

Chem. J. Chinese Universities ›› 2019, Vol. 40 ›› Issue (2): 254.doi: 10.7503/cjcu20180573
• Organic Chemistry • Previous Articles Next Articles
LÜ Mingjun, LI Wen, YANG Xinying, FANG Hao*( )
)
Received:2018-08-14
Online:2019-02-10
Published:2018-10-08
Contact:
FANG Hao
E-mail:haofangcn@sdu.edu.cn
Supported by:CLC Number:
TrendMD:
LÜ Mingjun,LI Wen,YANG Xinying,FANG Hao. Synthesis and Antitumor Activity of N9 Position Aromatic Substituted Purine-8-one Derivatives†[J]. Chem. J. Chinese Universities, 2019, 40(2): 254.
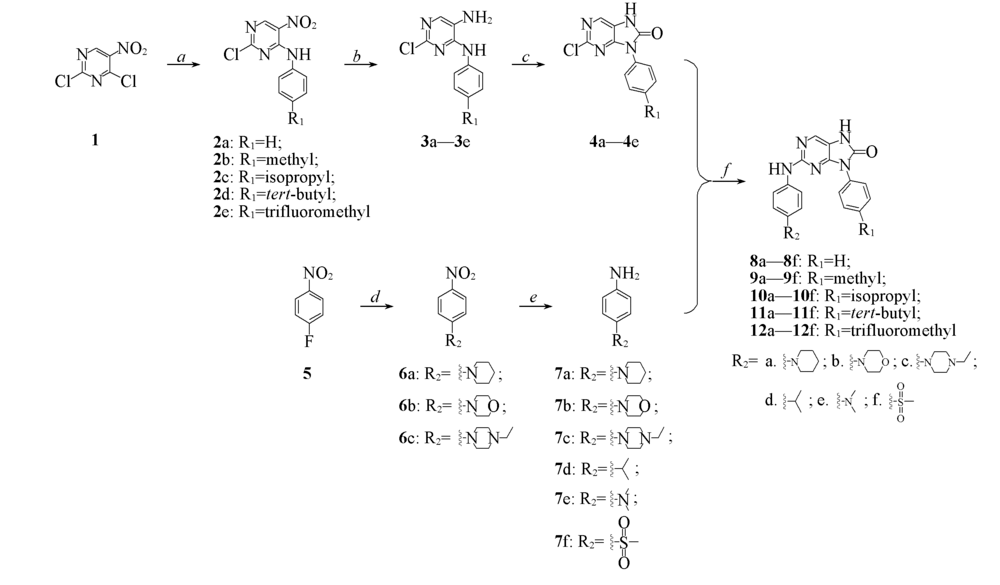
Scheme 1 Synthetic routes of target compounds 8—12a. Benzyl amine, DIPEA, dichloromethane, 0—25 ℃, 30 min; b. SnCl2·2H2O, ethyl acetate, 60 ℃, 4 h; c. phenyl carbonochloridate, NaHCO3, ethyl acetate and H2O, 0—60 ℃, 8 h; d. nitrogen heterocycle, triethylamine, DMSO, 110 ℃, 6 h; e. Pd/C, methanol, r. t., overnight; f. TsOH, n-butanol, 110 ℃, 6 h. Compounds 7d—7f were commercially available reagents.
| Compd. | ESI-MS(calcd.), m/z[M+H]+ | Compd. | ESI-MS(calcd.), m/z[M+H]+ |
|---|---|---|---|
| 2a | 251.05(251.03) | 3d | 277.11(277.12) |
| 2b | 265.06(265.04) | 3e | 289.07(289.05) |
| 2c | 293.13(293.08) | 6a | 207.05(207.11) |
| 2d | 307.11(307.10) | 6b | 209.05(209.09) |
| 2e | 319.01(319.02) | 6c | 236.12(236.14) |
| 3a | 221.03(221.06) | 7a | 177.05(177.14) |
| 3b | 235.02(235.07) | 7b | 179.02(179.12) |
| 3c | 263.07(263.10) | 7c | 206.10(206.16) |
Table 1 ESI-MS data of compounds 2a—2e, 3a—3e, 6a—6c and 7a—7c
| Compd. | ESI-MS(calcd.), m/z[M+H]+ | Compd. | ESI-MS(calcd.), m/z[M+H]+ |
|---|---|---|---|
| 2a | 251.05(251.03) | 3d | 277.11(277.12) |
| 2b | 265.06(265.04) | 3e | 289.07(289.05) |
| 2c | 293.13(293.08) | 6a | 207.05(207.11) |
| 2d | 307.11(307.10) | 6b | 209.05(209.09) |
| 2e | 319.01(319.02) | 6c | 236.12(236.14) |
| 3a | 221.03(221.06) | 7a | 177.05(177.14) |
| 3b | 235.02(235.07) | 7b | 179.02(179.12) |
| 3c | 263.07(263.10) | 7c | 206.10(206.16) |
| Compd. | Appearance | Yield(%) | m.p./℃ | ESI-MS(calcd.), m/z[M-H]- | 1H NMR(400 MHz), δ |
|---|---|---|---|---|---|
| 4a | Yellow solid | 92 | >280 | 245.09(245.02) | 8.20(s, 1H, pyrimidine H), 7.49(d, J=7.3 Hz, 2H, PhH), 7.36(d, J=7.3 Hz, 2H, PhH), 2.43(s, 3H, CH3) |
| 4b | Yellow solid | 98 | >280 | 259.14(259.04) | 8.20(s, 1H, pyrimidine H), 7.49(d, J=7.3 Hz, 2H, PhH), 7.36(d, J=7.3 Hz, 2H, PhH), 2.43(s, 3H, CH3) |
| 4c | Yellow solid | 90 | 267—269 | 287.20(287.07) | 11.83(s, 1H, NH), 8.24(s, 1H, pyrimidine H), 7.54—7.40(m, 4H, PhH), 2.99(Hept, J=6.8 Hz, 1H, CH), 1.26[d, J=6.9 Hz, 6H, (CH3)2] |
| 4d | Gray solid | 94 | 266—268 | 301.14(301.08) | 10.37(s, 1H, NH), 8.25(s, 1H, pyrimidine H), 7.60(d, J=8.6 Hz, 2H, PhH), 7.54(d, J=8.7 Hz, 2H, PhH), 1.38[s, 9H, C(CH3)3] |
| 4e | Gray solid | 90 | 218—220 | 313.11(313.01) | 9.64(s, 1H, NH), 8.29(s, 1H, pyrimidine H), 7.87(m, 4H, PhH) |
Table 2 Appearance, yields, melting points, ESI-MS and 1H NMR data of compounds 4a—4e*
| Compd. | Appearance | Yield(%) | m.p./℃ | ESI-MS(calcd.), m/z[M-H]- | 1H NMR(400 MHz), δ |
|---|---|---|---|---|---|
| 4a | Yellow solid | 92 | >280 | 245.09(245.02) | 8.20(s, 1H, pyrimidine H), 7.49(d, J=7.3 Hz, 2H, PhH), 7.36(d, J=7.3 Hz, 2H, PhH), 2.43(s, 3H, CH3) |
| 4b | Yellow solid | 98 | >280 | 259.14(259.04) | 8.20(s, 1H, pyrimidine H), 7.49(d, J=7.3 Hz, 2H, PhH), 7.36(d, J=7.3 Hz, 2H, PhH), 2.43(s, 3H, CH3) |
