

Chem. J. Chinese Universities ›› 2026, Vol. 47 ›› Issue (1): 20250303.doi: 10.7503/cjcu20250303
• Article • Previous Articles Next Articles
Received:2025-10-18
Online:2026-01-10
Published:2025-11-21
Contact:
WANG Dayang
E-mail:wangdayang@jlu.edu.cn
Supported by:CLC Number:
TrendMD:
GUO Zhuohuan, WANG Dayang. Thermodynamic Correlation Between Surface Carboxyl Configuration and Wettability[J]. Chem. J. Chinese Universities, 2026, 47(1): 20250303.
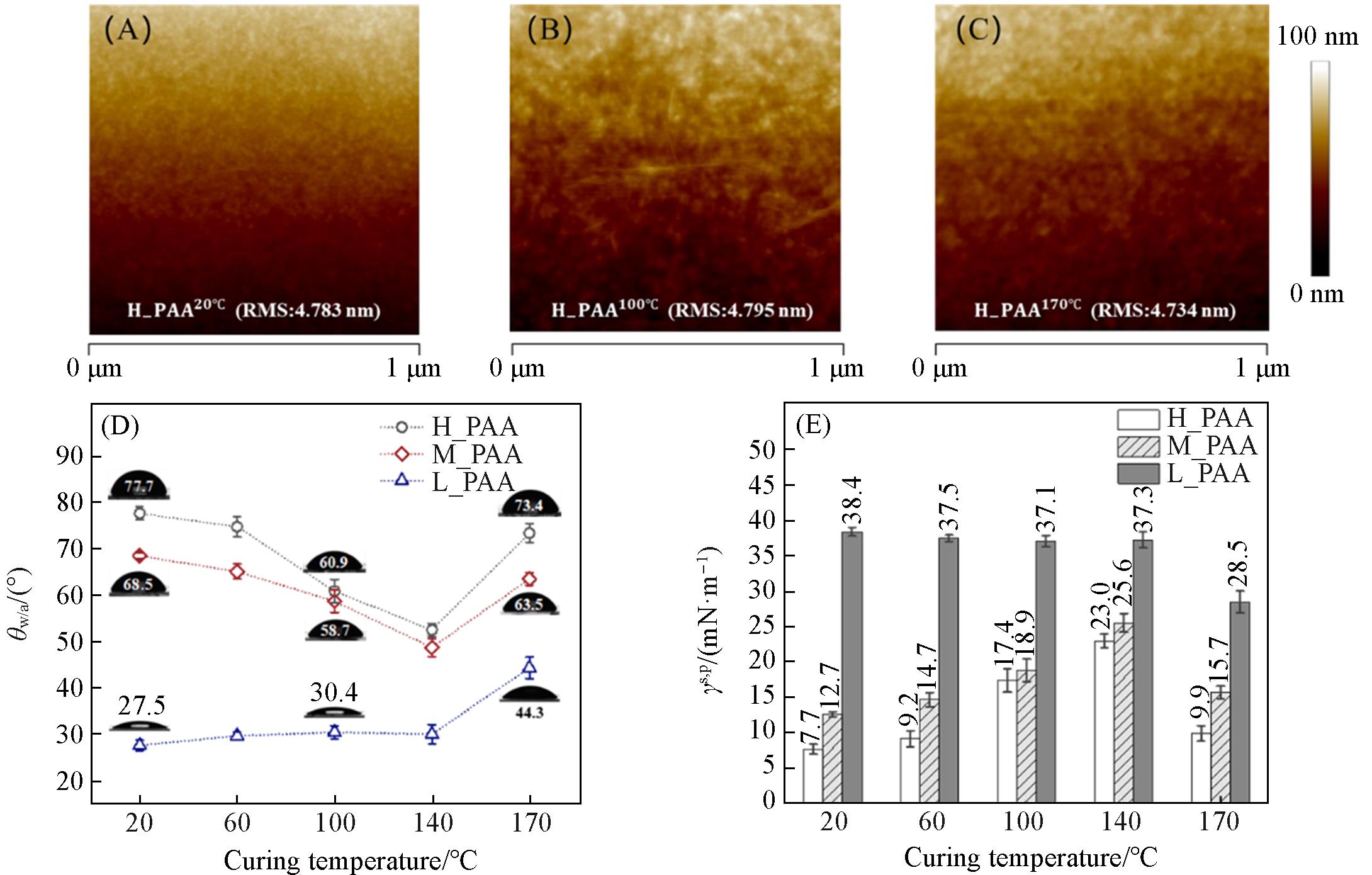
Fig.1 AFM images of as⁃prepared H-PAA20 ℃ thin film(A), H-PAA100 ℃ thin film(B) and H-PAA170 ℃ thin film(C) with the corresponding RMS values marked, plot of the θw/a values of as⁃prepared H-PAAT, M-PAAT and L-PAAT thin films versus the curing temperature(T), in which the photos of a 2 μL droplet of water placed on the corresponding thin films are shown(D) and histograms of the values of γs,p of as⁃prepared H-PAAT, M-PAAT and L-PAAT thin films(E)
| Wavenumber/cm-1 | Assignment |
|---|---|
| 3600—3000(broad) | O—H stretching |
| 2930 | CH2 stretching |
| 2500—2700(broad) | Overtones and combinations of bands near 1412 and 1238 cm-1 |
| enhanced by Fermi resonance | |
| 1695—1725(strong) | C=O stretching |
| 1526 | Acid anhydride stretching |
| 1457 | CH2 deformation |
| 1412, 1238, 1165 | C—O stretching coupled with O—H in⁃plane bending |
| 846 | C—COOH stretching |
Table 1 Infrared bands of PAA thin films
| Wavenumber/cm-1 | Assignment |
|---|---|
| 3600—3000(broad) | O—H stretching |
| 2930 | CH2 stretching |
