

高等学校化学学报 ›› 2018, Vol. 39 ›› Issue (8): 1759.doi: 10.7503/cjcu20170820
姜涛1( ), 王宁1, 彭述明1, 李梅2, 韩伟2, 陈一东3
), 王宁1, 彭述明1, 李梅2, 韩伟2, 陈一东3
收稿日期:2017-12-15
出版日期:2018-08-10
发布日期:2018-06-25
作者简介:联系人简介: 姜 涛, 男, 博士, 副研究员, 主要从事核燃料循环与材料研究. E-mail: 基金资助:
JIANG Tao1,*( ), WANG Ning1, PENG Shuming1, LI Mei2, HAN Wei2, CHEN Yitung3
), WANG Ning1, PENG Shuming1, LI Mei2, HAN Wei2, CHEN Yitung3
Received:2017-12-15
Online:2018-08-10
Published:2018-06-25
Contact:
JIANG Tao
E-mail:tjiang@caep.cn
Supported by:摘要:
采用多种电化学技术研究了在723~823 K范围内LiCl-KCl熔盐中Gd(Ⅲ)在液态Bi电极和Bi膜电极上的电化学行为. 利用开路计时电位法估算了Bi-Gd金属间化合物(Bi2Gd, BiGd, Bi3Gd4, Bi3Gd5)的活度、 相对偏摩尔吉布斯自由能、 生成吉布斯自由能、 生成焓和生成熵等热力学数据. 通过恒电流和恒电位电解, 在液态Bi电极上制备了Bi-Gd合金, 并采用X射线衍射(XRD)和扫描电子显微镜-能量散射谱(SEM-EDS)表征了其结构. 在恒电流电解中所得金属间化合物为BiGd, 而在恒电位电解中所得金属间化合物为Bi3Gd5.
中图分类号:
TrendMD:
姜涛, 王宁, 彭述明, 李梅, 韩伟, 陈一东. LiCl-KCl熔盐中Gd(Ⅲ)在Bi电极上的电化学行为及BixGdy金属间化合物的热力学数据. 高等学校化学学报, 2018, 39(8): 1759.
JIANG Tao, WANG Ning, PENG Shuming, LI Mei, HAN Wei, CHEN Yitung. Electrochemical Behaviour of Gd(Ⅲ) on Bi Electrode and Thermodynamic Data of BixGdy Intermetallic Compounds in LiCl-KCl Molten Salts†. Chem. J. Chinese Universities, 2018, 39(8): 1759.
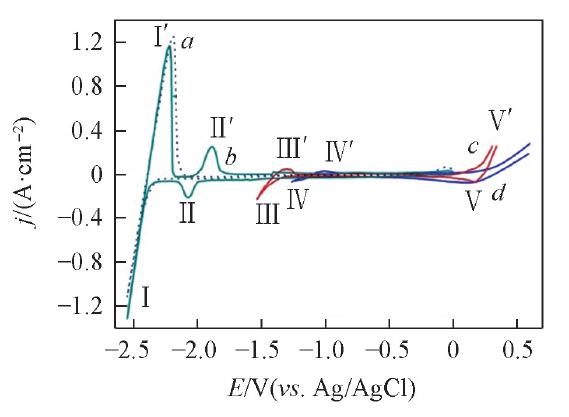
Fig.1 Cyclic voltammograms under different conditionsa. LiCl-KCl melts on W electrode; b. LiCl-KCl-GdCl3(5.2×10-5 mol/mL) melts on W electrode; c. LiCl-KCl melts on liquid Bi electrode; d. LiCl-KCl-GdCl3(5.2×10-5 mol/mL) melts on liquid Bi pool electrode. Scan rate: 0.1 V/s, SW=0.314 cm2, SBi=0.2 cm2, T=773 K.