| 4c | Yellow solid | 90 | 267—269 | 287.20(287.07) | 11.83(s, 1H, NH), 8.24(s, 1H, pyrimidine H), 7.54—7.40(m, 4H, PhH), 2.99(Hept, J=6.8 Hz, 1H, CH), 1.26[d, J=6.9 Hz, 6H, (CH3)2] |
| 4d | Gray solid | 94 | 266—268 | 301.14(301.08) | 10.37(s, 1H, NH), 8.25(s, 1H, pyrimidine H), 7.60(d, J=8.6 Hz, 2H, PhH), 7.54(d, J=8.7 Hz, 2H, PhH), 1.38[s, 9H, C(CH3)3] |
| 4e | Gray solid | 90 | 218—220 | 313.11(313.01) | 9.64(s, 1H, NH), 8.29(s, 1H, pyrimidine H), 7.87(m, 4H, PhH) |
| Compd. | Appearance | Yield(%) | m.p./℃ | HRMS(calcd.), m/z[M+H]+ |
|---|---|---|---|---|
| 8a | Gray solid | 54 | >280 | 387.1941(387.1928) |
| 8b | Gray solid | 58 | >280 | 389.1730(389.1721) |
| 8c | Gray solid | 46 | 244—246 | 416.2205(416.2193) |
| 8d | Yellowsolid | 51 | >280 | 346.1675(346.1662) |
| 8f | Yellow solid | 37 | >280 | 382.0984(382.0968) |
| 9a | Gray solid | 40 | 245—247 | 401.2095(401.2084) |
| 9b | Yellow solid | 58 | 270—272 | 403.1889(403.1877) |
| 9c | Gray solid | 28 | 246—248 | 430.2364(430.2350) |
| 9d | White solid | 53 | 276—278 | 360.1836(360.1819) |
| 9e | Gray solid | 40 | 264—266 | 361.1769(361.1771) |
| 9f | White solid | 33 | >280 | 396.1148(396.1125) |
| 10a | Gray solid | 42 | 252—254 | 429.2401(429.2397) |
| 10b | White solid | 63 | 259—261 | 431.2209(431.2190) |
| 10c | Gray solid | 51 | >280 | 458.2681(458.2663) |
| 10d | White solid | 43 | 260—262 | 388.2145(388.2132) |
| 10e | Gray solid | 51 | 242—244 | 389.2086(389.2084) |
| 10f | White solid | 28 | >280 | 424.1456(424.1438) |
| 11a | Gray solid | 59 | >280 | 443.2556(443.2554) |
| 11b | White solid | 54 | >280 | 445.2369(445.2347) |
| 11c | Gray solid | 41 | 260—262 | 472.2836(472.2819) |
| 11d | White solid | 50 | 254—256 | 402.2301(402.2288) |
| 11e | Gray solid | 53 | 264—266 | 403.2241(403.2241) |
| 11f | White solid | 15 | >280 | 438.1616(438.1594) |
| 12a | Gray solid | 40 | >280 | 455.1811(455.1802) |
| 12b | Gray solid | 45 | >280 | 457.1604(457.1594) |
| 12c | Gray solid | 29 | >280 | 484.2090(484.2067) |
| 12d | White solid | 36 | >280 | 414.1535(414.1536) |
| 12e | Gray solid | 48 | >280 | 415.1490(415.1489) |
| 12f | White solid | 56 | >280 | 450.0863(450.0842) |
Table 3 Appearance, yields, melting points and HRMS data of compounds 8—12
| Compd. | Appearance | Yield(%) | m.p./℃ | HRMS(calcd.), m/z[M+H]+ |
|---|---|---|---|---|
| 8a | Gray solid | 54 | >280 | 387.1941(387.1928) |
| 8b | Gray solid | 58 | >280 | 389.1730(389.1721) |
| 8c | Gray solid | 46 | 244—246 | 416.2205(416.2193) |
| 8d | Yellowsolid | 51 | >280 | 346.1675(346.1662) |
| 8f | Yellow solid | 37 | >280 | 382.0984(382.0968) |
| 9a | Gray solid | 40 | 245—247 | 401.2095(401.2084) |
| 9b | Yellow solid | 58 | 270—272 | 403.1889(403.1877) |
| 9c | Gray solid | 28 | 246—248 | 430.2364(430.2350) |
| 9d | White solid | 53 | 276—278 | 360.1836(360.1819) |
| 9e | Gray solid | 40 | 264—266 | 361.1769(361.1771) |
| 9f | White solid | 33 | >280 | 396.1148(396.1125) |
| 10a | Gray solid | 42 | 252—254 | 429.2401(429.2397) |
| 10b | White solid | 63 | 259—261 | 431.2209(431.2190) |
| 10c | Gray solid | 51 | >280 | 458.2681(458.2663) |
| 10d | White solid | 43 | 260—262 | 388.2145(388.2132) |
| 10e | Gray solid | 51 | 242—244 | 389.2086(389.2084) |
