

高等学校化学学报 ›› 2017, Vol. 38 ›› Issue (10): 1778.doi: 10.7503/cjcu20170094
张灵芝1, 蒋敏瑞2, 魏萍3, 朱启华1( ), 龚国清2, 卞学国3, 徐云根1(
), 龚国清2, 卞学国3, 徐云根1( )
)
收稿日期:2017-02-18
出版日期:2017-10-10
发布日期:2017-09-22
作者简介:联系人简介: 朱启华, 男, 博士, 副教授, 主要从事新药分子的设计与合成研究. E-mail:基金资助:
ZHANG Lingzhi1, JIANG Minrui2, WEI Ping3, ZHU Qihua1,*( ), GONG Guoqing2, BIAN Xueguo3, XU Yungen1,*(
), GONG Guoqing2, BIAN Xueguo3, XU Yungen1,*( )
)
Received:2017-02-18
Online:2017-10-10
Published:2017-09-22
Contact:
ZHU Qihua,XU Yungen
E-mail:zhuqihua@vip.126.com;xyg@cpu.edu.cn
Supported by:摘要:
将选择性5-HT2C受体激动剂类减肥药绿卡色林分子中的仲胺转化成氨基甲酸酯类前药, 设计合成了13个氨基甲酸酯类化合物. 新化合物的结构经核磁共振波谱、 红外光谱及高分辨质谱确证. 通过体外代谢稳定性实验, 筛选出半衰期长且可通过代谢持续产生绿卡色林的新化合物6b. 对化合物6b的大鼠减肥药理实验结果表明, 在日剂量相同的条件下, 化合物6b给药1次/d比绿卡色林给药2次/d的减肥效果略好.
中图分类号:
TrendMD:
张灵芝, 蒋敏瑞, 魏萍, 朱启华, 龚国清, 卞学国, 徐云根. 绿卡色林衍生物的合成、 体外代谢及体内活性研究. 高等学校化学学报, 2017, 38(10): 1778.
ZHANG Lingzhi, JIANG Minrui, WEI Ping, ZHU Qihua, GONG Guoqing, BIAN Xueguo, XU Yungen. Synthesis, Metabolic Stability and Biological Activity in vivo of Lorcaserin Derivatives†. Chem. J. Chinese Universities, 2017, 38(10): 1778.
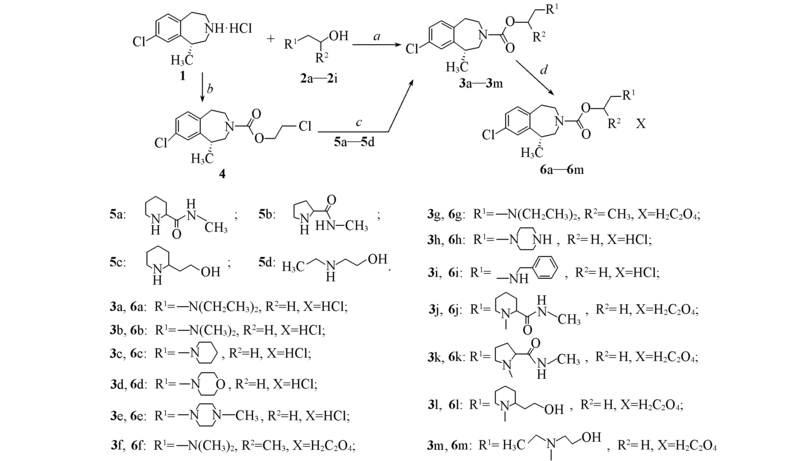
Scheme 1 Synthetic routes of compounds 6a—6mRegents and conditions: a. (i) CDI, dry CH2Cl2, N2, 0—20 ℃, 5 h, (ii) lorcaserin hydrochloride, triethylamine, r. t., CH2Cl2, overnight, (iii) CF3COOH, r. t., 30 min(3h and 3i); b. 2-chloroethyl chloroformate, triethylamine, dry CH2Cl2, 0 ℃, 4 h; c. K2CO3, NaI, 5a—5d; d. HCl, dry CH2Cl2 or H2C2O4, dry acetone.
| Compd. | Appearance | Yield(%) | Compd. | Appearance | Yield(%) | Compd. | Appearance | Yield(%) |
|---|---|---|---|---|---|---|---|---|
| 3a | Yellow oil | 80.4 | 3f | Yellow oil | 81.7 | 3j | Yellow oil | 72.5 |
| 3b | Yellow oil | 38.6 | 3g | Yellow oil | 85.8 | 3k | Yellow oil | 87.1 |
| 3c | Yellow oil | 42.3 | 3h | Yellow oil | 37.5 | 3l | Yellow oil | 78.1 |
| 3d | Yellow oil | 42.0 | 3i | Yellow oil | 81.5 | 3m | Yellow oil | 45.5 |
| 3e | Yellow oil | 43.8 |
Table 1 Appearances and yields of compounds 3a—3m*
| Compd. | Appearance | Yield(%) | Compd. | Appearance | Yield(%) | Compd. | Appearance | Yield(%) |
|---|---|---|---|---|---|---|---|---|
| 3a | Yellow oil | 80.4 | 3f | Yellow oil | 81.7 | 3j | Yellow oil | 72.5 |
| 3b | Yellow oil | 38.6 | 3g | Yellow oil | 85.8 | 3k | Yellow oil | 87.1 |
| 3c | Yellow oil | 42.3 | 3h | Yellow oil | 37.5 | 3l | Yellow oil | 78.1 |
| 3d | Yellow oil | 42.0 | 3i | Yellow oil | 81.5 | 3m | Yellow oil | 45.5 |
| 3e | Yellow oil | 43.8 |
| Compd. | 1H NMR(300 MHz, CDCl3), δ |
|---|---|
| 3a | 7.13—7.11(m, 1H), 7.08(dd, J1=8.0 Hz, J2=2.1 Hz, 1H), 7.02—6.99(m, 1H), 4.16(t, J=6.2 Hz, 2H), 3.84—3.33(m, 4H), 3.13—3.96(m, 2H), 2.81(dd, J=15.1, 6.2 Hz, 1H), 2.70(t, J=6.3 Hz, 2H), 2.58(q, J=7.1 Hz, 4H), 1.30—1.24(m, 3H), 1.03(t, J=7.1 Hz, 6H) |
| 3b | 7.13—7.10(m, 1H), 7.09(dd, J1=8.0 Hz, J2=2.1 Hz, 1H), 7.02—7.01(m, 1H), 4.20(t, J=5.9 Hz, 2H), 3.77—3.35(m, 4H), 3.03—3.00(m, 2H), 2.84—2.80(m, 1H), 2.56(t, J=5.9 Hz, 2H), 2.28(s, 6H), 1.30—1.24(m, 3H) |
| 3c | 7.14—7.07(m, 2H), 7.03—7.00(m, 1H), 4.23(t, J=6.0 Hz, 2H), 3.76—3.42(m, 4H), 3.08—3.00(m, 2H), 2.82(dd, J1=15.1 Hz, J2= 6.5 Hz, 1H), 2.61(t, J=5.9 Hz, 2H), 2.46—2.44(m, 4H), 1.60—1.55(m, 4H), 1.46—1.43(m, 2H), 1.30—1.28(m, 3H) |
| 3d | 7.14—7.08(m, 2H), 7.04—7.00(m, 1H), 4.23(t, J=5.8 Hz, 2H), 3.76—3.72(m, 1H), 3.70(t, J=4.5 Hz, 4H), 3.64—3.36(m, 3H), 3.08—3.00(m, 2H), 2.83(dd, J1=14.3 Hz, J2=4.7 Hz, 1H), 2.63(t, J=5.8 Hz, 2H), 2.52(t, J=4.6 Hz, 4H), 1.29(t, J=7.0 Hz, 3H) |
| 3e | 7.13—7.07(m, 2H), 7.03—7.01(m, 1H), 4.22(t, J=5.9 Hz, 2H), 3.74—3.40(m, 4H), 3.07—2.96(m, 2H), 2.82(dd, J1=15.2 Hz, J2=4.0 Hz, 1H), 2.64(t, J=5.9 Hz, 2H), 2.55—2.44(m, 8H), 2.28(s, 3H), 1.29—1.27(m, 3H) |