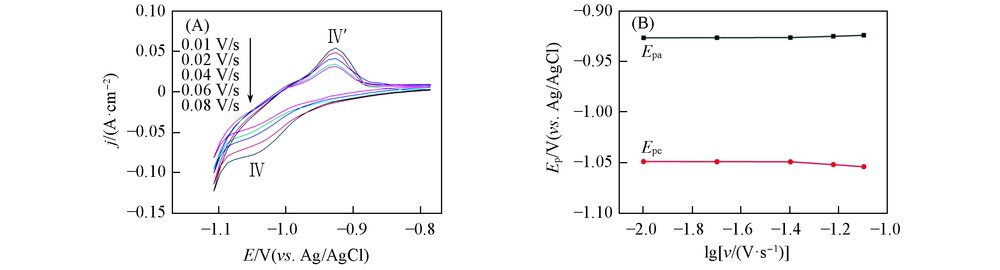
Fig.2 Cyclic voltammograms obtained in LiCl-KCl-GdCl3(5.2×10-5 mol/mL) melts on liquid Bi pool electrode at different scan rates(A) and relatioship between cathodic and anodic peak potentials with logarithm of scan rates(B)SBi=0.2 cm2, T=773 K.
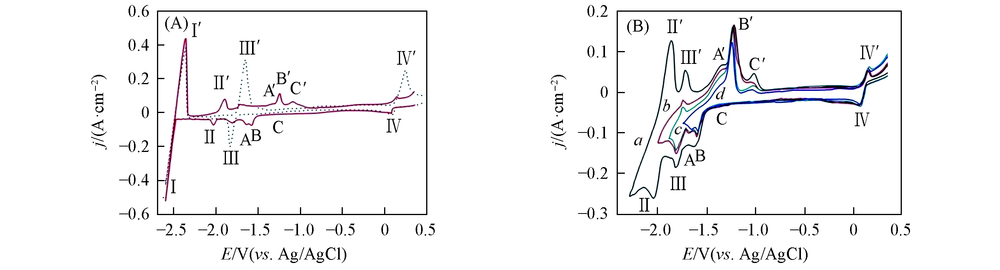
Fig.3 Cyclic voltammograms under different conditions(A) LiCl-KCl-BiCl3(3.37×10-6 mol/mL) melts on W electrode(dotted curve); LiCl-KCl-BiCl3(3.37×10-6 mol/mL)-GdCl3(8.67×10-5 mol/mL) melts on pre-deposited Bi film electrode(solid line); (B) LiCl-KCl-BiCl3(3.37×10-6 mol/mL)-GdCl3(8.67×10-5 mol/mL) melts on pre-deposited Bi film electrode at different terminal potentials. Terminal potential/V: a. -2.25; b. -2.00; c. -1.80; d. 1.75. Scan rate=0.1 V/s, S=0.314 cm2, T=773 K.
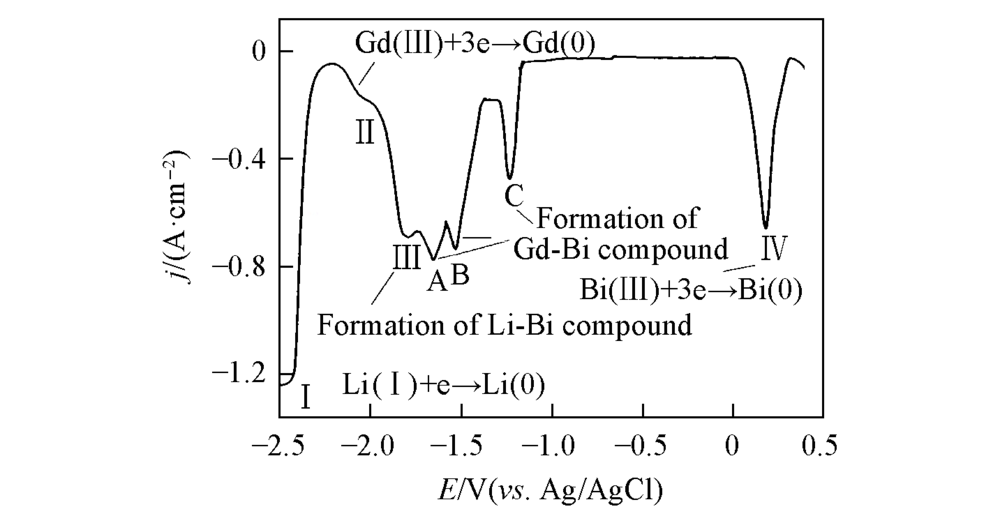
Fig.4 Square wave voltammogram obtained in LiCl-KCl-BiCl3(3.37×10-6 mol/mL)-GdCl3(8.67×10-5 mol/mL) melts on pre-deposited Bi film electrodePotential step=1 mV, frequency=20 Hz, S=0.314 cm2, T=773 K.