| 10f | White solid | 28 | >280 | 424.1456(424.1438) |
| 11a | Gray solid | 59 | >280 | 443.2556(443.2554) |
| 11b | White solid | 54 | >280 | 445.2369(445.2347) |
| 11c | Gray solid | 41 | 260—262 | 472.2836(472.2819) |
| 11d | White solid | 50 | 254—256 | 402.2301(402.2288) |
| 11e | Gray solid | 53 | 264—266 | 403.2241(403.2241) |
| 11f | White solid | 15 | >280 | 438.1616(438.1594) |
| 12a | Gray solid | 40 | >280 | 455.1811(455.1802) |
| 12b | Gray solid | 45 | >280 | 457.1604(457.1594) |
| 12c | Gray solid | 29 | >280 | 484.2090(484.2067) |
| 12d | White solid | 36 | >280 | 414.1535(414.1536) |
| 12e | Gray solid | 48 | >280 | 415.1490(415.1489) |
| 12f | White solid | 56 | >280 | 450.0863(450.0842) |
| Compd. | 1H NMR(400 MHz, DMSO-d6), δ | 13C NMR(101 MHz, DMSO-d6), δ |
|---|---|---|
| 8a | 11.14(s, 1H, NH), 9.02(s, 1H, NH), 8.01(s, 1H, pyrimidine H), 7.65(d, J=7.5 Hz, 2H, PhH), 7.55(q, 4H, PhH), 7.44(t, 1H, PhH), 6.80(d, J=7.4 Hz, 2H, PhH), 3.08—2.92[m, 4H, N(CH2)2], 1.61[m, 4H, CH2], 1.49(m, 2H, CH2) | 155.78, 152.78, 151.37, 147.00, 134.82, 133.76, 133.43, 129.35, 128.16, 127.00, 119.88, 117.06, 115.03, 51.22, 25.98, 24.34 |
| 8b | 11.16(s, 1H, NH), 9.07(s, 1H, NH), 8.02(s, 1H, pyrimidine H), 7.65(d, J=7.9 Hz, 2H, PhH), 7.62—7.51(m, 4H, PhH), 7.44(t, 1H, PhH), 6.82(d, J=7.9 Hz, 2H, PhH), 3.80—3.67[m, 4H, N(CH2)2], 3.07—2.93[m, 4H, O(CH2)2] | 155.73, 152.78, 151.37, 146.02, 134.80, 134.23, 129.36, 128.18, 127.01, 119.84, 116.14, 115.10, 66.65, 49.94 |
| 8c | 11.16(s, 1H, NH), 9.04(s, 1H, NH), 8.01(s, 1H, pyrimidine H), 7.65(d, J=7.9 Hz, 2H, PhH), 7.56(q, 4H, PhH), 7.45(t, 1H, PhH), 6.81(d, J=8.0 Hz, 2H, PhH), 3.02[m, 4H, N(CH2)2], 2.50[m, 4H, N(CH2)2], 2.37(q, J=7.1 Hz, 2H, CH2), 1.03(t, J=7.2 Hz, 3H, CH3) | 155.76, 152.90, 151.36, 146.08, 135.08, 134.81, 129.53, 129.36, 128.17, 126.97, 119.87, 116.35, 115.06, 52.88, 52.09, 49.60, 12.42 |
| 8d | 11.19(s, 1H, NH), 9.20(s, 1H, NH), 8.04(s, 1H, pyrimidine H), 7.73—7.52(m, 6H, PhH), 7.45(t, J=7.4 Hz, 1H, PhH), 7.06(d, 2H, J=8.4 Hz, PhH), 2.79(Hept, J=6.9 Hz, 1H, CH), 1.16[d, J=6.9 Hz, 6H, (CH3)2] | 155.54, 152.80, 151.36, 141.03, 139.29, 134.72, 133.39, 129.37, 128.23, 127.06, 126.45, 118.74, 115.40, 33.21, 24.56 |
| 8f | 11.37(s, 1H, NH), 9.94(s, 1H, NH), 8.15(s, 1H, pyrimidine H), 7.97(d, J=8.9 Hz, 2H, PhH), 7.73(d, J=8.9 Hz, 2H, PhH), 7.66(d, J=7.9 Hz, 2H, PhH), 7.59(t, J=7.7 Hz, 2H, PhH), 7.47(t, J=7.3 Hz, 1H, PhH), 3.12(s, 3H, CH3) | 154.40, 152.85, 151.42, 146.24, 134.33, 133.17, 131.75, 129.50, 128.49, 128.38, 127.21, 117.50, 116.70, 44.53 |
| 9a | 11.11(s, 1H, NH), 9.01(s, 1H, NH), 7.99(s, 1H, pyrimidine H), 7.60—7.45(dd, J=9.0, 8.2 Hz, 4H, PhH), 7.35(d, J=8.2 Hz, 2H, PhH), 6.80(d, J=9.0 Hz, 2H, PhH), 3.04—2.94[m, 4H, N(CH2)2], 2.39(s, 3H, CH3), 1.61(m, 4H, CH2), 1.49(m, 2H, CH2) | 155.78, 152.88, 151.50, 146.97, 137.72, 134.63, 133.82, 130.80, 129.82, 126.91, 119.82, 117.10, 115.05, 51.24, 25.98, 24.34, 21.22 |
| 9b | 11.12(s, 1H, NH), 9.05(s, 1H, NH), 8.00(s, 1H, pyrimidine H), 7.56(d, J=9.0 Hz, 2H, PhH), 7.50(d, J=8.2 Hz, 2H, PhH), 7.35(d, J=8.2 Hz, 2H, PhH), 6.82(d, J=9.0 Hz, 2H, PhH), 3.83—3.63[m, 4H, N(CH2)2], 3.08—2.93[m, 4H, O(CH2)2], 2.39(s, 3H, CH3) | 155.72, 152.88, 151.51, 146.00, 137.74, 134.62, 134.28, 130.79, 129.83, 126.92, 119.77, 116.16, 115.11, 66.66, 49.95, 21.23 |