| Compd. | 1H NMR(300 MHz, CDCl3), δ |
| 3f | 7.12—7.07(m, 2H), 7.01—6.98(m, 1H), 5.00—4.97(m, 1H), 3.84—3.34(m, 4H), 3.06—3.02(m, 2H), 2.84(dd, J1=14.0 Hz, J2=5.5 Hz, 1H), 2.56—2.41(m, 1H), 2.33—2.29(m, 1H), 2.27(s, 3H), 2.25(s, 3H), 1.30(d, J=6.9 Hz, 3H), 1.22(d, J=6.2 Hz, 3H) |
| 3g | 7.14—7.08(m, 2H), 7.04—7.00(m, 1H), 5.00—4.91(m, 1H), 3.65—3.43(m, 4H), 3.09—3.01(m, 2H), 2.82(dd, J1=15.0 Hz, J2=6.5 Hz, 1H), 2.64—2.50(m, 5H), 2.45—2.40(m, 1H), 1.30(d, J=7.1 Hz, 3H), 1.22(d, J=6.3 Hz, 3H), 1.01(td, J1=7.1 Hz, J2=2.8 Hz, 6H) |
| 3h | 7.17—7.14(m, 1H), 7.11(dd, J1=8.1 Hz, J2=2.0 Hz, 1H), 7.04—7.03(m, 1H), 4.23(t, J=5.9 Hz, 2H), 3.78—3.41(m, 4H), 3.08—3.00(m, 2H), 2.96—2.91(m, 2H), 2.84(dd, J1=14.5 Hz, J2=5.2 Hz, 1H), 2.65—2.61(m, 4H), 2.56—2.46(m, 4H), 1.31—1.28(m, 3H), 1.25(s, 1H) |
| 3i | 7.35—7.27(m, 5H), 7.15—7.08(m, 2H), 7.04—6.99(m, 1H), 4.27—4.23(t, J=5.4 Hz, 2H), 3.85(s, 2H), 3.65—3.39(m, 4H), 3.09—3.01(m, 2H), 2.90(t, J=5.3 Hz, 2H), 2.82(dd, J1=15.4 Hz, J2=6.5 Hz, 1H), 1.88(s, 1H), 1.31—1.2(m, 3H) |
| 3j | 7.15—7.10(m, 2H), 7.05—7.02(m, 1H), 6.79(s, 1H), 4.28—4.17(m, 2H), 3.81—3.40(m, 4H), 3.11—2.96(m, 3H), 2.87—2.82(m, 6H), 2.45—2.40(m, 1H), 2.15—2.12(m, 1H), 1.98—1.93(m, 2H), 1.72—1.63(m, 2H), 1.51—1.24(m, 2H), 1.33—1.28(m, 3H) |
| 3k | 7.45(s, 1H), 7.16—7.11(m, 2H), 7.05—7.00(m, 1H), 4.20—4.12(m, 2H), 3.74—3.30(m, 4H), 3.28—3.02(m, 3H), 2.99—2.70(m, 6H), 2.60—2.36(m, 1H), 2.26—2.08(m, 1H), 1.92—1.78(m, 4H), 1.34—1.28(m, 3H) |
| 3l | 7.14—7.09(m, 2H), 7.04—7.01(m, 1H), 4.21(t, J=5.8 Hz, 2H), 3.91—3.88(m, 4H), 3.22—2.94(m, 4H), 2.87—2.77(m, 4H), 2.44—2.40(m, 1H), 2.00—1.98(m, 1H), 1.73—1.53(m, 3H), 1.51—1.43(m, 3H), 1.32—1.26(m, 3H) |
| 3m | 7.18—7.08(m, 2H), 7.07—7.00(m, 1H), 4.21—4.18(m, 2H), 3.94—3.90(m, 2H), 3.76—3.47(m, 6H), 3.26—3.23(m, 4H), 3.08—3.01(m, 2H), 2.84(dd, J1=15.1 Hz, J2=5.9 Hz, 1H), 1.30—1.29(m, 3H), 1.29(t, J=6.8 Hz, 3H) |
Table 2 1H NMR data of compounds 3a—3m
| Compd. | 1H NMR(300 MHz, CDCl3), δ |
|---|---|
| 3a | 7.13—7.11(m, 1H), 7.08(dd, J1=8.0 Hz, J2=2.1 Hz, 1H), 7.02—6.99(m, 1H), 4.16(t, J=6.2 Hz, 2H), 3.84—3.33(m, 4H), 3.13—3.96(m, 2H), 2.81(dd, J=15.1, 6.2 Hz, 1H), 2.70(t, J=6.3 Hz, 2H), 2.58(q, J=7.1 Hz, 4H), 1.30—1.24(m, 3H), 1.03(t, J=7.1 Hz, 6H) |
| 3b | 7.13—7.10(m, 1H), 7.09(dd, J1=8.0 Hz, J2=2.1 Hz, 1H), 7.02—7.01(m, 1H), 4.20(t, J=5.9 Hz, 2H), 3.77—3.35(m, 4H), 3.03—3.00(m, 2H), 2.84—2.80(m, 1H), 2.56(t, J=5.9 Hz, 2H), 2.28(s, 6H), 1.30—1.24(m, 3H) |
| 3c | 7.14—7.07(m, 2H), 7.03—7.00(m, 1H), 4.23(t, J=6.0 Hz, 2H), 3.76—3.42(m, 4H), 3.08—3.00(m, 2H), 2.82(dd, J1=15.1 Hz, J2= 6.5 Hz, 1H), 2.61(t, J=5.9 Hz, 2H), 2.46—2.44(m, 4H), 1.60—1.55(m, 4H), 1.46—1.43(m, 2H), 1.30—1.28(m, 3H) |
| 3d | 7.14—7.08(m, 2H), 7.04—7.00(m, 1H), 4.23(t, J=5.8 Hz, 2H), 3.76—3.72(m, 1H), 3.70(t, J=4.5 Hz, 4H), 3.64—3.36(m, 3H), 3.08—3.00(m, 2H), 2.83(dd, J1=14.3 Hz, J2=4.7 Hz, 1H), 2.63(t, J=5.8 Hz, 2H), 2.52(t, J=4.6 Hz, 4H), 1.29(t, J=7.0 Hz, 3H) |
| 3e | 7.13—7.07(m, 2H), 7.03—7.01(m, 1H), 4.22(t, J=5.9 Hz, 2H), 3.74—3.40(m, 4H), 3.07—2.96(m, 2H), 2.82(dd, J1=15.2 Hz, J2=4.0 Hz, 1H), 2.64(t, J=5.9 Hz, 2H), 2.55—2.44(m, 8H), 2.28(s, 3H), 1.29—1.27(m, 3H) |
| Compd. | 1H NMR(300 MHz, CDCl3), δ |
| 3f | 7.12—7.07(m, 2H), 7.01—6.98(m, 1H), 5.00—4.97(m, 1H), 3.84—3.34(m, 4H), 3.06—3.02(m, 2H), 2.84(dd, J1=14.0 Hz, J2=5.5 Hz, 1H), 2.56—2.41(m, 1H), 2.33—2.29(m, 1H), 2.27(s, 3H), 2.25(s, 3H), 1.30(d, J=6.9 Hz, 3H), 1.22(d, J=6.2 Hz, 3H) |
