

高等学校化学学报 ›› 2017, Vol. 38 ›› Issue (3): 413.doi: 10.7503/cjcu20160546
收稿日期:2016-07-28
出版日期:2017-03-10
发布日期:2017-02-23
作者简介:联系人简介: 李 媛, 女, 教授, 主要从事有机合成方面的研究. E-mail: 基金资助:
MU Boshuai1, CHANG Suna2, BIAN Yanqing2,*( ), LI Yuan1,*(
), LI Yuan1,*( )
)
Received:2016-07-28
Online:2017-03-10
Published:2017-02-23
Contact:
BIAN Yanqing,LI Yuan
E-mail:bianyangqing151@sohu.com;liyuanhbsd@163.com
Supported by:摘要:
以(R)/(S)-4-苄基-2-噁唑烷酮为手性助剂, 采用不对称合成方法制备了18个具有光学活性的2-甲氧羰基-4-氟苯基-1,5-苯并硫氮杂卓类化合物9a~9i和14a~14i, 经HPLC分析e.e.值较为理想; 通过核磁共振谱、 红外光谱和高分辨质谱表征了其结构, 通过单晶X射线衍射法确定化合物9h的相对构型; 用抑菌圈法测试了目标化合物对新生隐球菌的抑菌活性. 研究结果表明, 由S型手性助剂诱导不对称合成的杂卓对新生隐球菌的抑制作用普遍高于由R型手性助剂诱导合成的杂卓及外消旋体. 测试了抑菌活性较好的化合物14a~14f的最小抑菌浓度(MIC)和最小杀菌浓度(MFC), 发现其对新生隐球菌的MIC和MFC均低于抗真菌药物氟康唑.
中图分类号:
TrendMD:
穆博帅, 常素娜, 边艳青, 李媛. 手性2-甲氧羰基-4-氟苯基-1,5-苯并硫氮杂卓的不对称合成及抑真菌活性. 高等学校化学学报, 2017, 38(3): 413.
MU Boshuai, CHANG Suna, BIAN Yanqing, LI Yuan. Asymmetric Synthesis and Antibacterial Activity of Chiral 2-Methoxycarbonyl-4-fluorophenyl-1,5-benzothiazepines†. Chem. J. Chinese Universities, 2017, 38(3): 413.
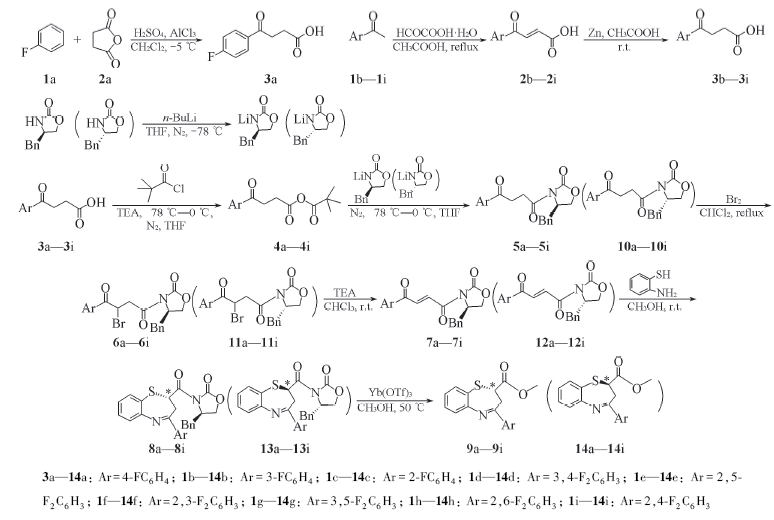
Scheme 1 Synthesis route of 1,5-benzothiazepines(9a—9i and 14a—14i) 3a—14a: Ar=4-FC6H4; 1b—14b: Ar=3-FC6H4; 1c—14c: Ar=2-FC6H4; 1d—14d: Ar=3,4-F2C6H3; 1e—14e: Ar=2,5-F2C6H3; 1f—14f: Ar=2,3-F2C6H3; 1g—14g: Ar=3,5-F2C6H3; 1h—14h: Ar=2,6-F2C6H3; 1i—14i: Ar=2,4-F2C6H3
| Compd. | Isolated yield(%) | m. p./℃ | HRMS(calcd., [M+Na]+), m/z | IR(KBr), | e.e.(%) |
|---|---|---|---|---|---|
| 9a | 32.0 | 93—96 | 338.0625(338.0621) | 1611, 1717 | 84 |
| 9b | 28.2 | 102—103 | 338.0623(338.0621) | 1610, 1733 | 65 |
| 9c | 23.5 | 83—85 | 338.0625(338.0621) | 1603, 1739 | 35 |
| 9d | 35.0 | 98—100 | 356.0531(356.0527) | 1598, 1733 | 93 |
| 9e | 24.8 | 111—113 | 356.0531(356.0527) | 1616, 1737 | 76 |
| 9f | 29.0 | 105—107 | 356.0528(356.0527) | 1607, 1728 | 67 |
| 9g | 36.3 | 86—88 | 356.0533(356.0527) | 1610, 1721 | 65 |
| 9h | 34.3 | 95—97 | 356.0532(356.0527) | 1615, 1728 | 71 |
| 9i | 25.7 | 76—78 | 356.0527(356.0527) | 1600, 1728 | 40 |
| 14a | 37.0 | 94—96 | 338.0629(338.0621) | 1595, 1708 | 60 |
| 14b | 25.8 | 102—103 | 338.0625(338.0621) | 1615, 1728 | 70 |
| 14c | 29.5 | 83—86 | 338.0625(338.0621) | 1621, 1746 | 34 |
| 14d | 33.4 | 98—99 | 356.0534(356.0527) | 1597, 1731 | 95 |
| 14e | 36.2 | 111—113 | 356.0534(356.0527) | 1605, 1731 | 70 |
| 14f | 38.2 | 106—108 | 356.0528(356.0527) | 1597, 1731 | 54 |
| 14g | 36.2 | 86—89 | 356.0525(356.0527) | 1601, 1729 | 73 |
| 14h | 42.1 | 95—97 | 356.0532(356.0527) | 1627, 1727 | 71 |
| 14i | 25.9 | 77—79 | 356.0534(356.0527) | 1611, 1731 | 40 |
Table 1 Yields, melting points, HRMS and IR data for compounds 9a—9i and 14a—14i
| Compd. | Isolated yield(%) | m. p./℃ | HRMS(calcd., [M+Na]+), m/z | IR(KBr), | e.e.(%) |
|---|---|---|---|---|---|
| 9a | 32.0 | 93—96 | 338.0625(338.0621) | 1611, 1717 | 84 |
| 9b | 28.2 | 102—103 | 338.0623(338.0621) | 1610, 1733 | 65 |
| 9c | 23.5 | 83—85 | 338.0625(338.0621) | 1603, 1739 | 35 |
| 9d | 35.0 | 98—100 | 356.0531(356.0527) | 1598, 1733 | 93 |
| 9e | 24.8 | 111—113 | 356.0531(356.0527) | 1616, 1737 | 76 |
| 9f | 29.0 | 105—107 | 356.0528(356.0527) | 1607, 1728 | 67 |