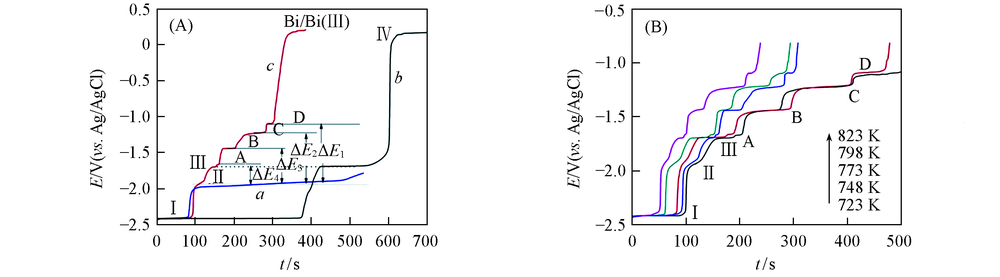
Fig.5 Open circuit chronopotentiograms of different conditions(A) a. LiCl-KCl-GdCl3(8.67×10-5 mol/mL) melts on W electrode; b. LiCl-KCl-BiCl3(3.37×10-6 mol/mL) melts on W electrode; c. LiCl-KCl-BiCl3(3.37×10-6 mol/mL)-GdCl3(8.67×10-5 mol/mL) melts on pre-deposited Bi film electrode; (B) LiCl-KCl-BiCl3(3.37×10-6 mol/mL)-GdCl3(8.67×10-5 mol/mL) melts on pre-deposited Bi film electrode at different temperatures. Deposition potential=-2.5 V, deposition time=60 s, S=0.314 cm2, T=773 K.
| Plateau* | T/K | Eeq/V(vs. Ag/AgCl) | ΔE/V[vs. Gd(Ⅲ)/Gd] | Δ | aGd |
|---|---|---|---|---|---|
| Ⅱ | 723 | -1.933±0.002 | — | — | — |
| 748 | -1.918±0.004 | — | — | — | |
| 773 | -1.903±0.002 | — | — | — | |
| 798 | -1.888±0.003 | — | — | — | |
| 823 | -1.881±0.002 | — | — | — | |
| A | 723 | -1.106±0.003 | 0.827±0.005 | -239.42±1.44 | 5.04×10-18 |
| 748 | -1.093±0.002 | 0.825±0.006 | -238.84±1.74 | 2.09×10-17 | |
| 773 | -1.081±0.002 | 0.822±0.004 | -237.97±1.16 | 8.30×10-17 | |
| 798 | -1.071±0.003 | 0.817±0.006 | -236.52±1.74 | 3.29×10-16 | |
| 823 | -1.067±0.003 | 0.814±0.005 | -235.65±1.45 | 1.10×10-15 | |
| B | 723 | -1.226±0.001 | 0.707±0.003 | -204.68±0.87 | 1.63×10-15 |
| 748 | -1.219±0.002 | 0.699±0.006 | -202.36±1.74 | 7.38×10-15 | |
| 773 | -1.209±0.003 | 0.694±0.005 | -200.91±1.45 | 2.65×10-14 | |
| 798 | -1.201±0.003 | 0.687±0.006 | -198.89±1.74 | 9.57×10-14 | |
| 823 | -1.197±0.001 | 0.684±0.003 | -198.02±0.87 | 2.70×10-13 | |
| C | 723 | -1.441±0.001 | 0.492±0.003 | -142.43±0.869 | 5.12×10-11 |
| 748 | -1.439±0.001 | 0.479±0.005 | -138.67±1.448 | 2.07×10-11 | |
| 773 | -1.438±0.002 | 0.465±0.004 | -134.62±1.158 | 8.00×10-10 | |
| 798 | -1.436±0.002 | 0.452±0.005 | -130.85±1.448 | 2.72×10-9 | |
| 823 | -1.433±0.001 | 0.448±0.003 | -129.70±0.869 | 5.86×10-9 | |
| D | 723 | -1.667±0.001 | 0.266±0.003 | -77.00±0.869 | 2.73×10-6 |
| 748 | -1.664±0.002 | 0.254±0.006 | -73.53±1.74 | 7.32×10-6 | |
| 773 | -1.662±0.003 | 0.241±0.005 | -69.77±1.45 | 1.93×10-5 | |
| 798 | -1.659±0.002 | 0.229±0.005 | -66.30±1.45 | 4.57×10-5 | |