| 3g | 7.14—7.08(m, 2H), 7.04—7.00(m, 1H), 5.00—4.91(m, 1H), 3.65—3.43(m, 4H), 3.09—3.01(m, 2H), 2.82(dd, J1=15.0 Hz, J2=6.5 Hz, 1H), 2.64—2.50(m, 5H), 2.45—2.40(m, 1H), 1.30(d, J=7.1 Hz, 3H), 1.22(d, J=6.3 Hz, 3H), 1.01(td, J1=7.1 Hz, J2=2.8 Hz, 6H) |
| 3h | 7.17—7.14(m, 1H), 7.11(dd, J1=8.1 Hz, J2=2.0 Hz, 1H), 7.04—7.03(m, 1H), 4.23(t, J=5.9 Hz, 2H), 3.78—3.41(m, 4H), 3.08—3.00(m, 2H), 2.96—2.91(m, 2H), 2.84(dd, J1=14.5 Hz, J2=5.2 Hz, 1H), 2.65—2.61(m, 4H), 2.56—2.46(m, 4H), 1.31—1.28(m, 3H), 1.25(s, 1H) |
| 3i | 7.35—7.27(m, 5H), 7.15—7.08(m, 2H), 7.04—6.99(m, 1H), 4.27—4.23(t, J=5.4 Hz, 2H), 3.85(s, 2H), 3.65—3.39(m, 4H), 3.09—3.01(m, 2H), 2.90(t, J=5.3 Hz, 2H), 2.82(dd, J1=15.4 Hz, J2=6.5 Hz, 1H), 1.88(s, 1H), 1.31—1.2(m, 3H) |
| 3j | 7.15—7.10(m, 2H), 7.05—7.02(m, 1H), 6.79(s, 1H), 4.28—4.17(m, 2H), 3.81—3.40(m, 4H), 3.11—2.96(m, 3H), 2.87—2.82(m, 6H), 2.45—2.40(m, 1H), 2.15—2.12(m, 1H), 1.98—1.93(m, 2H), 1.72—1.63(m, 2H), 1.51—1.24(m, 2H), 1.33—1.28(m, 3H) |
| 3k | 7.45(s, 1H), 7.16—7.11(m, 2H), 7.05—7.00(m, 1H), 4.20—4.12(m, 2H), 3.74—3.30(m, 4H), 3.28—3.02(m, 3H), 2.99—2.70(m, 6H), 2.60—2.36(m, 1H), 2.26—2.08(m, 1H), 1.92—1.78(m, 4H), 1.34—1.28(m, 3H) |
| 3l | 7.14—7.09(m, 2H), 7.04—7.01(m, 1H), 4.21(t, J=5.8 Hz, 2H), 3.91—3.88(m, 4H), 3.22—2.94(m, 4H), 2.87—2.77(m, 4H), 2.44—2.40(m, 1H), 2.00—1.98(m, 1H), 1.73—1.53(m, 3H), 1.51—1.43(m, 3H), 1.32—1.26(m, 3H) |
| 3m | 7.18—7.08(m, 2H), 7.07—7.00(m, 1H), 4.21—4.18(m, 2H), 3.94—3.90(m, 2H), 3.76—3.47(m, 6H), 3.26—3.23(m, 4H), 3.08—3.01(m, 2H), 2.84(dd, J1=15.1 Hz, J2=5.9 Hz, 1H), 1.30—1.29(m, 3H), 1.29(t, J=6.8 Hz, 3H) |
| Compd. | Appearance | m. p. /℃ | Yield(%) | HRMS(calcd.), m/z[M+H]+ |
|---|---|---|---|---|
| 6a | White solid | 156—158 | 78.2 | 339.1834(339.1840) |
| 6b | White solid | 150—152 | 37.5 | 311.1521(311.1529) |
| 6c | White solid | 152—154 | 41.2 | 351.1834(351.1841) |
| 6d | White solid | 157—158 | 41.0 | 353.1626(353.1625) |
| 6e | White solid | 222—224 | 42.4 | 366.1943(366.1949) |
| 6f | White solid | 103—105 | 78.7 | 325.1677(325.1674) |
| 6g | White solid | 98—100 | 82.4 | 353.1990(353.1985) |
| 6h | White solid | 178—180 | 36.4 | 352.1786(352.1795) |
| 6i | White solid | 178—180 | 79.0 | 373.1677(373.1674) |
| 6j | White solid | 85—86 | 69.9 | 408.2048(408.2045) |
| 6k | White solid | 127—129 | 84.2 | 394.1892(394.1892) |
| 6l | White solid | 75.1 | 395.2096(395.2091) | |
| 6m | White solid | 43.7 | 355.1783(355.1782) |
Table 3 Appearances, melting points, yield and HRMS data of compounds 6a—6m*
| Compd. | Appearance | m. p. /℃ | Yield(%) | HRMS(calcd.), m/z[M+H]+ |
|---|---|---|---|---|
| 6a | White solid | 156—158 | 78.2 | 339.1834(339.1840) |
| 6b | White solid | 150—152 | 37.5 | 311.1521(311.1529) |
| 6c | White solid | 152—154 | 41.2 | 351.1834(351.1841) |
| 6d | White solid | 157—158 | 41.0 | 353.1626(353.1625) |
| 6e | White solid | 222—224 | 42.4 | 366.1943(366.1949) |
| 6f | White solid | 103—105 | 78.7 | 325.1677(325.1674) |
| 6g | White solid | 98—100 | 82.4 | 353.1990(353.1985) |
| 6h | White solid | 178—180 | 36.4 | 352.1786(352.1795) |
| 6i | White solid | 178—180 | 79.0 | 373.1677(373.1674) |
| 6j | White solid | 85—86 | 69.9 | 408.2048(408.2045) |
| 6k | White solid | 127—129 | 84.2 | 394.1892(394.1892) |
| 6l | White solid | 75.1 | 395.2096(395.2091) | |
| 6m | White solid | 43.7 | 355.1783(355.1782) |
| Compd. | 1H NMR(300 MHz), δ* | 13C NMR(75 MHz), δ* |
|---|---|---|
| 6a | 12.55(bs, 1H), 7.16—7.12(m, 2H), 7.07—7.04(m, 1H), 4.62(t, J=5.0 Hz, 2H), 3.86—3.47(m, 4H), 3.28—3.02(m, 8H), 2.89—2.86(dd, J1=15.7Hz, J2=4.6 Hz, 1H), 1.47—1.37(m, 6H), 1.32—1.29(m, 3H) | 154.91, 145.40, 136.95, 131.18, 127.77, 126.38, 115.18, 59.16, 51.72, 49.69, 47.32, 46.34, 40.57, 35.11, 17.09, 8.32 |