| 9g | 36.3 | 86—88 | 356.0533(356.0527) | 1610, 1721 | 65 |
| 9h | 34.3 | 95—97 | 356.0532(356.0527) | 1615, 1728 | 71 |
| 9i | 25.7 | 76—78 | 356.0527(356.0527) | 1600, 1728 | 40 |
| 14a | 37.0 | 94—96 | 338.0629(338.0621) | 1595, 1708 | 60 |
| 14b | 25.8 | 102—103 | 338.0625(338.0621) | 1615, 1728 | 70 |
| 14c | 29.5 | 83—86 | 338.0625(338.0621) | 1621, 1746 | 34 |
| 14d | 33.4 | 98—99 | 356.0534(356.0527) | 1597, 1731 | 95 |
| 14e | 36.2 | 111—113 | 356.0534(356.0527) | 1605, 1731 | 70 |
| 14f | 38.2 | 106—108 | 356.0528(356.0527) | 1597, 1731 | 54 |
| 14g | 36.2 | 86—89 | 356.0525(356.0527) | 1601, 1729 | 73 |
| 14h | 42.1 | 95—97 | 356.0532(356.0527) | 1627, 1727 | 71 |
| 14i | 25.9 | 77—79 | 356.0534(356.0527) | 1611, 1731 | 40 |
| Compd. | 1H NMR(500 MHz, CDCl3), δ | 13C NMR(125 MHz, CDCl3), δ |
|---|---|---|
| 9a | 7.09—8.06(m, 8H, PhH), 4.34(dd, 1H, SCH, J=12.00, 5.00 Hz), 3.75(s, 3H, OCH3), 3.09—3.20(m, 2H, CH2) | 170.7, 168.1, 165.9, 163.9, 152.1, 135.4, 130.6, 129.6(d, JC—F=8.7 Hz), 125.5, 125.0, 121.0, 118.3, 115.8(d, JC—F=21.6 Hz), 125.2, 55.4, 52.6, 34.9 |
| 9b | 7.10—7.80(m, 8H, PhH), 4.34(dd, 1H, SCH, J=12.00, 6.00 Hz), 3.76(s, 3H, OCH3), 3.10—3.19(m, 2H, CH2) | 170.6, 168.0, 164.1, 162.1, 152.1 135.4, 130.5, 130.3(d, JC—F=8.0 Hz), 125.7, 125.0, 123.0, 121.0, 118.3, 114.2(d, JC—F=22.6 Hz), 55.8, 52.7, 31.6 |
| 9c | 7.10—8.08(m, 8H, PhH), 4.52(dd, 1H, SCH, J=11.50, 6.50 Hz), 3.73(s, 3H, OCH3), 3.09—3.18(m, 2H, CH2) | 170.9, 168.0, 162.3, 160.4, 151.5 135.5, 132.6(d, JC—F=8.7 Hz), 130.8, 130.5, 125.7, 124.9, 124.6, 121.5, 116.4(d, JC—F=23.1 Hz) 55.4, 52.6, 34.9 |
| 9d | 7.12—7.81(m, 7H, PhH), 4.51(dd, 1H, SCH, J=12.00, 5.50 Hz), 3.74(s, 3H, OCH3), 3.17(t, 1H, CH2, J=12.50 Hz), 3.08(dd, 1H, CH2, J=13.00, 5.50 Hz) | 170.7, 166.8, 151.3(dd, JC—F=145.5, 13.5 Hz), 151.2, 149.3(dd, JC—F=149.3, 13.5 Hz), 135.5, 130.5, 128.9(d, JC—F=8.1 Hz), 126.0, 125.2(d, JC—F=1.6 Hz), 125.0, 124.4(d, JC—F=6.8 Hz), 121.5, 119.4(d, JC—F=17.1 Hz), 55.5, 52.6, 34.9 |
| 9e | 7.12—7.82(m, 7H, PhH), 4.51(dd, 1H, SCH, J=11.50, 6.50 Hz), 3.75(s, 3H, OCH3), 3.08—3.16(m, 2H, CH2) | 170.7, 166.7, 159.7(dd, JC—F=164.37, 1.50 Hz), 157.1(dd, JC—F=167.00, 1.62 Hz), 151.1, 135.5, 130.5, 127.9(dd, JC—F=13.50, 7.38 Hz), 126.0, 125.0, 121.6, 119.2(dd, JC—F=24.38, 9.25 Hz), 117.7(dd, JC—F=26.50, 8.37 Hz), 116.7(dd, JC—F=25.37, 3.62 Hz), 55.5, 52.6, 34.7 |
| 9f | 7.13—7.82(m, 7H, PhH), 4.51(dd, 1H, SCH, J=12.00, 5.50 Hz), 3.74(s, 3H, OCH3), 3.17(t, 1H, CH2, J=12.75 Hz), 3.08(dd, 1H, CH2, J=13.00, 5.50 Hz) | 170.7, 167.0, 151.9, 151.3(dd, JC—F=145.5, 13.5 Hz), 149.8(dd, JC—F=149.3, 13.5 Hz), 135.5, 130.6, 128.8(d, JC—F=8.2 Hz), 126.0, 125.3(d, JC—F=1.6 Hz), 125.0, 124.4(d, JC—F=6.8 Hz), 121.5, 119.4(d, JC—F=17.1 Hz), 55.5, 52.6, 34.9 |
| 9g | 6.94—7.58(m, 7H, PhH), 4.35(dd, 1H, SCH, J=12.00, 6.00 Hz), 3.77(s, 3H, OCH3), 3.06—3.16(m, 2H, CH2) | 170.5, 166.7, 163.2(dd, JC—F=247.6, 12.5 Hz), 151.9, 140.8(t, JC—F=8.6 Hz), 135.5, 130.6, 125.9, 125.0, 120.9, 110.3(dd, JC—F=20.6, 6.1 Hz), 106.4(t, JC—F=25.7 Hz), 55.8, 52.7, 31.5 |