| 823 | -1.657±0.003 | 0.224±0.005 | -64.85±1.45 | 7.66×10-5 |
Table 1 Thermodynamic properties of Gd in two-phase coexisting states at different temperatures
| Plateau* | T/K | Eeq/V(vs. Ag/AgCl) | ΔE/V[vs. Gd(Ⅲ)/Gd] | Δ | aGd |
|---|---|---|---|---|---|
| Ⅱ | 723 | -1.933±0.002 | — | — | — |
| 748 | -1.918±0.004 | — | — | — | |
| 773 | -1.903±0.002 | — | — | — | |
| 798 | -1.888±0.003 | — | — | — | |
| 823 | -1.881±0.002 | — | — | — | |
| A | 723 | -1.106±0.003 | 0.827±0.005 | -239.42±1.44 | 5.04×10-18 |
| 748 | -1.093±0.002 | 0.825±0.006 | -238.84±1.74 | 2.09×10-17 | |
| 773 | -1.081±0.002 | 0.822±0.004 | -237.97±1.16 | 8.30×10-17 | |
| 798 | -1.071±0.003 | 0.817±0.006 | -236.52±1.74 | 3.29×10-16 | |
| 823 | -1.067±0.003 | 0.814±0.005 | -235.65±1.45 | 1.10×10-15 | |
| B | 723 | -1.226±0.001 | 0.707±0.003 | -204.68±0.87 | 1.63×10-15 |
| 748 | -1.219±0.002 | 0.699±0.006 | -202.36±1.74 | 7.38×10-15 | |
| 773 | -1.209±0.003 | 0.694±0.005 | -200.91±1.45 | 2.65×10-14 | |
| 798 | -1.201±0.003 | 0.687±0.006 | -198.89±1.74 | 9.57×10-14 | |
| 823 | -1.197±0.001 | 0.684±0.003 | -198.02±0.87 | 2.70×10-13 | |
| C | 723 | -1.441±0.001 | 0.492±0.003 | -142.43±0.869 | 5.12×10-11 |
| 748 | -1.439±0.001 | 0.479±0.005 | -138.67±1.448 | 2.07×10-11 | |
| 773 | -1.438±0.002 | 0.465±0.004 | -134.62±1.158 | 8.00×10-10 | |
| 798 | -1.436±0.002 | 0.452±0.005 | -130.85±1.448 | 2.72×10-9 | |
| 823 | -1.433±0.001 | 0.448±0.003 | -129.70±0.869 | 5.86×10-9 | |
| D | 723 | -1.667±0.001 | 0.266±0.003 | -77.00±0.869 | 2.73×10-6 |
| 748 | -1.664±0.002 | 0.254±0.006 | -73.53±1.74 | 7.32×10-6 | |
| 773 | -1.662±0.003 | 0.241±0.005 | -69.77±1.45 | 1.93×10-5 | |
| 798 | -1.659±0.002 | 0.229±0.005 | -66.30±1.45 | 4.57×10-5 | |
| 823 | -1.657±0.003 | 0.224±0.005 | -64.85±1.45 | 7.66×10-5 |
| Intermetallic compound | Equation | T/K | Δ |
|---|---|---|---|
| Bi2Gd | Δ | 723 | -239.42±1.45 |
| 748 | -238.84±1.74 | ||
| 773 | -237.97±1.16 | ||
| 798 | -236.52±1.74 | ||
| 823 | -235.65±1.45 | ||
| BiGd | Δ | 723 | -222.05±1.16 |