| 6b | 12.66(bs, 1H), 7.13—7.11(m, 2H), 7.04—7.01(m, 1H), 4.62—4.55(m, 2H), 3.82—3.45(m, 4H), 3.33—3.19(m, 2H), 3.14—3.01(m, 2H), 2.96—2.75(m, 7H), 1.30(d, J=7.0 Hz, 3H) | 154.37, 145.07, 136.17, 132.42, 130.70, 128.18, 125.23, 58.94, 55.51, 49.64, 45.39, 42.86, 39.52, 34.88, 17.59 |
| 6c | 12.45(bs, 1H), 7.16—7.12(m, 2H), 7.07—7.03(m, 1H), 4.65—4.64(m, 2H), 3.82—3.45(m, 6H), 3.24—3.22(m, 2H), 3.18—3.00(m, 2H), 2.88—2.83(m, 1H), 2.70—2.63(m, 2H), 2.33—2.30(m, 2H), 1.95—1.85(m, 3H), 1.50—1.37(m, 1H), 1.33—1.29(m, 3H) | 155.25, 145.55, 136.66, 132.35, 131.76, 127.85, 125.73, 59.43, 56.01, 53.48, 51.44, 46.30, 40.03, 34.97, 22.25, 21.44, 17.20 |
| 6d | 13.39(s, 1H), 7.15—7.11(m, 2H), 7.06—7.02(m, 1H), 4.64—4.40(m, 2H), 4.36—4.28(m, 2H), 4.01—3.98(m, 2H ), 3.81—3.50(m, 4H), 3.48—3.39(m, 2H), 3.30—3.22(m, 2H), 3.17—3.03(m, 2H), 2.99—2.89(m, 3H), 1.31—1.28(m, 3H) | 154.87, 145.75, 137.16, 132.16, 131.47, 128.06, 126.38, 63.92, 58.78, 56.01, 51.69, 50.95, 45.81, 39.95, 34.80, 17.11 |
| 6e | 7.15(d,J=1.9 Hz, 1H), 7.07(dd, J1=8.1 Hz, J2=1.9 Hz, 1H), 7.02(d, J=8.1 Hz, 1H), 4.19—4.17(m, 2H), 3.60—3.55(m, 4H), 3.44—3.24(m, 10H ), 3.12—3.06(m, 2H), 2.92(s, 3H), 2.79—2.74(m, 1H), 1.12(d, J=7.1 Hz, 3H) | 156.37, 145.13, 137.06, 131.19, 127.85, 126.14, 114.72, 59.43, 56.01, 50.96, 49.33, 48.83, 45.48, 42.55, 39.12, 33.26, 16.38 |
| 6f | 9.01(bs, 2H), 7.14—7.11(m, 2H), 7.07—7.03(m, 1H), 5.26—5.20(m, 1H), 3.84—3.48(m, 4H), 3.44—3.30(m, 2H), 3.12—3.00(m, 3H), 2.89(d, J=6.6 Hz, 3H),2.84(d, J=4.0 Hz, 3H), 1.31—1.29(m, 3H), 1.16(d, J=6.1 Hz, 3H) | 162.83, 154.43, 145.13, 136.66, 132.41, 127.84, 126.15, 68.73, 65.31, 60.25, 50.95, 46.29, 45.48, 42.55, 35.78, 22.25, 16.78 |
| 6g | 12.18(bs, 2H),7.16—7.12(m, 2H), 7.08—7.03(m, 1H),5.27—5.21(m, 1H), 3.78—3.44(m, 4H), 3.41—3.23(m, 6H), 3.07—2.93(m, 3H), 1.36—1.27(m, 12H) | 155.65, 145.96, 137.96, 132.01, 127.86, 126.14, 115.13, 72.97, 70.37, 58.12, 50.95, 47.60, 45.49, 40.44, 36.19, 18.91,17.35 11.73 |
| 6h | 7.11(m, 1H),7.05—6.98(m, 2H), 4.19—4.17(m, 2H), 3.60—3.57(m, 1H), 3.41—3.38(m, 6H), 3.25—3.15(m, 7H), 3.08—3.02(m, 1H), 2.96—2.88(m, 1H), 2.77—2.69(m, 1H), 1.10(d, J=7.1 Hz, 3H) | 145.95, 137.06, 132.71, 131.99, 127.84, 126.13, 114.72, 59.84, 56.00, 50.54, 48.43, 45.08, 41.66, 38.31, 33.25, 15.97. |
| 6i | 10.25(bs, 2H), 7.64—7.62(m, 2H), 7.43—7.41(m, 3H), 7.15—7.09(m, 2H), 7.04—7.01(m, 1H), 4.50—4.69(m, 2H), 4.19(s, 2H), 3.83—3.80(m, 1H), 3.60—3.55(m, 3H), 3.11—3.02(m, 4H), 2.91—2.79(m, 1H), 1.30—1.25(m, 3H) | 155.51, 145.80, 136.60, 131.30, 129.78, 129.41, 129.15, 128.79, 127.50, 125.82, 125.73, 59.90, 51.05, 49.99, 46.18, 44.92, 40.39, 35.47,17.17 |
| 6j | 11.49(bs, 0.5H), 10.83(bs, 0.5H), 9.11(bs, 0.5H), 8.79(bs, 0.5H), 7.15—7.11(m, 2H), 7.07—7.03(m, 1H), 4.60—4.37(m, 3H), 3.76—3.34(m, 7H), 3.21—3.01(m, 3H), 2.83(s, 3H), 2.72—2.61(m, 1H), 2.40—2.09(m, 3H), 1.99—1.81(m, 2H), 1.75—1.59(m, 1H), 1.36—1.30(m, 3H) | 175.06, 155.65, 145.13, 137.96, 132.00, 128.66, 126.13, 114.72, 67.84, 62.77, 55.20, 51.85, 51.30, 46.79, 41.38, 40.84, 36.60, 29.02, 25.68, 24.78, 23.15, 17.61 |
| 6k | 7.15—7.12(m, 1H), 7.09—7.01(m, 2H), 4.19—4.14(m, 2H), 3.69—3.63(m, 2H ), 3.53—3.36(m, 5H), 3.23—3.02(m, 3H), 3.02—2.88(m, 1H), 2.85—2.71(m, 1H), 2.63(d, J =5.6 Hz, 3H), 2.51—2.32(m, 1H), 2.09—1.93(m, 3H), 1.12(d, J=6.5 Hz, 3H) | 167.89, 156.56, 146.36, 137.06, 132.00, 127.85, 126.55, 115.13, 67.42, 64.49, 60.25, 56.50, 50.13, 46.31, 39.14, 32.85, 30.15, 29.42, 26.49, 22.25, 16.38 |