| 2500—2700(broad) | Overtones and combinations of bands near 1412 and 1238 cm-1 |
| enhanced by Fermi resonance | |
| 1695—1725(strong) | C=O stretching |
| 1526 | Acid anhydride stretching |
| 1457 | CH2 deformation |
| 1412, 1238, 1165 | C—O stretching coupled with O—H in⁃plane bending |
| 846 | C—COOH stretching |
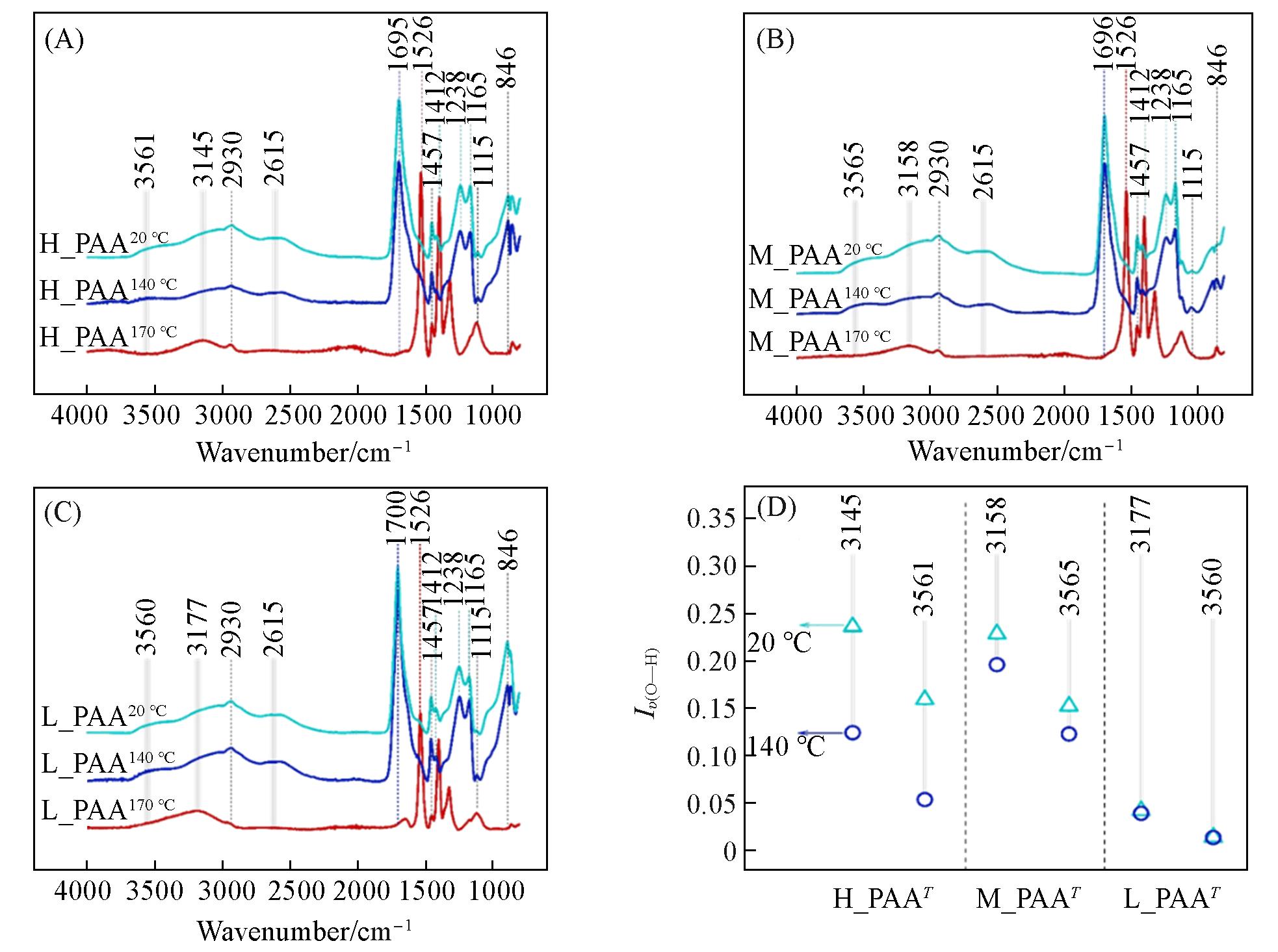
Fig.2 ATR⁃FTIR spectra of H-PAAT (A), M-PAAT (B) and L-PAAT (C) thin films in the range of 4000—400 cm-1 and the summary of the O—H stretching vibration peak intensity on the surface of H-PAAT, M-PAAT and L-PAAT thin films(D)
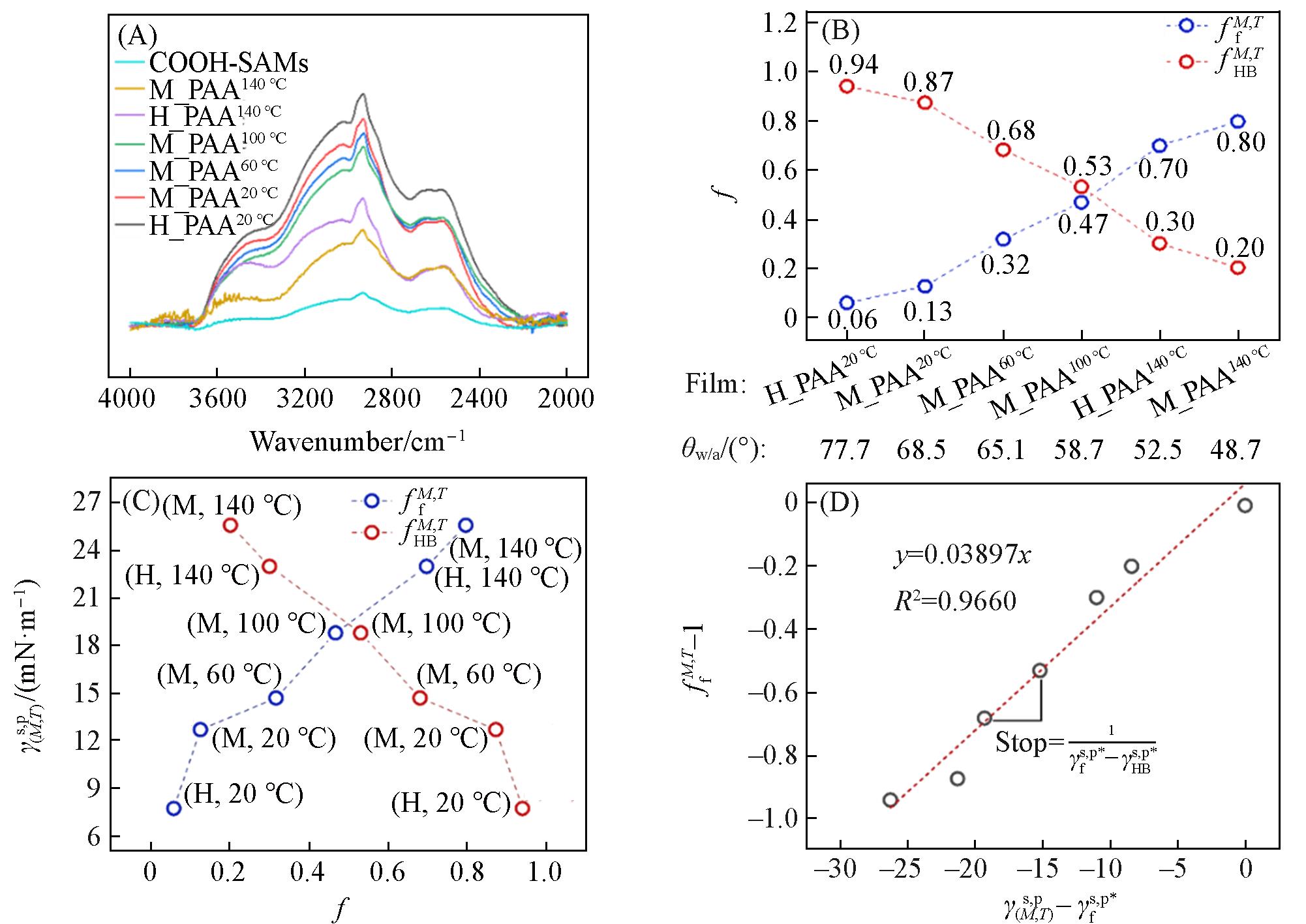
Fig.3 ATR⁃FTIR spectra of COOH⁃SAMs and PAA thin films in the range of 4000—2000 cm-1(A), plots of the calculated ffM,T and fHBM,T values of as⁃prepared PAA thin films versus the θw/a(B), plots of the γ(M,T)s,p values of as⁃prepared PAA thin films versus their ffM,T and fHBM,T values(C), and plots of the ffM,T-1 values of as⁃prepared COOH⁃SAMs and PAA thin films versus their γM,Ts,p-γfs,p* values(D)
| COOH Surface | COOH⁃SAMs | ||||||
|---|---|---|---|---|---|---|---|
| 2.02 | 190.10 | 176.40 | 137.80 | 107.40 | 60.87 | 40.89 | |
| 0.0100 | 0.9410 | 0.8736 | 0.6822 | 0.5317 | 0.3013 | 0.2024 | |
| 0.9900 | 0.0590 | 0.1264 | 0.3178 | 0.4683 | 0.6987 | 0.7976 | |
| 34.00 | 7.72 | 12.70 | 14.70 | 18.80 | 23.00 | 25.60 |
Table 2 Summary of the values of AHBM,T,fHBM,T,ffM,T and γM,Ts,p of as-prepared COOH⁃SAMs and PAA thin films
| COOH Surface | COOH⁃SAMs | ||||||
|---|---|---|---|---|---|---|---|