| 748 | -220.60±1.74 | ||
| 773 | -219.44±1.30 | ||
| 798 | -217.70±1.74 | ||
| 823 | -216.84±1.16 | ||
| Bi3Gd4 | Δ | 723 | -808.57±4.34 |
| 748 | -800.47±6.66 | ||
| 773 | -792.94±5.07 | ||
| 798 | -783.97±6.66 | ||
| 823 | -780.20±4.34 | ||
| Bi3Gd5 | Δ | 723 | -885.58±5.21 |
| 748 | -874.00±8.40 | ||
| 773 | -862.71±6.51 | ||
| 798 | -850.26±8.11 | ||
| 823 | -845.05±5.79 |
Table 2 Formulas and results of calculated Gibbs free energies of formation for Bi-Gd intermetallic compounds
| Intermetallic compound | Equation | T/K | Δ |
|---|---|---|---|
| Bi2Gd | Δ | 723 | -239.42±1.45 |
| 748 | -238.84±1.74 | ||
| 773 | -237.97±1.16 | ||
| 798 | -236.52±1.74 | ||
| 823 | -235.65±1.45 | ||
| BiGd | Δ | 723 | -222.05±1.16 |
| 748 | -220.60±1.74 | ||
| 773 | -219.44±1.30 | ||
| 798 | -217.70±1.74 | ||
| 823 | -216.84±1.16 | ||
| Bi3Gd4 | Δ | 723 | -808.57±4.34 |
| 748 | -800.47±6.66 | ||
| 773 | -792.94±5.07 | ||
| 798 | -783.97±6.66 | ||
| 823 | -780.20±4.34 | ||
| Bi3Gd5 | Δ | 723 | -885.58±5.21 |
| 748 | -874.00±8.40 | ||
| 773 | -862.71±6.51 | ||
| 798 | -850.26±8.11 | ||
| 823 | -845.05±5.79 |
| Intermetallic compound | Δ (kJ·mol-1) | Δ (J·mol-1·K-1) |
|---|---|---|
| Bi2Gd | -268.10±2.53 | -39.39±3.27 |
| BiGd | -387.34±3.74 | -87.38±4.83 |
| Bi3Gd4 | -1400.22±19.96 | -395.30±25.80 |
| Bi3Gd5 | -1568.07±27.40 | -521.52±35.30 |
Table 3 Thermodynamic data of Bi-Gd intermetallic compounds in temperature range of 723—823 K
| Intermetallic compound | Δ (kJ·mol-1) | Δ (J·mol-1·K-1) |
|---|---|---|
| Bi2Gd | -268.10±2.53 | -39.39±3.27 |
| BiGd | -387.34±3.74 | -87.38±4.83 |
| Bi3Gd4 | -1400.22±19.96 | -395.30±25.80 |
| Bi3Gd5 | -1568.07±27.40 | -521.52±35.30 |
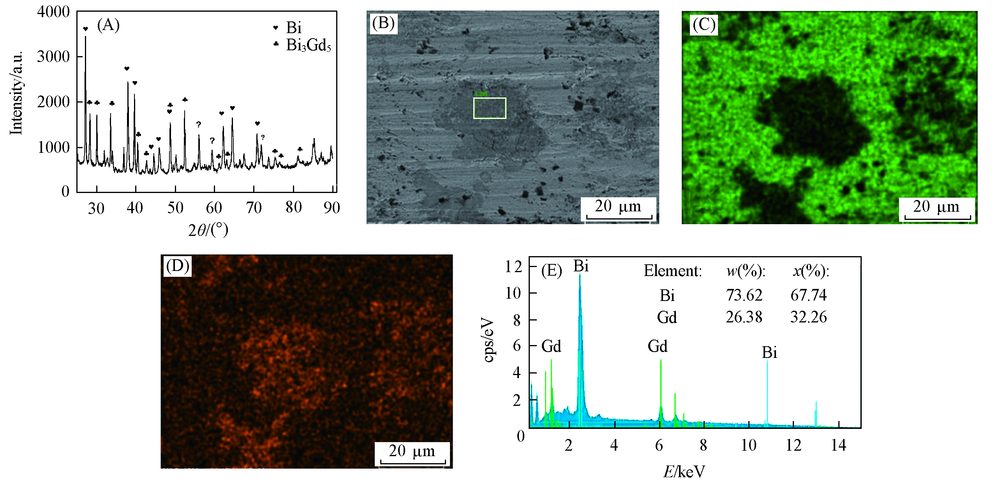
Fig.7 XRD pattern and SEM-EDS analysis of Bi-Gd alloy obtained in LiCl-KCl-GdCl3 melts by potentiostatic electrolysis(A) XRD pattern; (B) SEM image; (C) mapping analysis image of Bi; (D) mapping analysis image of Gd; (E) EDS spectrum of the framed area in (B). Electrolysis potential=-1.9 V, electrolysis time=4 h, Bi electrode, T=923 K.