| 6l | 7.13—7.09(m, 2H), 7.05—7.02(m, 1H), 4.50—4.18(m, 2H), 3.91—3.30(m, 10H), 3.23—3.01(m, 3H), 2.86—2.81(m, 1H), 2.09—1.82(m, 8H), 1.33—1.25(m, 3H) | 156.06, 146.36, 137.97, 132.41, 128.25, 126.13, 115.57, 69.14, 62.97, 62.37, 60.10, 53.35, 51.91, 51.25, 49.14, 46.35, 40.84, 36.20, 31.14, 27.31, 22.73, 17.19 |
| 6m | 7.13—7.02(m, 3H), 4.48—4.43(m, 2H), 3.94—3.90(m, 2H), 3.76—3.47(m, 6H), 3.26—3.23(m, 4H), 3.08—3.01(m, 2H), 2.83(dd, J1=14.6 Hz, J2= 4.2 Hz, 1H), 1.30—1.28(m, 6H) | 155.74, 146.18, 137.53, 131.42, 128.27, 125.68, 109.95, 63.09, 58.58, 55.75, 52.85, 50.63, 48.04, 46.12, 41.00, 36.17, 35.86, 17.06, 11.62 |
Table 4 1H NMR and 13C NMR data of compounds 6a—6m
| Compd. | 1H NMR(300 MHz), δ* | 13C NMR(75 MHz), δ* |
|---|---|---|
| 6a | 12.55(bs, 1H), 7.16—7.12(m, 2H), 7.07—7.04(m, 1H), 4.62(t, J=5.0 Hz, 2H), 3.86—3.47(m, 4H), 3.28—3.02(m, 8H), 2.89—2.86(dd, J1=15.7Hz, J2=4.6 Hz, 1H), 1.47—1.37(m, 6H), 1.32—1.29(m, 3H) | 154.91, 145.40, 136.95, 131.18, 127.77, 126.38, 115.18, 59.16, 51.72, 49.69, 47.32, 46.34, 40.57, 35.11, 17.09, 8.32 |
| 6b | 12.66(bs, 1H), 7.13—7.11(m, 2H), 7.04—7.01(m, 1H), 4.62—4.55(m, 2H), 3.82—3.45(m, 4H), 3.33—3.19(m, 2H), 3.14—3.01(m, 2H), 2.96—2.75(m, 7H), 1.30(d, J=7.0 Hz, 3H) | 154.37, 145.07, 136.17, 132.42, 130.70, 128.18, 125.23, 58.94, 55.51, 49.64, 45.39, 42.86, 39.52, 34.88, 17.59 |
| 6c | 12.45(bs, 1H), 7.16—7.12(m, 2H), 7.07—7.03(m, 1H), 4.65—4.64(m, 2H), 3.82—3.45(m, 6H), 3.24—3.22(m, 2H), 3.18—3.00(m, 2H), 2.88—2.83(m, 1H), 2.70—2.63(m, 2H), 2.33—2.30(m, 2H), 1.95—1.85(m, 3H), 1.50—1.37(m, 1H), 1.33—1.29(m, 3H) | 155.25, 145.55, 136.66, 132.35, 131.76, 127.85, 125.73, 59.43, 56.01, 53.48, 51.44, 46.30, 40.03, 34.97, 22.25, 21.44, 17.20 |
| 6d | 13.39(s, 1H), 7.15—7.11(m, 2H), 7.06—7.02(m, 1H), 4.64—4.40(m, 2H), 4.36—4.28(m, 2H), 4.01—3.98(m, 2H ), 3.81—3.50(m, 4H), 3.48—3.39(m, 2H), 3.30—3.22(m, 2H), 3.17—3.03(m, 2H), 2.99—2.89(m, 3H), 1.31—1.28(m, 3H) | 154.87, 145.75, 137.16, 132.16, 131.47, 128.06, 126.38, 63.92, 58.78, 56.01, 51.69, 50.95, 45.81, 39.95, 34.80, 17.11 |
| 6e | 7.15(d,J=1.9 Hz, 1H), 7.07(dd, J1=8.1 Hz, J2=1.9 Hz, 1H), 7.02(d, J=8.1 Hz, 1H), 4.19—4.17(m, 2H), 3.60—3.55(m, 4H), 3.44—3.24(m, 10H ), 3.12—3.06(m, 2H), 2.92(s, 3H), 2.79—2.74(m, 1H), 1.12(d, J=7.1 Hz, 3H) | 156.37, 145.13, 137.06, 131.19, 127.85, 126.14, 114.72, 59.43, 56.01, 50.96, 49.33, 48.83, 45.48, 42.55, 39.12, 33.26, 16.38 |
| 6f | 9.01(bs, 2H), 7.14—7.11(m, 2H), 7.07—7.03(m, 1H), 5.26—5.20(m, 1H), 3.84—3.48(m, 4H), 3.44—3.30(m, 2H), 3.12—3.00(m, 3H), 2.89(d, J=6.6 Hz, 3H),2.84(d, J=4.0 Hz, 3H), 1.31—1.29(m, 3H), 1.16(d, J=6.1 Hz, 3H) | 162.83, 154.43, 145.13, 136.66, 132.41, 127.84, 126.15, 68.73, 65.31, 60.25, 50.95, 46.29, 45.48, 42.55, 35.78, 22.25, 16.78 |
| 6g | 12.18(bs, 2H),7.16—7.12(m, 2H), 7.08—7.03(m, 1H),5.27—5.21(m, 1H), 3.78—3.44(m, 4H), 3.41—3.23(m, 6H), 3.07—2.93(m, 3H), 1.36—1.27(m, 12H) | 155.65, 145.96, 137.96, 132.01, 127.86, 126.14, 115.13, 72.97, 70.37, 58.12, 50.95, 47.60, 45.49, 40.44, 36.19, 18.91,17.35 11.73 |
| 6h | 7.11(m, 1H),7.05—6.98(m, 2H), 4.19—4.17(m, 2H), 3.60—3.57(m, 1H), 3.41—3.38(m, 6H), 3.25—3.15(m, 7H), 3.08—3.02(m, 1H), 2.96—2.88(m, 1H), 2.77—2.69(m, 1H), 1.10(d, J=7.1 Hz, 3H) | 145.95, 137.06, 132.71, 131.99, 127.84, 126.13, 114.72, 59.84, 56.00, 50.54, 48.43, 45.08, 41.66, 38.31, 33.25, 15.97. |
| 6i | 10.25(bs, 2H), 7.64—7.62(m, 2H), 7.43—7.41(m, 3H), 7.15—7.09(m, 2H), 7.04—7.01(m, 1H), 4.50—4.69(m, 2H), 4.19(s, 2H), 3.83—3.80(m, 1H), 3.60—3.55(m, 3H), 3.11—3.02(m, 4H), 2.91—2.79(m, 1H), 1.30—1.25(m, 3H) | 155.51, 145.80, 136.60, 131.30, 129.78, 129.41, 129.15, 128.79, 127.50, 125.82, 125.73, 59.90, 51.05, 49.99, 46.18, 44.92, 40.39, 35.47,17.17 |