| 9c | 11.12(s, 1H, NH), 9.02(s, 1H, NH), 7.99(s, 1H, pyrimidine H), 7.52(dd, J=9.0, 8.2 Hz, 4H, PhH), 7.35(d, J=8.2 Hz, 2H, PhH), 6.81(d, J=9.0 Hz, 2H, PhH), 3.07—2.92[m, 4H, N(CH2)2], 2.50[m, 4H, N(CH2)2], 2.42—2.32(m, 5H, CH3, CH2), 1.02(t, J=7.2 Hz, 3H, CH3) | 155.76, 152.88, 151.50, 146.09, 137.72, 134.63, 133.95, 130.80, 129.82, 126.90, 119.82, 116.36, 115.07, 52.93, 52.12, 49.67, 21.23, 12.49 |
| 9d | 11.15(s, 1H, NH), 9.18(s, 1H, NH), 8.02(s, 1H, pyrimidine H), 7.61(d, J=8.5 Hz, 2H, PhH), 7.51(d, J=8.2 Hz, 2H, PhH), 7.36(d, J=8.2 Hz, 2H, PhH), 7.07(d, J=8.5 Hz, 2H, PhH), 2.79(Hept, J=6.9 Hz, 1H, CH), 2.39(s, 3H, CH3), 1.16[d, J=6.9 Hz, 6H, (CH3)2] | 155.54, 152.90, 151.49, 140.99, 139.32, 137.79, 134.55, 130.76, 129.83, 126.95, 126.46, 118.69, 115.40, 33.21, 24.57, 21.22 |
| 9e | 11.08(s, 1H, NH), 8.91(s, 1H, NH), 7.97(s, 1H, pyrimidine H), 7.50(dd, J=9.0, 8.2 Hz, 4H, PhH), 7.35(d, J=8.2 Hz, 2H, PhH), 6.65(d, J=9.0 Hz, 2H, PhH), 2.80[s, 6H, N(CH3)2], 2.39(s, 3H, CH3) | 155.98, 152.87, 151.51, 146.17, 137.67, 134.70, 131.86, 130.83, 129.80, 126.88, 120.42, 114.88, 113.53, 41.37, 21.22 |
| 9f | 11.33(s, 1H, NH), 9.92(s, 1H, NH), 8.13(s, 1H, pyrimidine H), 7.96(d, J=8.9 Hz, 2H, PhH), 7.73(d, J=8.9 Hz, 2H, PhH), 7.51(d, J=8.2 Hz, 2H, PhH), 7.38(d, J=8.2 Hz, 2H, PhH), 3.12(s, 3H, CH3), 2.40(s, 3H, CH3) | 154.41, 152.94, 151.53, 146.27, 138.07, 134.19, 131.72, 130.56, 129.94, 128.38, 127.06, 117.46, 116.70, 44.55, 21.24 |
| 10a | 11.09(s, 1H, NH), 9.00(s, 1H, NH), 7.99(s, 1H, pyrimidine H), 7.52(dd, J=9.0, 8.4 Hz, 4H, PhH), 7.41(d, J=8.4 Hz, 2H, PhH), 6.80(d, J=9.0 Hz, 2H, PhH), 3.02—2.97[m, 5H, N(CH2)2, CH], 1.61(m, 4H, CH2), 1.50(m, 2H, CH2), 1.26[d, J=6.9 Hz, 6H, (CH3)2] | 155.79, 152.92, 151.59, 148.57, 146.98, 134.54, 133.79, 131.04, 127.23, 127.14, 119.94, 117.06, 115.11, 51.23, 33.68, 25.96, 24.34 |
| Compd. | 1H NMR(400 MHz, DMSO-d6), δ | 13C NMR(101 MHz, DMSO-d6), δ |
| 10b | 11.11(s, 1H, NH), 9.04(s, 1H, NH), 8.00(s, 1H, pyrimidine H), 7.56(d, J=9.0 Hz, 2H, PhH), 7.52(d, J=8.4 Hz, 2H, PhH), 7.41(d, J=8.4 Hz, 2H, PhH), 6.82(d, J=9.0 Hz, 2H, PhH), 3.78—3.67[m, 4H, N(CH2)2], 3.04—2.94[m, 5H, O(CH2)2, CH], 1.26[d, J=6.9 Hz, 6H, (CH3)2] | 155.73, 152.93, 151.60, 148.58, 146.03, 134.50, 134.28, 127.24, 127.14, 119.88, 116.15, 115.18, 66.65, 49.96, 33.69, 24.35 |
| 10c | 11.09(s, 1H, NH), 9.01(s, 1H, NH), 7.99(s, 1H, pyrimidine H), 7.62—7.47(dd, J=9.0, 8.4 Hz, 4H, PhH), 7.41(d, J=8.4 Hz, 2H, PhH), 6.81(d, J=9.0 Hz, 2H, PhH), 3.10—2.89[m, 5H, N(CH2)2, CH], 2.47[m, 4H, N(CH2)2], 2.35(q, J=7.2 Hz, 2H, CH2), 1.26[d, J=6.9 Hz, 6H, (CH3)2], 1.02(t, J=7.2 Hz, 3H, CH3) | 155.77, 152.92, 151.59, 148.57, 146.12, 134.53, 133.94, 131.03, 127.23, 127.13, 119.93, 116.35, 115.13, 52.92, 52.11, 49.68, 33.68, 24.35, 12.49 |
| 10d | 11.15(s, 1H, NH), 9.18(s, 1H, NH), 8.02(s, 1H, pyrimidine H), 7.60(d, J=8.5 Hz, 2H, PhH), 7.53(d, J=8.3 Hz, 2H, PhH), 7.42(d, J=8.4 Hz, 2H, PhH), 7.06(d, J=8.5 Hz, 2H, PhH), 2.98(Hept, J=6.6 Hz, 1H, CH), 2.80(Hept, J=6.8 Hz, 1H, CH), 1.26[d, J=6.9 Hz, 6H, (CH3)2], 1.16[d, J=6.9 Hz, 6H, (CH3)2] | 155.54, 152.93, 151.57, 148.63, 141.02, 139.31, 134.46, 130.99, 127.24, 127.17, 126.43, 118.81, 115.45, 33.69, 33.21, 24.56, 24.34 |