| 9h | 7.01—7.60(m, 7H, PhH), 4.49(dd, 1H, SCH, J=12.0, 5.5 Hz), 3.73(s, 3H, OCH3), 3.23(t, 1H, CH2, J=12.5 Hz), 2.87(dd, 1H, CH2, J=13.0, 5.0 Hz) | 170.6, 163.8, 160.3(dd, JC—F=250.2, 6.7 Hz), 151.4, 135.4, 131.1(t, JC—F=10.1 Hz), 130.4, 126.1, 125.3, 121.5, 118.1(t, JC—F=18.1 Hz), 112.1(dd, JC—F=20.2, 4.8 Hz), 55.6, 52.6, 35.8 |
| 9i | 6.84—8.15(m, 7H, PhH), 4.48(dd, 1H, SCH, J=12.00, 5.50 Hz), 3.74(s, 3H, OCH3), 3.06—3.17(m, 2H, CH2) | 170.7, 167.0, 164.7(dd, JC—F=253.2, 12.5 Hz), 161.7(dd, JC—F=251.6, 12.2 Hz), 151.0, 135.5, 132.3(dd, JC—F=9.8, 4.5 Hz), 130.6, 127.9, 127.3, 126.0, 125.0, 112.3(dd, JC—F=21.0, 3.0 Hz), 104.6(t, JC—F=26.1 Hz), 55.4, 52.6, 34.8 |
| 14a | 7.09—8.06(m, 8H, PhH), 4.34(dd, 1H, SCH, J=12.00, 5.50 Hz), 3.75(s, 3H, OCH3), 3.09—3.20(m, 2H, CH2) | 170.7, 168.1, 165.9, 163.9, 152.1, 135.4, 130.6, 129.6(d, JC—F=8.7 Hz), 125.5, 125.0, 121.0, 118.3, 115.8(d, JC—F=21.2 Hz) , 125.2, 55.6, 52.7, 31.5 |
| 14b | 7.11—7.81(m, 8H, PhH), 4.36(dd, 1H, SCH, J=12.00, 5.50 Hz), 3.76(s, 3H, OCH3), 3.10—3.20(m, 2H, CH2) | 170.6, 168.1, 164.1, 162.1, 152.0, 135.0, 130.6, 130.3(d, JC—F=7.2 Hz), 125.7, 125.0, 123.0, 121.0, 118.3, 114.2(d, JC—F=22.7 Hz) 55.8, 52.7, 31.6 |
| 14c | 7.12—8.08(m, 8H, PhH), 4.51(dd, 1H, SCH, J=12.00, 6.00 Hz), 3.73(s, 3H, OCH3), 3.09—3.18(m, 2H, CH2) | 170.8, 168.1, 162.3, 160.4, 151.4, 135.5, 132.70(d, JC—F=8.8 Hz), 130.8, 130.5, 125.8, 125.0, 124.6, 121.5, 116.4(d, JC—F=23.1 Hz) 55.4, 52.6, 34.9 |
| 14d | 7.13—8.01(m, 7H, PhH), 4.50(dd, 1H, SCH, J=12.00, 5.50 Hz), 3.74(s, 3H, OCH3), 3.16(t, 1H, CH2, J=12.50 Hz), 3.07(dd, 1H, CH2, J=13.00, 5.50 Hz) | 170.6, 166.7, 152.5(dd, JC—F=145.7, 12.8 Hz), 152.1, 150.5(dd, JC—F=149.1, 13.5 Hz), 135.5, 134.6(d, JC—F=8.1 Hz), 130.6, 125.7, 125.0, 123.8(d, JC—F=6.5 Hz), 120.8, 117.4(d, JC—F=17.5 Hz), 116.5(d, JC—F=18.5 Hz), 55.7, 52.7, 31.3 |
| 14e | 7.12—7.84(m, 7H, PhH), 4.52(dd, 1H, SCH, J=11.00, 7.00 Hz), 3.75(s, 3H, OCH3), 3.12—3.18(m, 2H, CH2) | 170.8, 166.7, 159.7(dd, JC—F=164.4, 1.5 Hz), 157.1(dd, JC—F=166.7, 1.6 Hz), 151.1, 135.5, 130.5, 127.9(dd, JC—F=13.5, 7.3 Hz), 126.0, 125.0, 121.6, 119.2(dd, JC—F=24.3, 9.2 Hz), 117.7(dd, JC—F=26.5, 8.2 Hz), 116.7(dd, JC—F=25.3, 3.6 Hz), 55.4, 52.6, 34.7 |
| 14f | 7.12—7.81(m, 7H, PhH), 4.50(dd, 1H, SCH, J=12.00, 5.50 Hz), 3.74(s, 3H, OCH3), 3.17(t, 1H, CH2, J=12.75 Hz), 3.08(dd, 1H, CH2, J=13.00, 5.50 Hz) | 170.7, 166.9, 151.4, 151.3(dd, JC—F=144.6, 13.5 Hz), 149.3(dd, JC—F=153.0, 13.5 Hz), 135.5, 130.6, 128.8(d, JC—F=8.1 Hz), 126.0, 125.3(d, JC—F=1.6 Hz), 125.0, 124.4(d, JC—F=6.8 Hz), 121.5, 119.4(d, JC—F=17.0 Hz), 55.5, 52.6, 34.9 |
| 14g | 6.94—7.58(m, 7H, PhH), 4.35(dd, 1H, SCH, J=12.00, 6.00 Hz), 3.77(s, 3H, OCH3), 3.07—3.16(m, 2H, CH2) | 170.5, 166.7, 163.2(dd, JC—F=247.2, 12.6 Hz), 151.9, 140.8(t, JC—F=8.8 Hz), 135.5, 130.6, 125.9, 125.0, 120.9, 110.3(dd, JC—F=20.6, 6.1 Hz), 106.4(t, JC—F=25.7 Hz), 55.8, 52.7, 31.5 |
| 14h | 7.00—7.60(m, 7H, PhH), 4.49(dd, 1H, SCH, J=12.0, 5.5 Hz), 3.73(s, 3H, OCH3), 3.23(t, 1H, CH2, J=12.5 Hz), 2.87(dd, 1H, CH2, J=13.0, 5.0 Hz) | 170.7, 163.9, 160.3(dd, JC—F=250.1, 6.7 Hz), 151.3, 135.4, 131.1(t, JC—F=10.1 Hz), 130.4, 126.1, 125.3, 121.5, 118.1(t, JC—F=18.1 Hz), 112.1(dd, JC—F=20.2, 4.8 Hz), 55.6, 52.6, 35.8 |