| 6j | 11.49(bs, 0.5H), 10.83(bs, 0.5H), 9.11(bs, 0.5H), 8.79(bs, 0.5H), 7.15—7.11(m, 2H), 7.07—7.03(m, 1H), 4.60—4.37(m, 3H), 3.76—3.34(m, 7H), 3.21—3.01(m, 3H), 2.83(s, 3H), 2.72—2.61(m, 1H), 2.40—2.09(m, 3H), 1.99—1.81(m, 2H), 1.75—1.59(m, 1H), 1.36—1.30(m, 3H) | 175.06, 155.65, 145.13, 137.96, 132.00, 128.66, 126.13, 114.72, 67.84, 62.77, 55.20, 51.85, 51.30, 46.79, 41.38, 40.84, 36.60, 29.02, 25.68, 24.78, 23.15, 17.61 |
| 6k | 7.15—7.12(m, 1H), 7.09—7.01(m, 2H), 4.19—4.14(m, 2H), 3.69—3.63(m, 2H ), 3.53—3.36(m, 5H), 3.23—3.02(m, 3H), 3.02—2.88(m, 1H), 2.85—2.71(m, 1H), 2.63(d, J =5.6 Hz, 3H), 2.51—2.32(m, 1H), 2.09—1.93(m, 3H), 1.12(d, J=6.5 Hz, 3H) | 167.89, 156.56, 146.36, 137.06, 132.00, 127.85, 126.55, 115.13, 67.42, 64.49, 60.25, 56.50, 50.13, 46.31, 39.14, 32.85, 30.15, 29.42, 26.49, 22.25, 16.38 |
| 6l | 7.13—7.09(m, 2H), 7.05—7.02(m, 1H), 4.50—4.18(m, 2H), 3.91—3.30(m, 10H), 3.23—3.01(m, 3H), 2.86—2.81(m, 1H), 2.09—1.82(m, 8H), 1.33—1.25(m, 3H) | 156.06, 146.36, 137.97, 132.41, 128.25, 126.13, 115.57, 69.14, 62.97, 62.37, 60.10, 53.35, 51.91, 51.25, 49.14, 46.35, 40.84, 36.20, 31.14, 27.31, 22.73, 17.19 |
| 6m | 7.13—7.02(m, 3H), 4.48—4.43(m, 2H), 3.94—3.90(m, 2H), 3.76—3.47(m, 6H), 3.26—3.23(m, 4H), 3.08—3.01(m, 2H), 2.83(dd, J1=14.6 Hz, J2= 4.2 Hz, 1H), 1.30—1.28(m, 6H) | 155.74, 146.18, 137.53, 131.42, 128.27, 125.68, 109.95, 63.09, 58.58, 55.75, 52.85, 50.63, 48.04, 46.12, 41.00, 36.17, 35.86, 17.06, 11.62 |
| Compd. | Metabolic results | ||||
|---|---|---|---|---|---|
| Regression equation (1) | Half-intial concentration/ (μmol·L-1) | Regression equation (2) | |||
| Lorcaserin | y=0.0625x-0.1457 | 0.9806 | 7.958 | y=0.0028x+0.048 | 0.9724 |
| 6a | y=0.0486x-0.0756 | 0.9850 | 9.946 | y=0.0014x+0.0571 | 0.9774 |
| 6b | y=0.0607x-0.1028 | 0.9945 | 9.068 | y=0.003x+0.0415 | 0.9788 |
| 6c | y=0.0725x+0.1009 | 0.9719 | 7.477 | y=0.0725x+0.1009 | 0.9402 |
| 6d | y=0.0527x-0.0604 | 0.9896 | 8.429 | y=0.0032x+0.0874 | 0.9595 |
| 6e | y=0.0757x+0.0892 | 0.9889 | 10.349 | y=0.0009x+0.0664 | 0.9634 |
| 6f | y=0.0572x-0.1324 | 0.9813 | 8.457 | y=0.0016x+0.0546 | 0.9935 |
| 6g | y=0.0451x-0.0776 | 0.9973 | 9.297 | y=0.0115x+0.0138 | 0.9411 |
| 6h | y=0.0561x+0.2282 | 0.9960 | 12.253 | y=0.047x-0.3031 | 0.9393 |
| 6i | y=0.0585x-0.0448 | 0.9874 | 8.939 | y=0.0046x+0.0421 | 0.9655 |
| 6j | y=0.0535x-0.0584 | 0.9994 | 9.892 | y=0.0035x+0.0724 | 0.9165 |
| 6k | y=0.0500x-0.0300 | 0.9988 | 14.680 | y=0.0049x+0.0038 | 0.9474 |
| 6l | y=0.0623x+0.0208 | 0.9962 | 10.443 | y=0.0083x-0.0347 | 0.9384 |
| 6m | y=0.0663x+0.2536 | 0.9862 | 9.392 | y=24.877 | 0.9806 |
Table 5 Metabolic results of compounds 6a—6m
| Compd. | Metabolic results | ||||
|---|---|---|---|---|---|
| Regression equation (1) | Half-intial concentration/ (μmol·L-1) | Regression equation (2) | |||
| Lorcaserin | y=0.0625x-0.1457 | 0.9806 | 7.958 | y=0.0028x+0.048 | 0.9724 |
| 6a | y=0.0486x-0.0756 | 0.9850 | 9.946 | y=0.0014x+0.0571 | 0.9774 |
| 6b | y=0.0607x-0.1028 | 0.9945 | 9.068 | y=0.003x+0.0415 | 0.9788 |
| 6c | y=0.0725x+0.1009 | 0.9719 | 7.477 | y=0.0725x+0.1009 | 0.9402 |
| 6d | y=0.0527x-0.0604 | 0.9896 | 8.429 | y=0.0032x+0.0874 | 0.9595 |
| 6e | y=0.0757x+0.0892 | 0.9889 | 10.349 | y=0.0009x+0.0664 | 0.9634 |
| 6f | y=0.0572x-0.1324 | 0.9813 | 8.457 | y=0.0016x+0.0546 | 0.9935 |
| 6g | y=0.0451x-0.0776 | 0.9973 | 9.297 | y=0.0115x+0.0138 | 0.9411 |
| 6h | y=0.0561x+0.2282 | 0.9960 | 12.253 | y=0.047x-0.3031 | 0.9393 |
| 6i | y=0.0585x-0.0448 | 0.9874 | 8.939 | y=0.0046x+0.0421 | 0.9655 |
| 6j | y=0.0535x-0.0584 | 0.9994 | 9.892 | y=0.0035x+0.0724 | 0.9165 |