| 10e | 11.07(s, 1H, NH), 8.91(s, 1H, NH), 7.97(s, 1H, pyrimidine H), 7.59—7.46(dd, J=9.0, 8.3 Hz, 4H, PhH), 7.41(d, J=8.3 Hz, 2H, PhH), 6.64(d, J=9.0 Hz, 2H, PhH), 2.98(Hept, J=6.8 Hz, 1H, CH), 2.80[s, 6H, N(CH3)2], 1.25[d, J=6.9 Hz, 6H, (CH3)2] | 155.98, 152.91, 151.58, 148.52, 146.18, 134.62, 131.87, 131.06, 127.21, 127.10, 120.50, 114.92, 113.50, 41.35, 33.68, 24.34 |
| 10f | 11.29(s, 1H, NH), 9.92(s, 1H, NH), 8.14(s, 1H, pyrimidine H), 7.97(d, J=8.8 Hz, 2H, PhH), 7.73(d, J=8.8 Hz, 2H, PhH), 7.53(d, J=8.2 Hz, 2H, PhH), 7.44(d, J=8.4 Hz, 2H, PhH), 3.12(s, 3H, CH3), 3.02—2.95(Hept, J=6.9 Hz, 1H, CH), 1.26[d, J=6.9 Hz, 6H, (CH3)2] | 154.40, 153.00, 151.64, 148.89, 146.28, 134.04, 131.73, 130.80, 128.37, 127.36, 127.29, 117.51, 116.77, 44.55, 33.71, 24.35 |
| 11a | 11.10(s, 1H, NH), 9.00(s, 1H, NH), 7.99(s, 1H, pyrimidine H), 7.54(m, 6H, PhH), 6.80(d, J=9.0 Hz, 2H, PhH), 3.03—2.95[m, 4H, N(CH2)2], 1.61(m, 4H, CH2), 1.50(m, 2H, CH2), 1.34[s, 9H, C(CH3)3] | 155.79, 152.92, 151.61, 150.75, 146.98, 134.51, 133.78, 130.75, 126.82, 126.16, 119.99, 117.04, 115.13, 51.22, 34.94, 31.58, 25.95, 24.35 |
| 11b | 11.11(s, 1H, NH), 9.04(s, 1H, NH), 8.00(s, 1H, pyrimidine H), 7.54(m, 6H, PhH), 6.82(d, J=9.0 Hz, 2H, PhH), 3.78—3.66[m, 4H, N(CH2)2], 3.06—2.92[m, 4H, O(CH2)2], 1.34[s, 9H, C(CH3)3] | 155.73, 152.93, 151.62, 150.77, 146.03, 134.47, 134.28, 130.74, 126.83, 126.18, 119.92, 116.15, 115.21, 66.65, 49.96, 34.94, 31.58 |
| 11c | 11.08(s, 1H, NH), 9.00(s, 1H, NH), 7.99(s, 1H, pyrimidine H), 7.53(m, 6H, PhH), 6.81(d, J=9.0 Hz, 2H, PhH), 3.05—2.99[m, 4H, N(CH2)2], 2.47[m, 4H, N(CH2)2], 2.35(q, J=7.1 Hz, 2H, CH2), 1.34[s, 9H, C(CH3)3], 1.02(t, J=7.2 Hz, 3H, CH3) | 155.77, 152.92, 151.60, 150.75, 146.12, 134.50, 133.93, 130.74, 126.81, 126.17, 119.97, 116.34, 115.15, 52.92, 52.11, 49.67, 34.94, 31.58, 12.49 |
| 11d | 11.15(s, 1H, NH), 9.18(s, 1H, NH), 8.02(s, 1H, pyrimidine H), 7.71—7.47(m, 6H, PhH), 7.06(d, J=8.5 Hz, 2H, PhH), 2.80(Hept, J=7.1 Hz, 1H, CH), 1.34[s, 9H, C(CH3)3], 1.16[d, J=6.9 Hz, 6H, (CH3)2] | 155.54, 152.93, 151.58, 150.81, 141.03, 139.31, 134.44, 130.70, 126.83, 126.42, 126.18, 118.86, 115.47, 34.94, 33.21, 31.58, 24.56 |
| 11e | 11.07(s, 1H, NH), 8.91(s, 1H, NH), 7.97(s, 1H, pyrimidine H), 7.64—7.43(m, 6H, PhH), 6.64(d, J=9.0 Hz, 2H, PhH), 2.80[s, 6H, N(CH3)2], 1.34[s, 9H, C(CH3)3] | 155.98, 152.90, 151.58, 150.69, 146.19, 134.61, 131.85, 130.78, 126.76, 126.14, 120.54, 114.94, 113.49, 41.34, 34.93, 31.58 |
| 11f | 11.22(s, 1H, NH), 9.92(s, 1H, NH), 8.14(s, 1H, pyrimidine H), 7.97(d, J=8.8 Hz, 2H, PhH), 7.73(d, J=8.8 Hz, 2H, PhH), 7.59(d, J=8.6 Hz, 2H, PhH), 7.54(d, J=8.8 Hz, 2H, PhH), 3.12(s, 3H, CH3), 1.35[s, 9H, C(CH3)3] | 154.39, 153.01, 151.68, 151.08, 146.29, 133.99, 131.73, 130.53, 128.37, 126.98, 126.30, 117.53, 116.83, 44.55, 34.98, 31.58 |
| Compd. | 1H NMR(400 MHz, DMSO-d6), δ | 13C NMR(101 MHz, DMSO-d6), δ |
| 12a | 11.27(s, 1H, NH), 9.05(s, 1H, NH), 8.05(s, 1H, pyrimidine H), 7.97(dd, J=8.7 Hz, 4H, PhH), 7.52(d, J=8.9 Hz, 2H, PhH), 6.84(d, J=8.9 Hz, 2H, PhH), 3.11—2.92[m, 4H, N(CH2)2], 1.73—1.57(m, 4H, CH2), 1.57—1.40(m, 2H, CH2) | 155.76, 152.36, 150.90, 147.12, 137.20, 135.32, 133.55, 128.18, 127.86, 127.06, 126.42, 126.38, 125.87, 123.17, 120.06, 117.07, 115.04, 51.18, 25.95, 24.35 |
