离中的作用[J]. 高等学校化学学报, 2009, 30(5): 988. |
| [13] | 黄荣谊, 陈宏, 严娟, 朱坤, 刘光祥, 任小明. 三种新型铜配合物的合成、结构及理论计算[J]. 高等学校化学学报, 2009, 30(4): 655. |
| [14] | 王一, 王永, 韩克利. 非血红素配合物[FeⅣ(O)(TMC)(NCMe)]2+与[FeⅣ(O)(TMCS)]+的几何结构、电子结构、成键性和反应活性比较[J]. 高等学校化学学报, 2008, 29(12): 2469. |
| [15] | 毛华平,于有海,陈梁,卢晓峰,张万金,张红星 . “哑铃状”偶氮化合物的合成与表征[J]. 高等学校化学学报, 2006, 27(10): 1999. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||