| 6k | y=0.0500x-0.0300 | 0.9988 | 14.680 | y=0.0049x+0.0038 | 0.9474 |
| 6l | y=0.0623x+0.0208 | 0.9962 | 10.443 | y=0.0083x-0.0347 | 0.9384 |
| 6m | y=0.0663x+0.2536 | 0.9862 | 9.392 | y=24.877 | 0.9806 |
| Group | Animal number | Weight/g | |
|---|---|---|---|
| Before experimenta | After experimentb | ||
| Normal diet control | 6 | 222.33±9.29 | 322.17±7.96 |
| High fat control | 6 | 230.83±5.11 | 502.50±22.83** |
| Lorcaserin control | 6 | 228.67±6.00 | 470.17±15.17** |
| Compound 6b | 6 | 228.33±8.56 | 496.00±20.67** |
Table 6 Effect of fodder on body-weigh in model rats(x?±s, n=6)
| Group | Animal number | Weight/g | |
|---|---|---|---|
| Before experimenta | After experimentb | ||
| Normal diet control | 6 | 222.33±9.29 | 322.17±7.96 |
| High fat control | 6 | 230.83±5.11 | 502.50±22.83** |
| Lorcaserin control | 6 | 228.67±6.00 | 470.17±15.17** |
| Compound 6b | 6 | 228.33±8.56 | 496.00±20.67** |
| Compd. | Half-life period/min | Compd. | Half-life period/min | Compd. | Half-life period/min |
|---|---|---|---|---|---|
| Lorcaserin | 27.736 | 6e | 33.586 | 6j | 8.198 |
| 6a | 31.031 | 6f | 39.778 | 6k | 13.127 |
| 6b | 22.926 | 6g | 8.153 | 6l | 15.718 |
| 6c | 14.754 | 6h | 8.185 | 6m | 12.488 |
| 6d | 9.762 | 6i | 15.167 |
Table 7 Half-life period of compounds 6a—6m
| Compd. | Half-life period/min | Compd. | Half-life period/min | Compd. | Half-life period/min |
|---|---|---|---|---|---|
| Lorcaserin | 27.736 | 6e | 33.586 | 6j | 8.198 |
| 6a | 31.031 | 6f | 39.778 | 6k | 13.127 |
| 6b | 22.926 | 6g | 8.153 | 6l | 15.718 |
| 6c | 14.754 | 6h | 8.185 | 6m | 12.488 |
| 6d | 9.762 | 6i | 15.167 |
| Group | Animal number | Weight/g | Weight increase/g | |
|---|---|---|---|---|
| Before experimenta | After experimentb | |||
| Normal diet control | 6 | 322.17±7.96 | 325.00±15.43 | 2.83±13.54 |
| High fat control | 6 | 502.50±22.83** | 550.33±19.00** | 47.83±15.89** |
| Lorcaserin control | 6 | 470.17±15.17** | 481.83±18.22**## | 11.67±11.67# |
| Compound 6b | 6 | 496.00±20.67** | 490.83±28.50**## | -5.17±17.28## |
Table 8 Effect of compound 6b on body-weigh in high-fat model rats(xˉ±s, n=6)
| Group | Animal number | Weight/g | Weight increase/g | |
|---|---|---|---|---|
| Before experimenta | After experimentb | |||
| Normal diet control | 6 | 322.17±7.96 | 325.00±15.43 | 2.83±13.54 |
| High fat control | 6 | 502.50±22.83** | 550.33±19.00** | 47.83±15.89** |
| Lorcaserin control | 6 | 470.17±15.17** | 481.83±18.22**## | 11.67±11.67# |
| Compound 6b | 6 | 496.00±20.67** | 490.83±28.50**## | -5.17±17.28## |
| Group | Animal number | Liver index | Lipid index | Lees index |
|---|---|---|---|---|
| Normal diet control | 6 | 2.95±0.53 | 0.85±0.33 | 290.66±5.05 |
| High fat control | 6 | 3.89±0.27** | 3.04±0.53** | 311.77±4.18** |
| Lorcaserin control | 6 | 3.97±0.25** | 2.70±0.68** | 306.66±4.08** |
| Compound 6b | 6 | 3.61±0.34* | 2.09±0.48** | 304.45±5.20** |
Table 9 Effect of new compound in high-fat model rats(xˉ±s, n=6)
| Group | Animal number | Liver index | Lipid index | Lees index |
|---|---|---|---|---|
| Normal diet control | 6 | 2.95±0.53 | 0.85±0.33 | 290.66±5.05 |
| High fat control | 6 | 3.89±0.27** | 3.04±0.53** | 311.77±4.18** |
| Lorcaserin control | 6 | 3.97±0.25** | 2.70±0.68** | 306.66±4.08** |
| Compound 6b | 6 | 3.61±0.34* | 2.09±0.48** | 304.45±5.20** |
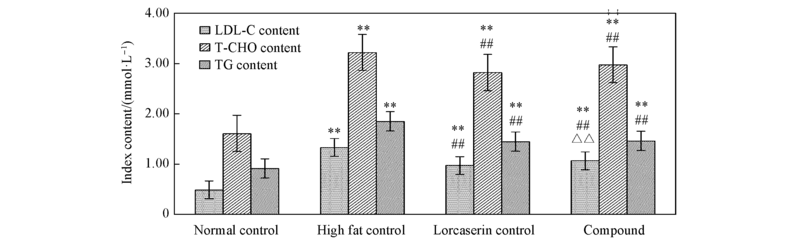
Fig.3 Effect of compound 6b on blood serum T-CHO, TG, LDL-C in model rats**P<0.01 compared with the normal diet control group; ## P<0.01 compared with the high fat control group; △△ P<0.01 compared with the compound control group.