| 12b | 11.28(s, 1H, NH), 9.09(s, 1H, NH), 8.05(s, 1H, pyrimidine H), 7.98(dd, J=8.7 Hz, 4H, PhH), 7.56(d, J=9.0 Hz, 2H, PhH), 6.85(d, J=8.9 Hz, 2H, PhH), 3.75—3.71[m, 4H, N(CH2)2], 3.02—2.96[m, 4H, O(CH2)2] | 155.70, 152.37, 150.91, 146.17, 137.19, 135.29, 134.05, 127.06, 126.43, 126.39, 119.99, 116.17, 116.01, 115.11, 66.65, 49.91 |
| 12c | 11.27(s, 1H, NH), 9.06(s, 1H, NH), 8.05(s, 1H, pyrimidine H), 7.98(dd, J=8.6 Hz, 4H, PhH), 7.53(d, J=8.8 Hz, 2H, PhH), 6.83(d, J=8.7 Hz, 2H, PhH), 3.06—3.01[m, 4H, N(CH2)2], 2.40—2.34(q, J=7.2 Hz, 2H, CH2), 1.03(t, J=7.2 Hz, 3H, CH3) | 155.74, 152.37, 150.91, 146.25, 135.31, 133.71, 127.05, 126.42, 126.39, 121.17, 120.05, 116.37, 116.20, 115.08, 52.90, 52.11, 49.62, 12.46 |
| 12d | 11.32(s, 1H, NH), 9.22(s, 1H, pyrimidineH), 8.08(s, 1H, pyrimidine H), 7.97(dd, J=8.9 Hz, 4H, PhH), 7.60(d, J=8.5 Hz, 2H, PhH), 7.09(d, J=8.5 Hz, 2H, PhH), 2.81(Hept, J=6.9 Hz, 1H, CH), 1.17[d, J=6.9 Hz, 6H, (CH3)2] | 155.50, 152.39, 150.91, 141.24, 139.13, 137.13, 135.21, 127.16, 126.51, 126.44, 126.40, 118.89, 115.42, 33.22, 24.55 |
| 12e | 11.24(s, 1H, NH), 8.95(s, 1H, NH), 8.03(s, 1H, pyrimidine H), 8.01—7.92(dd, J=8.7 Hz, 4H, PhH, NH), 7.48(d, J=9.0 Hz, 2H, PhH), 6.67(d, J=9.0 Hz, 2H, PhH), 2.81[s, 6H, N(CH3)2] | 155.96, 152.36, 150.90, 146.34, 137.22, 135.41, 131.57, 127.02, 126.40, 120.68, 114.86, 113.51, 99.99, 41.32 |
| 12f | 11.51(s, 1H, NH), 9.96(s, 1H, NH), 8.19(s, 1H, pyrimidine H), 7.97(m, 6H, PhH), 7.76(d, J=8.8 Hz, 2H, PhH), 3.13(s, 3H, CH3) | 154.34, 152.47, 150.98, 146.12, 136.94, 134.81, 131.89, 128.46, 127.34, 126.58, 117.55, 116.78, 44.53 |
Table 4 1H NMR and 13C NMR data of compounds 8—12
| Compd. | 1H NMR(400 MHz, DMSO-d6), δ | 13C NMR(101 MHz, DMSO-d6), δ |
|---|---|---|
| 8a | 11.14(s, 1H, NH), 9.02(s, 1H, NH), 8.01(s, 1H, pyrimidine H), 7.65(d, J=7.5 Hz, 2H, PhH), 7.55(q, 4H, PhH), 7.44(t, 1H, PhH), 6.80(d, J=7.4 Hz, 2H, PhH), 3.08—2.92[m, 4H, N(CH2)2], 1.61[m, 4H, CH2], 1.49(m, 2H, CH2) | 155.78, 152.78, 151.37, 147.00, 134.82, 133.76, 133.43, 129.35, 128.16, 127.00, 119.88, 117.06, 115.03, 51.22, 25.98, 24.34 |
| 8b | 11.16(s, 1H, NH), 9.07(s, 1H, NH), 8.02(s, 1H, pyrimidine H), 7.65(d, J=7.9 Hz, 2H, PhH), 7.62—7.51(m, 4H, PhH), 7.44(t, 1H, PhH), 6.82(d, J=7.9 Hz, 2H, PhH), 3.80—3.67[m, 4H, N(CH2)2], 3.07—2.93[m, 4H, O(CH2)2] | 155.73, 152.78, 151.37, 146.02, 134.80, 134.23, 129.36, 128.18, 127.01, 119.84, 116.14, 115.10, 66.65, 49.94 |
| 8c | 11.16(s, 1H, NH), 9.04(s, 1H, NH), 8.01(s, 1H, pyrimidine H), 7.65(d, J=7.9 Hz, 2H, PhH), 7.56(q, 4H, PhH), 7.45(t, 1H, PhH), 6.81(d, J=8.0 Hz, 2H, PhH), 3.02[m, 4H, N(CH2)2], 2.50[m, 4H, N(CH2)2], 2.37(q, J=7.1 Hz, 2H, CH2), 1.03(t, J=7.2 Hz, 3H, CH3) | 155.76, 152.90, 151.36, 146.08, 135.08, 134.81, 129.53, 129.36, 128.17, 126.97, 119.87, 116.35, 115.06, 52.88, 52.09, 49.60, 12.42 |
| 8d | 11.19(s, 1H, NH), 9.20(s, 1H, NH), 8.04(s, 1H, pyrimidine H), 7.73—7.52(m, 6H, PhH), 7.45(t, J=7.4 Hz, 1H, PhH), 7.06(d, 2H, J=8.4 Hz, PhH), 2.79(Hept, J=6.9 Hz, 1H, CH), 1.16[d, J=6.9 Hz, 6H, (CH3)2] | 155.54, 152.80, 151.36, 141.03, 139.29, 134.72, 133.39, 129.37, 128.23, 127.06, 126.45, 118.74, 115.40, 33.21, 24.56 |
| 8f | 11.37(s, 1H, NH), 9.94(s, 1H, NH), 8.15(s, 1H, pyrimidine H), 7.97(d, J=8.9 Hz, 2H, PhH), 7.73(d, J=8.9 Hz, 2H, PhH), 7.66(d, J=7.9 Hz, 2H, PhH), 7.59(t, J=7.7 Hz, 2H, PhH), 7.47(t, J=7.3 Hz, 1H, PhH), 3.12(s, 3H, CH3) | 154.40, 152.85, 151.42, 146.24, 134.33, 133.17, 131.75, 129.50, 128.49, 128.38, 127.21, 117.50, 116.70, 44.53 |