| 14i | 6.86—8.19(m, 7H, PhH), 4.49(dd, 1H, SCH, J=12.00, 6.50 Hz), 3.74(s, 3H, OCH3), 3.10—3.20(m, 2H, CH2) | 170.7, 167.1, 164.7(dd, JC—F=246.7, 12.5 Hz), 161.7(dd, JC—F=251.6, 12.2 Hz), 151.3, 135.6, 132.2(dd, JC—F=9.8, 4.5 Hz), 130.5, 127.9, 127.3, 126.7, 125.0, 112.3(dd, JC—F=21.0, 3.0 Hz), 104.3(t, JC—F=26.1 Hz), 55.4, 52.6, 34.8 |
Table 2 1H NMR and 13C NMR data for compounds 9a—9i and 14a—14i
| Compd. | 1H NMR(500 MHz, CDCl3), δ | 13C NMR(125 MHz, CDCl3), δ |
|---|---|---|
| 9a | 7.09—8.06(m, 8H, PhH), 4.34(dd, 1H, SCH, J=12.00, 5.00 Hz), 3.75(s, 3H, OCH3), 3.09—3.20(m, 2H, CH2) | 170.7, 168.1, 165.9, 163.9, 152.1, 135.4, 130.6, 129.6(d, JC—F=8.7 Hz), 125.5, 125.0, 121.0, 118.3, 115.8(d, JC—F=21.6 Hz), 125.2, 55.4, 52.6, 34.9 |
| 9b | 7.10—7.80(m, 8H, PhH), 4.34(dd, 1H, SCH, J=12.00, 6.00 Hz), 3.76(s, 3H, OCH3), 3.10—3.19(m, 2H, CH2) | 170.6, 168.0, 164.1, 162.1, 152.1 135.4, 130.5, 130.3(d, JC—F=8.0 Hz), 125.7, 125.0, 123.0, 121.0, 118.3, 114.2(d, JC—F=22.6 Hz), 55.8, 52.7, 31.6 |
| 9c | 7.10—8.08(m, 8H, PhH), 4.52(dd, 1H, SCH, J=11.50, 6.50 Hz), 3.73(s, 3H, OCH3), 3.09—3.18(m, 2H, CH2) | 170.9, 168.0, 162.3, 160.4, 151.5 135.5, 132.6(d, JC—F=8.7 Hz), 130.8, 130.5, 125.7, 124.9, 124.6, 121.5, 116.4(d, JC—F=23.1 Hz) 55.4, 52.6, 34.9 |
| 9d | 7.12—7.81(m, 7H, PhH), 4.51(dd, 1H, SCH, J=12.00, 5.50 Hz), 3.74(s, 3H, OCH3), 3.17(t, 1H, CH2, J=12.50 Hz), 3.08(dd, 1H, CH2, J=13.00, 5.50 Hz) | 170.7, 166.8, 151.3(dd, JC—F=145.5, 13.5 Hz), 151.2, 149.3(dd, JC—F=149.3, 13.5 Hz), 135.5, 130.5, 128.9(d, JC—F=8.1 Hz), 126.0, 125.2(d, JC—F=1.6 Hz), 125.0, 124.4(d, JC—F=6.8 Hz), 121.5, 119.4(d, JC—F=17.1 Hz), 55.5, 52.6, 34.9 |
| 9e | 7.12—7.82(m, 7H, PhH), 4.51(dd, 1H, SCH, J=11.50, 6.50 Hz), 3.75(s, 3H, OCH3), 3.08—3.16(m, 2H, CH2) | 170.7, 166.7, 159.7(dd, JC—F=164.37, 1.50 Hz), 157.1(dd, JC—F=167.00, 1.62 Hz), 151.1, 135.5, 130.5, 127.9(dd, JC—F=13.50, 7.38 Hz), 126.0, 125.0, 121.6, 119.2(dd, JC—F=24.38, 9.25 Hz), 117.7(dd, JC—F=26.50, 8.37 Hz), 116.7(dd, JC—F=25.37, 3.62 Hz), 55.5, 52.6, 34.7 |
| 9f | 7.13—7.82(m, 7H, PhH), 4.51(dd, 1H, SCH, J=12.00, 5.50 Hz), 3.74(s, 3H, OCH3), 3.17(t, 1H, CH2, J=12.75 Hz), 3.08(dd, 1H, CH2, J=13.00, 5.50 Hz) | 170.7, 167.0, 151.9, 151.3(dd, JC—F=145.5, 13.5 Hz), 149.8(dd, JC—F=149.3, 13.5 Hz), 135.5, 130.6, 128.8(d, JC—F=8.2 Hz), 126.0, 125.3(d, JC—F=1.6 Hz), 125.0, 124.4(d, JC—F=6.8 Hz), 121.5, 119.4(d, JC—F=17.1 Hz), 55.5, 52.6, 34.9 |
| 9g | 6.94—7.58(m, 7H, PhH), 4.35(dd, 1H, SCH, J=12.00, 6.00 Hz), 3.77(s, 3H, OCH3), 3.06—3.16(m, 2H, CH2) | 170.5, 166.7, 163.2(dd, JC—F=247.6, 12.5 Hz), 151.9, 140.8(t, JC—F=8.6 Hz), 135.5, 130.6, 125.9, 125.0, 120.9, 110.3(dd, JC—F=20.6, 6.1 Hz), 106.4(t, JC—F=25.7 Hz), 55.8, 52.7, 31.5 |
| 9h | 7.01—7.60(m, 7H, PhH), 4.49(dd, 1H, SCH, J=12.0, 5.5 Hz), 3.73(s, 3H, OCH3), 3.23(t, 1H, CH2, J=12.5 Hz), 2.87(dd, 1H, CH2, J=13.0, 5.0 Hz) | 170.6, 163.8, 160.3(dd, JC—F=250.2, 6.7 Hz), 151.4, 135.4, 131.1(t, JC—F=10.1 Hz), 130.4, 126.1, 125.3, 121.5, 118.1(t, JC—F=18.1 Hz), 112.1(dd, JC—F=20.2, 4.8 Hz), 55.6, 52.6, 35.8 |