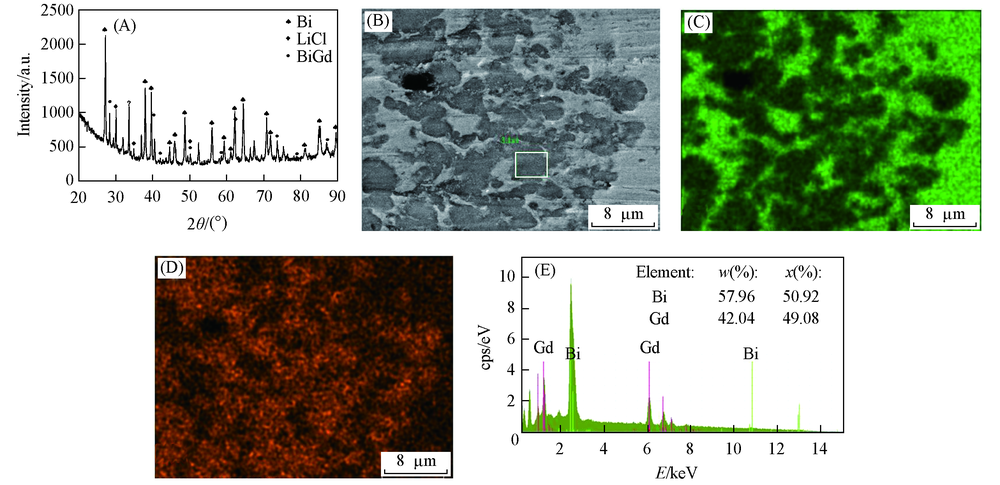
Fig.8 XRD pattern and SEM-EDS analysis of Bi-Gd alloy obtained in LiCl-KCl-GdCl3 melts by galvanostatic electrolysis(A) XRD pattern; (B) SEM image; (C) mapping analysis image of Bi; (D) mapping analysis image of Gd; (E) EDS spectrum of the framed area in (B). Current intensity=-0.5 A, electrolysis time=5.5 h, Bi electrode, T=923 K.