| 2.02 | 190.10 | 176.40 | 137.80 | 107.40 | 60.87 | 40.89 | |
| 0.0100 | 0.9410 | 0.8736 | 0.6822 | 0.5317 | 0.3013 | 0.2024 | |
| 0.9900 | 0.0590 | 0.1264 | 0.3178 | 0.4683 | 0.6987 | 0.7976 | |
| 34.00 | 7.72 | 12.70 | 14.70 | 18.80 | 23.00 | 25.60 |
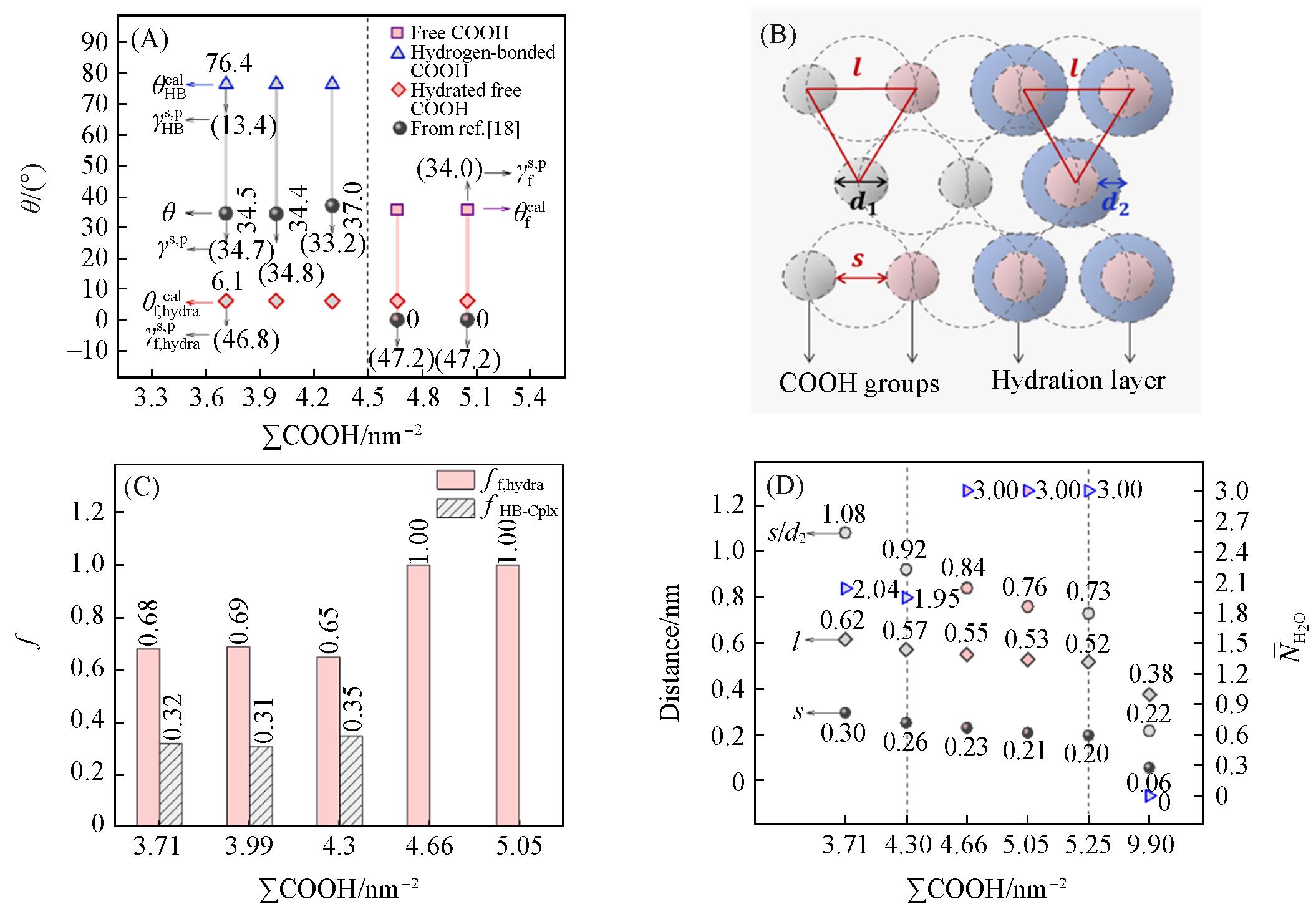
Fig.4 Summary of the literature⁃reported water contact angles(θ) and corresponding polar components of surface free energy(γs,p) for COOH⁃SAMs surfaces at different ∑COOH values, together with theoretical γs,p values calculated without hydration(γHBs,p and γfs,p) and with hydration (γf, hydras,p), as well as the corresponding theoretical contact angles θHBcal, θfcal and θf,hydracal(A), schematic illustration of the monolayer structure of COOH groups organized in a hexagonal arrangement(B), histogram of ff,hydra andfHB⁃Cplx values for COOH⁃SAMs surfaces at different ∑C OOH values(C) and summary of l, s, s/d2 and N¯H2O for COOH⁃SAMs surfaces at different ∑COOH values(D)
| [1] | Lee H., Alcaraz M. L., Rubner M. F., Cohen R. E., ACS Nano, 2013, 7(3), 2172—2185 |
| [2] | Arima Y., Iwata H., Biomaterials, 2007, 28(20), 3074—3082 |
| [3] | Cheng Y., Feng F., Zhu T. X., Zheng Y. H., Gou Y. K., Yang D. P., Huang J. Y., Lai Y. K., Jiang Z. Y., Chem. Eng. J., 2025, 504, 158421 |
| [4] | Rac V., Lević S., Balanč B., Graells B. O., Bijelić G., Colloid. Surface. B, 2019, 180, 441—448 |
| [5] | Xu Z., Song K., Yuan S. L., Liu C. B., Langmuir, 2011, 27(14), 8611—8620 |
| [6] | Major R. C., Houston J. E., McGrath M. J., Siepmann J. I., Zhu X. Y., Phys. Rev. Lett., 2006, 96, 177803 |