| 9i | 6.84—8.15(m, 7H, PhH), 4.48(dd, 1H, SCH, J=12.00, 5.50 Hz), 3.74(s, 3H, OCH3), 3.06—3.17(m, 2H, CH2) | 170.7, 167.0, 164.7(dd, JC—F=253.2, 12.5 Hz), 161.7(dd, JC—F=251.6, 12.2 Hz), 151.0, 135.5, 132.3(dd, JC—F=9.8, 4.5 Hz), 130.6, 127.9, 127.3, 126.0, 125.0, 112.3(dd, JC—F=21.0, 3.0 Hz), 104.6(t, JC—F=26.1 Hz), 55.4, 52.6, 34.8 |
| 14a | 7.09—8.06(m, 8H, PhH), 4.34(dd, 1H, SCH, J=12.00, 5.50 Hz), 3.75(s, 3H, OCH3), 3.09—3.20(m, 2H, CH2) | 170.7, 168.1, 165.9, 163.9, 152.1, 135.4, 130.6, 129.6(d, JC—F=8.7 Hz), 125.5, 125.0, 121.0, 118.3, 115.8(d, JC—F=21.2 Hz) , 125.2, 55.6, 52.7, 31.5 |
| 14b | 7.11—7.81(m, 8H, PhH), 4.36(dd, 1H, SCH, J=12.00, 5.50 Hz), 3.76(s, 3H, OCH3), 3.10—3.20(m, 2H, CH2) | 170.6, 168.1, 164.1, 162.1, 152.0, 135.0, 130.6, 130.3(d, JC—F=7.2 Hz), 125.7, 125.0, 123.0, 121.0, 118.3, 114.2(d, JC—F=22.7 Hz) 55.8, 52.7, 31.6 |
| 14c | 7.12—8.08(m, 8H, PhH), 4.51(dd, 1H, SCH, J=12.00, 6.00 Hz), 3.73(s, 3H, OCH3), 3.09—3.18(m, 2H, CH2) | 170.8, 168.1, 162.3, 160.4, 151.4, 135.5, 132.70(d, JC—F=8.8 Hz), 130.8, 130.5, 125.8, 125.0, 124.6, 121.5, 116.4(d, JC—F=23.1 Hz) 55.4, 52.6, 34.9 |
| 14d | 7.13—8.01(m, 7H, PhH), 4.50(dd, 1H, SCH, J=12.00, 5.50 Hz), 3.74(s, 3H, OCH3), 3.16(t, 1H, CH2, J=12.50 Hz), 3.07(dd, 1H, CH2, J=13.00, 5.50 Hz) | 170.6, 166.7, 152.5(dd, JC—F=145.7, 12.8 Hz), 152.1, 150.5(dd, JC—F=149.1, 13.5 Hz), 135.5, 134.6(d, JC—F=8.1 Hz), 130.6, 125.7, 125.0, 123.8(d, JC—F=6.5 Hz), 120.8, 117.4(d, JC—F=17.5 Hz), 116.5(d, JC—F=18.5 Hz), 55.7, 52.7, 31.3 |
| 14e | 7.12—7.84(m, 7H, PhH), 4.52(dd, 1H, SCH, J=11.00, 7.00 Hz), 3.75(s, 3H, OCH3), 3.12—3.18(m, 2H, CH2) | 170.8, 166.7, 159.7(dd, JC—F=164.4, 1.5 Hz), 157.1(dd, JC—F=166.7, 1.6 Hz), 151.1, 135.5, 130.5, 127.9(dd, JC—F=13.5, 7.3 Hz), 126.0, 125.0, 121.6, 119.2(dd, JC—F=24.3, 9.2 Hz), 117.7(dd, JC—F=26.5, 8.2 Hz), 116.7(dd, JC—F=25.3, 3.6 Hz), 55.4, 52.6, 34.7 |
| 14f | 7.12—7.81(m, 7H, PhH), 4.50(dd, 1H, SCH, J=12.00, 5.50 Hz), 3.74(s, 3H, OCH3), 3.17(t, 1H, CH2, J=12.75 Hz), 3.08(dd, 1H, CH2, J=13.00, 5.50 Hz) | 170.7, 166.9, 151.4, 151.3(dd, JC—F=144.6, 13.5 Hz), 149.3(dd, JC—F=153.0, 13.5 Hz), 135.5, 130.6, 128.8(d, JC—F=8.1 Hz), 126.0, 125.3(d, JC—F=1.6 Hz), 125.0, 124.4(d, JC—F=6.8 Hz), 121.5, 119.4(d, JC—F=17.0 Hz), 55.5, 52.6, 34.9 |
| 14g | 6.94—7.58(m, 7H, PhH), 4.35(dd, 1H, SCH, J=12.00, 6.00 Hz), 3.77(s, 3H, OCH3), 3.07—3.16(m, 2H, CH2) | 170.5, 166.7, 163.2(dd, JC—F=247.2, 12.6 Hz), 151.9, 140.8(t, JC—F=8.8 Hz), 135.5, 130.6, 125.9, 125.0, 120.9, 110.3(dd, JC—F=20.6, 6.1 Hz), 106.4(t, JC—F=25.7 Hz), 55.8, 52.7, 31.5 |
| 14h | 7.00—7.60(m, 7H, PhH), 4.49(dd, 1H, SCH, J=12.0, 5.5 Hz), 3.73(s, 3H, OCH3), 3.23(t, 1H, CH2, J=12.5 Hz), 2.87(dd, 1H, CH2, J=13.0, 5.0 Hz) | 170.7, 163.9, 160.3(dd, JC—F=250.1, 6.7 Hz), 151.3, 135.4, 131.1(t, JC—F=10.1 Hz), 130.4, 126.1, 125.3, 121.5, 118.1(t, JC—F=18.1 Hz), 112.1(dd, JC—F=20.2, 4.8 Hz), 55.6, 52.6, 35.8 |
| 14i | 6.86—8.19(m, 7H, PhH), 4.49(dd, 1H, SCH, J=12.00, 6.50 Hz), 3.74(s, 3H, OCH3), 3.10—3.20(m, 2H, CH2) | 170.7, 167.1, 164.7(dd, JC—F=246.7, 12.5 Hz), 161.7(dd, JC—F=251.6, 12.2 Hz), 151.3, 135.6, 132.2(dd, JC—F=9.8, 4.5 Hz), 130.5, 127.9, 127.3, 126.7, 125.0, 112.3(dd, JC—F=21.0, 3.0 Hz), 104.3(t, JC—F=26.1 Hz), 55.4, 52.6, 34.8 |