| [1] | Gibilaro M., Massot L., Chamelot P., Taxil P., Electrochim. Acta, 2009, 54(22), 5300—5306 |
| [2] | Kinoshita K., Koyama T., Inoue T., Ougier M., Glatz J.P., J. Phys. Chem. Solids, 2005, 66(2—4), 619—624 |
| [3] | SouČek P., Cassayre L., Malmbeck R., Mendes E., Jardin R., Glatz J. P., Radiochim. Acta, 2008, 96(4/5), 315—322 |
| [4] | Shirai O., Iwai T., Shiozawa K., Suzuki Y., Sakamura Y., Inoue T., J. Nucl. Mater., 2000, 277(2), 226—230 |
| [5] | McFarlane H. F., Lineberry M., J. Prog. Nucl. Energ., 1997, 31(1), 155—173 |
| [6] | Chen L.J., Zhang M. L., Han W., Yan Y. D., Cao P., Chem. J. Chinese Universities, 2012, 33(2), 327—330 |
| (陈丽军, 张密林, 韩伟, 颜永得, 曹鹏. 高等学校化学学报, 2012, 33(2), 327—330) | |
| [7] | Li M., Li W., Han W., Zhang M.L., Yan Y. D., Chem. J. Chinese Universities, 2014, 35(12), 2662—2667 |
| (李梅, 李炜, 韩伟, 张密林, 颜永得. 高等学校化学学报, 2014, 35(12), 2662—2667) | |
| [8] | Li M., Gu Q.Q., Han W., Zhang X. M., Sun Y., Zhang M. L., Yan Y. D, RSC Adv., 2015, 5(100), 82471—82480 |
| [9] | Castrillejo Y., Bermejo M.R., Diaz-Arocas P., Rosa F. D. L., Barrado E., Electrochemistry, 2005, 73(8), 636—643 |
| [10] | Castrillejo Y., Bermejo M.R., Arocas P. D., Martínez A. M., Barrado E., J. Electroanal. Chem., 2005, 579(2), 343—358 |
| [11] | Jiang T., Peng S.M., Li M., Pei T. T., Han W., Sun Y., Zhang M. L, Acta Phys.-Chim. Sin., 2016, 32(7), 1708—17140 |
| (姜涛, 彭述明, 李梅, 裴婷婷, 韩伟, 孙杨, 张密林. 物理化学学报, 2016, 32(7), 1708—1714) | |
| [12] | Han W., Li Z.Y., Li M., Li W. L., Zhang X. M., Yang X. G., Zhang M. L., Sun Y. J., Electrochem. Soc., 2017, 164(4), E62—E70 |
| [13] | Han W., Ji N., Wang J., Li M., Yang X., Sun Y., Zhang M., RSC Adv., 2017, 7(50), 31682—31690 |
| [14] | Vandarkuzhali S., Chandra M., Ghosh S., Samanta N., Nedumaran S., Prabhakara Reddy B., Nagarajan K., Electrochim. Acta, 2014, 145, 86—98 |
| [15] | Kato T., Inoue T., Iwai T., Arai Y., J. Nucl. Mater., 2006, 357(1), 105—114 |
| [16] | Wang L., Liu Y.L., Liu K., Tang S. L., Yuan L. Y., Lu T., Chai Z. F., Shi W. Q., J. Electrochem. Soc., 2015, 162(9), E179—E184 |
| [17] | Liu Y.L., Yuan L. Y., Kui L., Ye G. A., Zhang M. L., He H., Tang H. B., Lin R. S., Chai Z. F., Shi W. Q., Electrochim. Acta, 2014, 120, 369—378 |
| [18] | Li M., Wang J., Han W., Yang X.G., Zhang M., Sun Y., Zhang M. L., Yan Y. D., Electrochim. Acta, 2017, 228, 299—307 |
| [19] | Iizuka M., J. Electrochem. Soc., 1998, 145(1), 84—88 |
| [20] | Caravaca C., de Córdoba G., Tomás M. J., Rosado M., J. Nucl. Mater., 2007, 360(1), 25—31 |