| [7] | Tu A., Kwag H. R., Barnette A. L., Kim S. H., Langmuir, 2012, 28(43), 15263—15269 |
| [8] | Arima Y., Iwata H., J. Mater. Chem., 2007, 17, 4079—4087 |
| [9] | Choi S., Yang Y., Chae J., Biosens. Bioelectron., 2008, 24(4), 893—899 |
| [10] | Ohnuki H., Izumi M., Lenfant S., Guerin D., Imakubo T., Vuillaume D., Appl. Surf. Sci., 2005, 246(4), 392—396 |
| [11] | James M., Darwish T. A., Ciampi S., Sylvester S. O., Zhang Z. M., Ng A., Gooding J. J., Hanley T. L., Soft Matter, 2011, 7, 5039 |
| [12] | Winter N., Vieceli J., Benjamin I., J. Phys. Chem. B, 2008, 112(2), 227—231 |
| [13] | Xu Z., Song K., Yuan S. L., Liu C. B., Langmuir, 2011, 27(14), 8611—8620 |
| [14] | Szori M., Jedlovszky P., Roeselova M., Phys. Chem. Chem. Phys., 2010, 12, 4604—4616 |
| [15] | Osnis A., Sukenik C. N., Major D. T., J. Phys. Chem. C, 2012, 116(14), 770—782 |
| [16] | Stevens M. J., Grest G. S., Biointerphases, 2008, 3, FC13—FC22 |
| [17] | Ma Z. Y., Wang D. Y., Colloid. Surface. A, 2023, 675, 132031 |
| [18] | Guo P., Tu Y. S., Yang J. R., Wang C. L., Sheng N., Fang H. P., Phys. Rev. Lett., 2015, 115, 186101 |
| [19] | Fowkes M., J. Ind. Eng. Chem., 1960, 12, 40—52 |
| [20] | Yamakawa H., Modern Theory of Polymer Solutions, Harper & Row, New York, 1971 |
| [21] | Cranford S. W., Buehler M. J., Macromolecules, 2012, 45(19), 8067—8082 |
| [22] | Han T. Y., Ma Z. Y., Wang D. Y., ACS Macro Lett., 2021, 10, 354—358 |
| [23] | Kurapati R., Natarajan U., Ind. Eng. Chem. Res., 2020, 59, 16099—16111 |