| [1] | Lam D. D., Przydzial M. J., Ridley S. H., Yeo G. S., Rochford J. J., O’Rahilly S., Heisler L. K., Endocrinology,2008, 149(3), 1323—1328 |
| [2] | Ioannides-Demos L. L., Proietto J., Tonkin A. M., McNeil J. J., Drug Safety,2006, 29(4), 277—302 |
| [3] | Kim G. W., Lin J. E., Blomain E. S., Waldman S. A., Expert Opin. Drug Discov., 2013, 8(6), 655—671 |
| [4] | Topol E. J., Bousser M. G., Fox K. A., Creager M. A., Despres J. P., Easton J. D., Hamm C. W., Montalescot G., Steg P. G., Pearson T. A., Cohen E., Gaudin C., Job B., Murphy J. H., Bhatt D. L., Lancet,2010, 376(9740), 517—523 |
| [5] | Chaudhari D., Crisostomo C., Ganote C., Youngberg G., Case Rep. Nephrol., 2013, 2013, 124604 |
| [6] | Khazaal Y., Zullino D. F., Eur. J. Clin. Pharmacol., 2007, 63(9), 891—892 |
| [7] | Gadde K. M., Parker C. B., Maner L. G., Wagner H. R., Logue E. J., Drezner M. K., Krishnan K. R., Obes. Res., 2001, 9(9), 544—551 |
| [8] | Plodkowski R. A., Nguyen Q., Sundaram U., Nguyen L., Chau D. L., St Jeor S., Expert Opin. Pharmacother., 2009, 10(6), 1069—1081 |
| [9] | Waser B., Beetschen K., Pellegata N. S., Reubi J. C., Neuroendocrinology,2011, 94(4), 291—301 |
| [10] | Wang Y., Zheng Q. C., Chem. J. Chinese Universities,2015, 36(11), 2226—2235 |
| (王衍, 郑清川. 高等学校化学学报, 2015, 36(11), 2226—2235) | |
| [11] | Wang Y., Zheng Q. C., Zhang J. L., Xie M., Zhan J. Y., Zhang H. X., Chem. Res. Chinese Universities,2015, 31(6), 1029—1038 |
| [12] | Usmani K. A., Chen W. G., Sadeque A. J., Drug Metab. Dispos., 2012, 40(4), 761—771 |
| [13] | Arena Pharmaceuticals, Belviq(Lorcaserin Hydrochloride) Prescribing Information., 2012, 2012-06-29, |
| [14] | Jr S. J. D., FotschC., J. Med. Chem., 2012, 55(13), 6002—6020 |
| [15] | Sadeque A. J., Usmani K. A., Palamar S., Cerny M. A., Chen W. G., Drug Metab. Dispos., 2012, 40(4), 772—778 |
| [16] | Wang X. Y., Ye J., Sun G. X., Xue B. J., Zhao Y. Y., Miao P. P., Su J., Zhang Y. J., Chinese Journal of Chinese Materia Medica,2014, 39(19), 3855—3859 |
| (杨晓燕, 叶静, 孙桂霞, 薛宝娟, 赵圆圆, 苗培培, 苏瑾, 张玉杰. 中国中药杂志, 2014, 39(19), 3855—3859) | |
| [17] | Usmani K. A., Chen W. G., Sadeque A. J., Drug Metab. Dispos., 2012, 40(4), 761—771 |
| [18] | Thomsen W. J., Grottick A. J., Menzaghi F., Reyes-Saldana H., Espitia S., Yuskin D., Whelan K., Martin M., Morgan M., Chen W., Al-Shamma H., Smith B., Chalmers D., Behan D., J. Pharmacol. Exp. Ther., 2008, 325(2), 577—587 |
| [19] | Smith B. M., Smith J. M., Tsai J. H., Schultz J. A., Gilson C. A., Estrada S. A., Chen R. R., Park D. M., Prieto E. B., Gallardo C. S., Sengupta D., Dosa P. I., Covel J. A., Ren A., Webb R. R., Beeley N. R., Martin M., Morgan M., Espitia S., Saldana H. R., Bjenning C., Whelan K. T., Grottick A. J., Menzaghi F., Thomsen W. J., J. Med. Chem., 2008, 51(2), 305—313 |
| [20] | Tian H., Wang Y. T., Tao L., Ding H., Chinese Pharmacological Bulletin,2013, 29(7), 1016—1024 |
| (田辉, 王玉婷, 陶莉, 丁虹. 中国药理学通报, 2013, 29(7), 1016—1024) |
| [1] | 任玉双, 郭园园, 刘学怡, 宋杰, 张川. 顺铂前药接枝修饰硫代DNA及其自组装靶向纳米药物研究[J]. 高等学校化学学报, 2020, 41(8): 1721. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||