| [21] | Tang H., Pesic B., J. Electrochem. Soc., 2014, 161(9), D429—D436 |
| [22] | Bermejo M.R., Gómez J., Medina J., Martínez A. M., Castrillejo Y., J. Electroanal. Chem., 2006, 588(2), 253—266 |
| [23] | Nourry C., Massot L., Chamelot P., Taxil P., Electrochim. Acta, 2008, 53(5), 2650—2655 |
| [24] | Nourry C., Massot L., Chamelot P., Taxil P., J. Appl. Electrochem., 2009, 39(12), 2359—2367 |
| [25] | Nourry C., Massot L., Chamelot P., Taxil P., J. Appl. Electrochem., 2009, 39(6), 927—933 |
| [26] | Zhou W., Liu Y.L., Liu K., Liu Z. R., Yuan L. Y., Wang L., Feng Y. X., Chai Z. F., Shi W. Q., J. Electrochem. Soc., 2015, 162(10), D531—D539 |
| [27] | Seon F., Picard G., Tremillon B., Electrochim. Acta, 1983, 28(2), 209—215 |
| [28] | Shibata H., Hayashi H., Akabori M., Arai Y., Kurata M., J. Phys. Chem. Solids, 2014, 75(8), 972—976 |
| [29] | Castrillejo Y., Vega A., Vega M., Hernández P., Rodriguez J.A., Barrado E., Electrochim. Acta, 2014, 118, 58—66 |
| [30] | Wang J.S., Li C. R., Guo C. P., Du Z. M., Wu B, Calphad., 2013, 41, 1—5 |
| [1] | 马鉴新, 刘晓东, 徐娜, 刘国成, 王秀丽. 一种具有发光传感、 安培传感和染料吸附性能的多功能Zn(II)配位聚合物[J]. 高等学校化学学报, 2022, 43(1): 20210585. |
| [2] | 宋丽, 林家祥, 黄定海. 步进扫描差示扫描量热法研究不同链结构的聚乙烯类聚烯烃热力学特性[J]. 高等学校化学学报, 2019, 40(8): 1740. |
| [3] | 李梅, 李炜, 韩伟, 张密林, 颜永得. LiCl-KCl熔盐体系中镨(Ⅲ)在镍电极上的电化学行为[J]. 高等学校化学学报, 2014, 35(12): 2662. |
| [4] | 夏妍, 曹发和, 常林荣, 刘文娟, 张鉴清. 锈层下碳钢和耐候钢的微区和宏观腐蚀电化学行为[J]. 高等学校化学学报, 2013, 34(5): 1246. |
| [5] | 徐智策 王建英 王晓玲 胡永琪. 1-烷基-3-甲基咪唑硫酸氢盐系列离子液体的热力学性质[J]. 高等学校化学学报, 2011, 32(8): 1860. |
| [6] | 程杰, 文越华, 徐艳, 曹高萍, 杨裕生. 基体对流动锌酸钾碱液中锌电沉积的影响[J]. 高等学校化学学报, 2011, 32(11): 2640. |
| [7] | 刘美, 闫伟伟, 臧娜, 阮文娟, 朱志昂. 新型吡啶基修饰的尾式卟啉的合成及性质[J]. 高等学校化学学报, 2009, 30(8): 1501. |
| [8] | 董社英, 王远, 黄廷林, 周元臻, 郑建斌. 芦丁在离子液体[bmim]BF4中的电化学行为及其影响因素[J]. 高等学校化学学报, 2009, 30(11): 2165. |
| [9] | 高红旭,赵凤起,胡荣祖,徐抗震,张海,王鹏,杜志明,徐司雨,仪建华,马海霞,常春然,宋纪蓉, . 3,4-二硝基呋咱基氧化呋咱的比热容、热力学性质、绝热至爆时间及热感度概率密度分布[J]. 高等学校化学学报, 2008, 29(5): 981. |
| [10] | 邢海青,郭占成,,王志,王明涌 . 超重力场中水溶液的电化学反应特性[J]. 高等学校化学学报, 2007, 28(9): 1765. |
| [11] | 郑建斌, 张宏芳, 张秀琦, 高鸿 . 白藜芦醇的电化学行为及其与DNA的相互作用[J]. 高等学校化学学报, 2006, 27(9): 1635. |
| [12] | 温靖邦, 周海晖, 罗胜联, 庞新宇, 陈金华, 旷亚非 . 基底材料对脉冲电流法制备的聚苯胺膜性能的影响[J]. 高等学校化学学报, 2006, 27(5): 948. |
| [13] | 谭学才, 麦智彬, 邹小勇, 邱俊明, 蔡沛祥. 壳聚糖与茜素红相互作用的电化学研究[J]. 高等学校化学学报, 2005, 26(6): 1055. |
| [14] | 夏其英, 肖鹤鸣, 居学海, 贡雪东. 第ⅢA族金属叠氮多聚体结构和性质的理论研究[J]. 高等学校化学学报, 2005, 26(5): 922. |
| [15] | 居学海, 肖继军, 肖鹤鸣. 硝仿肼离子对相互作用的密度泛函理论研究[J]. 高等学校化学学报, 2003, 24(6): 1067. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||