| [24] | Zhang W., Zhang Z. N., Wang X. P., J. Colloid Interface Sci., 2009, 333, 346—353 |
| [25] | Maura J. J., Eustance D. J., Ratcliffe C. T., Macromolecules, 1987, 20, 196—202 |
| [26] | Bardet L., Cassanas⁃Fabre G., Alain M., J. Mol. Struct., 1975, 24(1), 153—164 |
| [27] | Leyte J. C., Zuiderweg L. H., Vledder H. J., Spectrochim. Acta, 1967, 23(5), 1397—1407 |
| [28] | Lin⁃Vien D., Colthup N. B., Fately W. G., Grasselli J. G., The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules, Academic Press, San Diego, 1991 |
| [29] | Chen H. C., Lin H. C., Chen H. H., Mai F. D., Liu Y. C., Lin C. M., Chang C. C., Tsai H. Y., Yang C. P., Sci. Rep., 2014, 4, 4425 |
| [30] | Kim T. H., Ahn J. H., Choi H. K., Choi Y. J., Cho C. S., Arch. Pharm. Res., 2007, 30, 381—386 |
| [31] | Hoerter M., Oprea A., Barsan N., Weimar U., Sens. Actuat. B, 2008, 134(2), 743—749 |
| [32] | Itoh K., Yaita M., Hasegawa T., Fujii S., Misono Y., J. Electron Spectrosc. Relat. Phenom., 1990, 54/55, 923—932 |
| [33] | Benoit R. L., Louis C., Fre’chette M., Thermochimica Acta, 1991, 176, 221—232 |
| [34] | Du D. M., Qin M., Zhou Z. Y., Fu A. P., Int. J. Quantum Chemistry, 2012, 112(2), 351—358 |
| [35] | Marcus Y., Chem. Rev., 1988, 88, 1475—1498 |
| [1] | CHEN Qingqing, LI Jiangtao, HUANG Xinrong, GU Fang, WANG Haijun. Excess Entropy of Janus Particles Immersed in Hydrogen Bonding Fluids [J]. Chem. J. Chinese Universities, 2024, 45(2): 20230443. |