| Empirical formula | C17H13F2NO2S | T/K | 298(2) |
|---|---|---|---|
| Formula mass | 333.34 | F(000) | 688 |
| Crystal system | Monoclinic | θ range for data collection/(°) | 2.83—25.02 |
| Space group | P21/n | Limiting indices | -8≤h≤9, -16≤k≤25, -11≤l≤10 |
| a/nm | 7.6497(7) | Reflections collected/unique | 7578/2704(Rint=0.0294) |
| b/nm | 21.5632(19) | Completeness to θ=25.02°(%) | 99.80 |
| c/nm | 9.3170(8) | Absorption correction | Semi-empirical from equivalents |
| α/(°) | 90 | Max. and min. transmission | 0.9249 and 0.9037 |
| β/(°) | 90.9530(10) | Refinement method | Full-matrix least-squares on F2 |
| γ/(°) | 90 | Data/restraints/parameters | 2704/0/209 |
| Volume/nm3 | 1536.6(2) | Goodness-of-fit on F2 | 1.07 |
| Z | 4 | FinalR indices [I>2σ(I)] | R1=0.0447, wR2=0.1060 |
| Calculated density/(g·cm-3) | 1.441 | R indices(all data) | R1=0.0686, wR2=0.1164 |
| Absorption coefficient/mm-1 | 0.240 | Largest diff. peak and hole/(e·nm-3) | 0.218 and -0.225 |
Table 3 X-ray crystallographic data of compound 9h
| Empirical formula | C17H13F2NO2S | T/K | 298(2) |
|---|---|---|---|
| Formula mass | 333.34 | F(000) | 688 |
| Crystal system | Monoclinic | θ range for data collection/(°) | 2.83—25.02 |
| Space group | P21/n | Limiting indices | -8≤h≤9, -16≤k≤25, -11≤l≤10 |
| a/nm | 7.6497(7) | Reflections collected/unique | 7578/2704(Rint=0.0294) |
| b/nm | 21.5632(19) | Completeness to θ=25.02°(%) | 99.80 |
| c/nm | 9.3170(8) | Absorption correction | Semi-empirical from equivalents |
| α/(°) | 90 | Max. and min. transmission | 0.9249 and 0.9037 |
| β/(°) | 90.9530(10) | Refinement method | Full-matrix least-squares on F2 |
| γ/(°) | 90 | Data/restraints/parameters | 2704/0/209 |
| Volume/nm3 | 1536.6(2) | Goodness-of-fit on F2 | 1.07 |
| Z | 4 | FinalR indices [I>2σ(I)] | R1=0.0447, wR2=0.1060 |
| Calculated density/(g·cm-3) | 1.441 | R indices(all data) | R1=0.0686, wR2=0.1164 |
| Absorption coefficient/mm-1 | 0.240 | Largest diff. peak and hole/(e·nm-3) | 0.218 and -0.225 |
| Compd. | e.e.(%) | C. Neofonmans | Compd. | e.e.(%) | C. Neofonmans | ||
|---|---|---|---|---|---|---|---|
| ATCC 34874 | Clinical isolate | ATCC 34874 | Clinical isolate | ||||
| 9a, 14a(Raceme) | 0 | 18.30 | 19.30 | 14e | 70 | 21.47 | 21.90 |
| 9a | 84 | 12.03 | 12.97 | 9f, 14f(Raceme) | 0 | 18.23 | 18.87 |
| 14a | 60 | 21.60 | 22.70 | 9f | 67 | 15.30 | 15.23 |
| 9b, 14b(Raceme) | 0 | 18.57 | 19.23 | 14f | 54 | 20.07 | 19.70 |
| 9b | 65 | 13.20 | 12.60 | 9g, 14g(Raceme) | 0 | 14.67 | 17.53 |
| 14b | 70 | 21.37 | 21.63 | 9g | 65 | 13.23 | 16.20 |
| 9c, 14c(Raceme) | 0 | 20.07 | 22.50 | 14g | 73 | 15.23 | 17.50 |
| 9c | 35 | 18.73 | 20.13 | 9h, 14h(Raceme) | 0 | 6.00 | 6.00 |
| 14c | 34 | 21.83 | 24.10 | 9h | 71 | 6.00 | 6.00 |
| 9d, 14d(Raceme) | 0 | 19.47 | 18.67 | 14h | 40 | 6.00 | 6.00 |
| 9d | 93 | 12.90 | 13.03 | 9i, 14i(Raceme) | 0 | 11.70 | 10.57 |
| 14d | 95 | 23.33 | 20.47 | 9i | 71 | 6.00 | 6.00 |
| 9e, 14e(Raceme) | 0 | 17.60 | 18.33 | 14i | 40 | 13.83 | 14.20 |
| 9e | 76 | 16.73 | 17.03 | DMSO | 6.00 | 6.00 | |
Table 4 Zone(mm) of growth inhibition of compounds 9a—9i and 14a—14i*