| 9a | 11.11(s, 1H, NH), 9.01(s, 1H, NH), 7.99(s, 1H, pyrimidine H), 7.60—7.45(dd, J=9.0, 8.2 Hz, 4H, PhH), 7.35(d, J=8.2 Hz, 2H, PhH), 6.80(d, J=9.0 Hz, 2H, PhH), 3.04—2.94[m, 4H, N(CH2)2], 2.39(s, 3H, CH3), 1.61(m, 4H, CH2), 1.49(m, 2H, CH2) | 155.78, 152.88, 151.50, 146.97, 137.72, 134.63, 133.82, 130.80, 129.82, 126.91, 119.82, 117.10, 115.05, 51.24, 25.98, 24.34, 21.22 |
| 9b | 11.12(s, 1H, NH), 9.05(s, 1H, NH), 8.00(s, 1H, pyrimidine H), 7.56(d, J=9.0 Hz, 2H, PhH), 7.50(d, J=8.2 Hz, 2H, PhH), 7.35(d, J=8.2 Hz, 2H, PhH), 6.82(d, J=9.0 Hz, 2H, PhH), 3.83—3.63[m, 4H, N(CH2)2], 3.08—2.93[m, 4H, O(CH2)2], 2.39(s, 3H, CH3) | 155.72, 152.88, 151.51, 146.00, 137.74, 134.62, 134.28, 130.79, 129.83, 126.92, 119.77, 116.16, 115.11, 66.66, 49.95, 21.23 |
| 9c | 11.12(s, 1H, NH), 9.02(s, 1H, NH), 7.99(s, 1H, pyrimidine H), 7.52(dd, J=9.0, 8.2 Hz, 4H, PhH), 7.35(d, J=8.2 Hz, 2H, PhH), 6.81(d, J=9.0 Hz, 2H, PhH), 3.07—2.92[m, 4H, N(CH2)2], 2.50[m, 4H, N(CH2)2], 2.42—2.32(m, 5H, CH3, CH2), 1.02(t, J=7.2 Hz, 3H, CH3) | 155.76, 152.88, 151.50, 146.09, 137.72, 134.63, 133.95, 130.80, 129.82, 126.90, 119.82, 116.36, 115.07, 52.93, 52.12, 49.67, 21.23, 12.49 |
| 9d | 11.15(s, 1H, NH), 9.18(s, 1H, NH), 8.02(s, 1H, pyrimidine H), 7.61(d, J=8.5 Hz, 2H, PhH), 7.51(d, J=8.2 Hz, 2H, PhH), 7.36(d, J=8.2 Hz, 2H, PhH), 7.07(d, J=8.5 Hz, 2H, PhH), 2.79(Hept, J=6.9 Hz, 1H, CH), 2.39(s, 3H, CH3), 1.16[d, J=6.9 Hz, 6H, (CH3)2] | 155.54, 152.90, 151.49, 140.99, 139.32, 137.79, 134.55, 130.76, 129.83, 126.95, 126.46, 118.69, 115.40, 33.21, 24.57, 21.22 |
| 9e | 11.08(s, 1H, NH), 8.91(s, 1H, NH), 7.97(s, 1H, pyrimidine H), 7.50(dd, J=9.0, 8.2 Hz, 4H, PhH), 7.35(d, J=8.2 Hz, 2H, PhH), 6.65(d, J=9.0 Hz, 2H, PhH), 2.80[s, 6H, N(CH3)2], 2.39(s, 3H, CH3) | 155.98, 152.87, 151.51, 146.17, 137.67, 134.70, 131.86, 130.83, 129.80, 126.88, 120.42, 114.88, 113.53, 41.37, 21.22 |
| 9f | 11.33(s, 1H, NH), 9.92(s, 1H, NH), 8.13(s, 1H, pyrimidine H), 7.96(d, J=8.9 Hz, 2H, PhH), 7.73(d, J=8.9 Hz, 2H, PhH), 7.51(d, J=8.2 Hz, 2H, PhH), 7.38(d, J=8.2 Hz, 2H, PhH), 3.12(s, 3H, CH3), 2.40(s, 3H, CH3) | 154.41, 152.94, 151.53, 146.27, 138.07, 134.19, 131.72, 130.56, 129.94, 128.38, 127.06, 117.46, 116.70, 44.55, 21.24 |
| 10a | 11.09(s, 1H, NH), 9.00(s, 1H, NH), 7.99(s, 1H, pyrimidine H), 7.52(dd, J=9.0, 8.4 Hz, 4H, PhH), 7.41(d, J=8.4 Hz, 2H, PhH), 6.80(d, J=9.0 Hz, 2H, PhH), 3.02—2.97[m, 5H, N(CH2)2, CH], 1.61(m, 4H, CH2), 1.50(m, 2H, CH2), 1.26[d, J=6.9 Hz, 6H, (CH3)2] | 155.79, 152.92, 151.59, 148.57, 146.98, 134.54, 133.79, 131.04, 127.23, 127.14, 119.94, 117.06, 115.11, 51.23, 33.68, 25.96, 24.34 |
| Compd. | 1H NMR(400 MHz, DMSO-d6), δ | 13C NMR(101 MHz, DMSO-d6), δ |
| 10b | 11.11(s, 1H, NH), 9.04(s, 1H, NH), 8.00(s, 1H, pyrimidine H), 7.56(d, J=9.0 Hz, 2H, PhH), 7.52(d, J=8.4 Hz, 2H, PhH), 7.41(d, J=8.4 Hz, 2H, PhH), 6.82(d, J=9.0 Hz, 2H, PhH), 3.78—3.67[m, 4H, N(CH2)2], 3.04—2.94[m, 5H, O(CH2)2, CH], 1.26[d, J=6.9 Hz, 6H, (CH3)2] | 155.73, 152.93, 151.60, 148.58, 146.03, 134.50, 134.28, 127.24, 127.14, 119.88, 116.15, 115.18, 66.65, 49.96, 33.69, 24.35 |