| [2] | ZHANG Yong, XU Jun, BAO Yu, CUI Shuxun. Quantifying the Degree of Weakening Effect of Nonpolar Organic Solvent on the Strength of Intramolecular Hydrogen Bonding by Single-molecule Force Spectroscopy [J]. Chem. J. Chinese Universities, 2022, 43(4): 20210863. |
| [3] | BAI Lan, ZHAI Lei, WANG Changou, HE Minhui, MO Song, FAN Lin. Thermal Expansion Behavior of Amide-containing Polyimide Films with Ultralow Thermal Expansion Coefficient † [J]. Chem. J. Chinese Universities, 2020, 41(4): 795. |
| [4] | XU Yu,HUA Er. Hydrogen Bonding Study on Protic Ionic Liquids Composed of N-Alkyl Ethylenediaminum Cations with Trifluoroacetic Anion† [J]. Chem. J. Chinese Universities, 2018, 39(9): 1954. |
| [5] | YAN Fei, ZHANG Min, ZHANG Lu, LI Chengtao, LI Yichen. Interfacial Interaction and Compatibilizing Mechanism of PVDF/TPU Blends [J]. Chem. J. Chinese Universities, 2017, 38(5): 888. |
| [6] | LI Lei, LI Shushi, WANG Changsheng. Theoretical Studies on Noncovalent Interactions Between Charged Histidine Side Chain and DNA Base† [J]. Chem. J. Chinese Universities, 2017, 38(1): 56. |
| [7] | LI Jiangtao, LIU Shujing, GU Fang, WANG Haijun. Adsorption-desorption of Hydrogen Bonding Fluid Confined in a Spherical Cavity: the Role of Surface Regulation† [J]. Chem. J. Chinese Universities, 2016, 37(9): 1710. |
| [8] | ZHANG Qingzhong, ZHANG Junmei, LIU Yuping, REN Xiao, HUANG Wei. Synthesis and Characterization of Electro-optic Materials Based on Intermolecular Lateral Hydrogen Bonding† [J]. Chem. J. Chinese Universities, 2015, 36(10): 2053. |
| [9] | WANG Xi, LI Li, CHEN Ning, WANG Qi. Effect of Hydrogen Bonding on Water States in Sorbitol Modified PVA System [J]. Chem. J. Chinese Universities, 2012, 33(04): 813. |