| Compd. | e.e.(%) | C. Neofonmans | Compd. | e.e.(%) | C. Neofonmans | ||
|---|---|---|---|---|---|---|---|
| ATCC 34874 | Clinical isolate | ATCC 34874 | Clinical isolate | ||||
| 9a, 14a(Raceme) | 0 | 18.30 | 19.30 | 14e | 70 | 21.47 | 21.90 |
| 9a | 84 | 12.03 | 12.97 | 9f, 14f(Raceme) | 0 | 18.23 | 18.87 |
| 14a | 60 | 21.60 | 22.70 | 9f | 67 | 15.30 | 15.23 |
| 9b, 14b(Raceme) | 0 | 18.57 | 19.23 | 14f | 54 | 20.07 | 19.70 |
| 9b | 65 | 13.20 | 12.60 | 9g, 14g(Raceme) | 0 | 14.67 | 17.53 |
| 14b | 70 | 21.37 | 21.63 | 9g | 65 | 13.23 | 16.20 |
| 9c, 14c(Raceme) | 0 | 20.07 | 22.50 | 14g | 73 | 15.23 | 17.50 |
| 9c | 35 | 18.73 | 20.13 | 9h, 14h(Raceme) | 0 | 6.00 | 6.00 |
| 14c | 34 | 21.83 | 24.10 | 9h | 71 | 6.00 | 6.00 |
| 9d, 14d(Raceme) | 0 | 19.47 | 18.67 | 14h | 40 | 6.00 | 6.00 |
| 9d | 93 | 12.90 | 13.03 | 9i, 14i(Raceme) | 0 | 11.70 | 10.57 |
| 14d | 95 | 23.33 | 20.47 | 9i | 71 | 6.00 | 6.00 |
| 9e, 14e(Raceme) | 0 | 17.60 | 18.33 | 14i | 40 | 13.83 | 14.20 |
| 9e | 76 | 16.73 | 17.03 | DMSO | 6.00 | 6.00 | |
| Compd. | MIC(C. Neofonmans) | MFC(C. Neofonmans) | ||
|---|---|---|---|---|
| ATCC 34874 | Clinical isolate | ATCC 34874 | Clinical isolate | |
| 14a | 10.0 | 4.0 | 22.0 | 20.0 |
| 14b | 8.0 | 8.0 | 22.0 | 22.0 |
| 14c | 10.0 | 8.0 | 22.0 | 22.0 |
| 14d | 8.0 | 6.0 | 20.0 | 22.0 |
| 14e | 10.0 | 8.0 | 20.0 | 24.0 |
| 14f | 10.0 | 4.0 | 22.0 | 20.0 |
| A | 20.0 | 22.0 | >128.0 | >128.0 |
Table 5 MIC and MFC values(μg/mL) for compounds 14a—14f and fluconazole
| Compd. | MIC(C. Neofonmans) | MFC(C. Neofonmans) | ||
|---|---|---|---|---|
| ATCC 34874 | Clinical isolate | ATCC 34874 | Clinical isolate | |
| 14a | 10.0 | 4.0 | 22.0 | 20.0 |
| 14b | 8.0 | 8.0 | 22.0 | 22.0 |
| 14c | 10.0 | 8.0 | 22.0 | 22.0 |
| 14d | 8.0 | 6.0 | 20.0 | 22.0 |
| 14e | 10.0 | 8.0 | 20.0 | 24.0 |
| 14f | 10.0 | 4.0 | 22.0 | 20.0 |
| A | 20.0 | 22.0 | >128.0 | >128.0 |
| [1] | Morton G. C., Salvino J. M., Labaudiniere R. F., Herpin T. F., Tetrahedron Lett., 2000, 41(17), 3029—3033 |
| [2] | Geyer H. M., Watzman N., Buckley J. P., J. Pharm. Sci., 1970, 59(7), 964—968 |
| [3] | Bedos P., Amblard M., Subra G., Dodey P., Luccarini J., Paquet J., Pruneau D., Aumelas A., Martinez J., J. Med. Chem., 2000, 43(12), 2387—2394 |
| [4] | Atwal K. S., Bergey J. L., Moreland S., J. Med. Chem., 1987, 30(4), 627—635 |
| [5] | Tarabova B., Lacinova L., Engel J., Eur. J. Pharmaco., 2007, 573(1—3), 39—48 |
| [6] | Dandia A., Singh R., Sharma R., Phosphorus, Sulfur Silicon Relat. Elem., 2008, 183(12), 3116—3126 |
| [7] | Santo R. D., Costi R., Il Farmaco, 2005, 60(5), 385—392 |
| [8] | Desai M. D., Desai K. K., Asian J. Chem., 2002, 14(2), 974—978 |
| [9] | Bariwal J. B., Upadhyay K. D., Manvar A. T., Trivedia J. C., Singha J. S., Jainc K. S., Shah A. K., Eur. J. Med. Chem., 2008, 43(11), 2279—2290 |
| [10] | Shen S., Ye J., Liu F., Phosphorus, Sulfur Silicon Relat. Elem.,2010, 185(11), 2366—2374 |
| [11] | Yan Y., Yang X., Wu L., Phosphorus, Sulfur Silicon Relat. Elem.,2012, 187(5), 573—579 |
| [12] | Khouzani H. L., Tamjidi P., Mohammadpoor-Baltork I., Yaeghoobi M., Rahman N., Khosropour A., Moghadam M., Tangestaninejad S., Mirkhani V., Habibi M. H., Kashima A., Suzuki T., Heterocycl. Chem., 2014, 51(1), 138—150 |
| [13] | Kamble R. R., Sudha B. S., Phosphorus, Sulfur Silicon Relat. Elem.,2008, 183(7), 1691—1709 |
| [14] | Gan J. G., Ma D. M., Org. Lett., 2009, 11(13), 2788—2790 |
| [15] | Lancelot J.C., Letosis B., Saturnio C., Robba M., Synth. Commun., 1991, 21, 1901—1908 |
| [16] | Yaccoubi F., Efrit M. L. E., Zantour H., Phosphorus, Sulfur Silicon Relat. Elem., 2002, 177(10), 2321—2330 |
| [17] | Mohamed M. A. A., Synth. Commun., 2011, 41(3), 331—340 |
| [18] | Qiu Z. L., Li W. H., Zhu H. F., Liu Q., Li Y., Chem. J. Chinese Universities, 2013, 34(3), 579—589 |
| (邱召来, 李文红, 朱海菲, 刘倩, 李媛. 高等学校化学学报, 2013, 34(3, 579—589) | |
| [19] | Sun N., Zhang P., Li Y., Chin. J. Org. Chem., 2000, 20(5), 735—737 |
| (孙娜, 张萍, 李媛. 有机化学, 2000, 20(5),, 735—737) | |
| [20] | Fan S. L., Zhang B., Gao L. Y., Wang L. Z., Bian Y. Q., Li Y., Chem. J. Chinese Universities, 2014, 32(12), 2574—2583 |
| (范世丽, 张博, 高丽叶, 王兰芝, 边艳青, 李媛. 高等学校化学学报, 2014, 32(12), 2574—2583) | |
| [21] | Zhang J., Mu B. S., Wu M., Bian Y. Q., Li Y., Chem. J. Chinese Universities, 2015, 36(4), 687—697 |
| (张静, 穆博帅, 吴萌, 边艳青, 李媛. 高等学校化学学报, 2015, 36(4), 687—697) | |
| [22] | Lutz R. E., Scott G. W., J. Org. Chem., 1948, 13(2), 284—296 |
| [23] | Wang H. Y., Wang Z., Li S. H., Qiu Y. T., Liu B., Song Z. G., Liu Z. H., Chem. Res. Chinese Universities, 2016, 32(3), 373—379 |
| [24] | Pavel I., Couve-Bonnaire S., Jubault P., Pannecoucke X., Org. Lett., 2012, 14(19), 5130—5133 |
| [25] | Sun B., Yin X., Zhang J., Huang J., Xu Y., Zhang F., Wang J. H., Wang G. Q., Hu C., Chem. Res. Chinese Universities, 2016, 32(1), 1—7 |
| [1] | 李霄, 高立国, 弓莹, 马亚军, 马向荣. 杂双金属复合物ZABDP催化芳香酮与芳香醛的直接不对称Aldol反应[J]. 高等学校化学学报, 2017, 38(5): 778. |
| [2] | 张静, 穆博帅, 吴萌, 边艳青, 李媛. 4-氟苯基-2,3-二氢-1,5-苯并[b]硫氮杂䓬类化合物的合成、结构和抑真菌活性[J]. 高等学校化学学报, 2015, 36(4): 687. |
| [3] | 康旺, 卜辉娟, 李文红, 李媛. 具有杀菌活性的2-乙氧羰基-4-芳基-1,5-苯并硫氮杂卓的初步构效关系[J]. 高等学校化学学报, 2014, 35(4): 766. |
| [4] | 邱召来, 李文红, 朱海菲, 刘倩, 李媛. 3-(CH2)n CO2 C2 H5-1,5-苯并硫氮杂卓的合成、晶体结构及抑真菌活性[J]. 高等学校化学学报, 2013, 34(3): 579. |
| [5] | 肖涛, 周旋, 宋颢. 氧吲哚生物碱Humantenine关键骨架的立体选择性构建[J]. 高等学校化学学报, 2012, 33(12): 2676. |
| [6] | 夏亚穆, 常亮. (-)-(1R,2S)-肉豆蔻木脂素的不对称合成[J]. 高等学校化学学报, 2010, 31(9): 1780. |
| [7] | 许柏岩, 宋娜, 李文泽, 辛志君, 李瀛. (7R,10S)-Boivinianin B的不对称合成[J]. 高等学校化学学报, 2009, 30(7): 1329. |
| [8] | 何兰 张唯 刘玉美 李铭 陈庆华. 多功能光学活性丁二醇衍生物的合成和结构[J]. 高等学校化学学报, 2006, 27(3): 464. |
| [9] | 孙彬, 梅天胜, 刘佐胜, 李裕林, 李瀛, 彭立增. (+)-MethylCembra-1,3,7,11-tetraene-16-carboxynate的不对称合成研究[J]. 高等学校化学学报, 2005, 26(5): 880. |
| [10] | 陈小川, 任新锋, 彭坤, 武同兴, 潘鑫复. 8-O-4′新木脂素化合物的不对称合成[J]. 高等学校化学学报, 2003, 24(10): 1811. |
| [11] | 陈芬儿, 傅晗, 孟歌, 罗有富, 鄢明国. d-生物素的不对称全合成研究(Ⅴ)[J]. 高等学校化学学报, 2002, 23(6): 1060. |
| [12] | 胡爱国, 王善韦, 赵雪梅, 王积涛. 新型α-氨基酸不对称合成体系的性质研究[J]. 高等学校化学学报, 2001, 22(S1): 124. |
| [13] | 姜茹, 南鹏娟, 柳巍, 李晓晔, 张生勇. 新型手性膦-硼烷配合物的合成及其在不对称催化氢化反应中的应用[J]. 高等学校化学学报, 2001, 22(6): 936. |
| [14] | 胡爱国, 王善韦, 韩军, 王积涛. α-氨基酸的不对称合成[J]. 高等学校化学学报, 2001, 22(3): 421. |
| [15] | 李媛, 张萍, 李旭, 张丽君. 1,5-苯并硫氮杂-β-内酰胺衍生物的不对称合成及晶体结构[J]. 高等学校化学学报, 2001, 22(11): 1829. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||