| [10] | CUI Ying*, YE Bao-Hui, NIU Yan-Li, ZHONG Yong-Rui. Selective Interaction Based on Complex [Ru(phen)2(H2biim)](PF6)2 and Anions [J]. Chem. J. Chinese Universities, 2010, 31(9): 1706. |
| [11] |
XU Zhong1, LI Ning1, CUI Yan-Ping1, LIU Hong-Mei2, WANG Hong-Bo1, YE Yuan-Feng2, ZHAO Jian-Wei2*.
Theoretical Investigations of Electric Field Effect on the Hydrogen Bonding and Electron Transport in DNA Base Pairs [J]. Chem. J. Chinese Universities, 2009, 30(3): 588. |
| [12] | FENG Feng, YU Jian-Guo*, FANG Wei-Hai. Theoretical Study for DNA and RNA on Their Stability Difference [J]. Chem. J. Chinese Universities, 2009, 30(12): 2445. |
| [13] | XU Nai-Ku1, XIAO Chang-Fa1*, FENG Yan2. Preparation and Characterization of Absorptive Functional Fiber Copolymerized by n-Butyl Methacrylate with Hydroxyethyl Methacrylate [J]. Chem. J. Chinese Universities, 2008, 29(8): 1677. |
| [14] | MENG Xiang-Hua, SHI Ji-Cheng*, TONG Qing-Song, YANG Yu-Rong, JIA Li. Synthesis and Crystal Structure of a Novel Chiral Coordination Polymer [Zn2(C7H8O6)2(bipy)2(H2O)2]·4H2O [J]. Chem. J. Chinese Universities, 2008, 29(6): 1086. |
| [15] | YANG Li-Min1*, ZHAO Guo-Zhong2, ZHAO Kui1, SHI Xiao-Xi2, JIA Xin-Feng2, WENG Shi-Fu3, XU Yi-Zhuang3, LU Xiang-Yang1, XIE Da-Tao1, WU Jin-Guang3, CHEN Jia-Er1. Far-IR and THz Absorption Spectra Studies of Cholic Acid and Deoxycholic Acid [J]. Chem. J. Chinese Universities, 2008, 29(6): 1116. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