| 10c | 11.09(s, 1H, NH), 9.01(s, 1H, NH), 7.99(s, 1H, pyrimidine H), 7.62—7.47(dd, J=9.0, 8.4 Hz, 4H, PhH), 7.41(d, J=8.4 Hz, 2H, PhH), 6.81(d, J=9.0 Hz, 2H, PhH), 3.10—2.89[m, 5H, N(CH2)2, CH], 2.47[m, 4H, N(CH2)2], 2.35(q, J=7.2 Hz, 2H, CH2), 1.26[d, J=6.9 Hz, 6H, (CH3)2], 1.02(t, J=7.2 Hz, 3H, CH3) | 155.77, 152.92, 151.59, 148.57, 146.12, 134.53, 133.94, 131.03, 127.23, 127.13, 119.93, 116.35, 115.13, 52.92, 52.11, 49.68, 33.68, 24.35, 12.49 |
| 10d | 11.15(s, 1H, NH), 9.18(s, 1H, NH), 8.02(s, 1H, pyrimidine H), 7.60(d, J=8.5 Hz, 2H, PhH), 7.53(d, J=8.3 Hz, 2H, PhH), 7.42(d, J=8.4 Hz, 2H, PhH), 7.06(d, J=8.5 Hz, 2H, PhH), 2.98(Hept, J=6.6 Hz, 1H, CH), 2.80(Hept, J=6.8 Hz, 1H, CH), 1.26[d, J=6.9 Hz, 6H, (CH3)2], 1.16[d, J=6.9 Hz, 6H, (CH3)2] | 155.54, 152.93, 151.57, 148.63, 141.02, 139.31, 134.46, 130.99, 127.24, 127.17, 126.43, 118.81, 115.45, 33.69, 33.21, 24.56, 24.34 |
| 10e | 11.07(s, 1H, NH), 8.91(s, 1H, NH), 7.97(s, 1H, pyrimidine H), 7.59—7.46(dd, J=9.0, 8.3 Hz, 4H, PhH), 7.41(d, J=8.3 Hz, 2H, PhH), 6.64(d, J=9.0 Hz, 2H, PhH), 2.98(Hept, J=6.8 Hz, 1H, CH), 2.80[s, 6H, N(CH3)2], 1.25[d, J=6.9 Hz, 6H, (CH3)2] | 155.98, 152.91, 151.58, 148.52, 146.18, 134.62, 131.87, 131.06, 127.21, 127.10, 120.50, 114.92, 113.50, 41.35, 33.68, 24.34 |
| 10f | 11.29(s, 1H, NH), 9.92(s, 1H, NH), 8.14(s, 1H, pyrimidine H), 7.97(d, J=8.8 Hz, 2H, PhH), 7.73(d, J=8.8 Hz, 2H, PhH), 7.53(d, J=8.2 Hz, 2H, PhH), 7.44(d, J=8.4 Hz, 2H, PhH), 3.12(s, 3H, CH3), 3.02—2.95(Hept, J=6.9 Hz, 1H, CH), 1.26[d, J=6.9 Hz, 6H, (CH3)2] | 154.40, 153.00, 151.64, 148.89, 146.28, 134.04, 131.73, 130.80, 128.37, 127.36, 127.29, 117.51, 116.77, 44.55, 33.71, 24.35 |
| 11a | 11.10(s, 1H, NH), 9.00(s, 1H, NH), 7.99(s, 1H, pyrimidine H), 7.54(m, 6H, PhH), 6.80(d, J=9.0 Hz, 2H, PhH), 3.03—2.95[m, 4H, N(CH2)2], 1.61(m, 4H, CH2), 1.50(m, 2H, CH2), 1.34[s, 9H, C(CH3)3] | 155.79, 152.92, 151.61, 150.75, 146.98, 134.51, 133.78, 130.75, 126.82, 126.16, 119.99, 117.04, 115.13, 51.22, 34.94, 31.58, 25.95, 24.35 |
| 11b | 11.11(s, 1H, NH), 9.04(s, 1H, NH), 8.00(s, 1H, pyrimidine H), 7.54(m, 6H, PhH), 6.82(d, J=9.0 Hz, 2H, PhH), 3.78—3.66[m, 4H, N(CH2)2], 3.06—2.92[m, 4H, O(CH2)2], 1.34[s, 9H, C(CH3)3] | 155.73, 152.93, 151.62, 150.77, 146.03, 134.47, 134.28, 130.74, 126.83, 126.18, 119.92, 116.15, 115.21, 66.65, 49.96, 34.94, 31.58 |
| 11c | 11.08(s, 1H, NH), 9.00(s, 1H, NH), 7.99(s, 1H, pyrimidine H), 7.53(m, 6H, PhH), 6.81(d, J=9.0 Hz, 2H, PhH), 3.05—2.99[m, 4H, N(CH2)2], 2.47[m, 4H, N(CH2)2], 2.35(q, J=7.1 Hz, 2H, CH2), 1.34[s, 9H, C(CH3)3], 1.02(t, J=7.2 Hz, 3H, CH3) | 155.77, 152.92, 151.60, 150.75, 146.12, 134.50, 133.93, 130.74, 126.81, 126.17, 119.97, 116.34, 115.15, 52.92, 52.11, 49.67, 34.94, 31.58, 12.49 |
| 11d | 11.15(s, 1H, NH), 9.18(s, 1H, NH), 8.02(s, 1H, pyrimidine H), 7.71—7.47(m, 6H, PhH), 7.06(d, J=8.5 Hz, 2H, PhH), 2.80(Hept, J=7.1 Hz, 1H, CH), 1.34[s, 9H, C(CH3)3], 1.16[d, J=6.9 Hz, 6H, (CH3)2] | 155.54, 152.93, 151.58, 150.81, 141.03, 139.31, 134.44, 130.70, 126.83, 126.42, 126.18, 118.86, 115.47, 34.94, 33.21, 31.58, 24.56 |
| 11e | 11.07(s, 1H, NH), 8.91(s, 1H, NH), 7.97(s, 1H, pyrimidine H), 7.64—7.43(m, 6H, PhH), 6.64(d, J=9.0 Hz, 2H, PhH), 2.80[s, 6H, N(CH3)2], 1.34[s, 9H, C(CH3)3] | 155.98, 152.90, 151.58, 150.69, 146.19, 134.61, 131.85, 130.78, 126.76, 126.14, 120.54, 114.94, 113.49, 41.34, 34.93, 31.58 |
| 11f | 11.22(s, 1H, NH), 9.92(s, 1H, NH), 8.14(s, 1H, pyrimidine H), 7.97(d, J=8.8 Hz, 2H, PhH), 7.73(d, J=8.8 Hz, 2H, PhH), 7.59(d, J=8.6 Hz, 2H, PhH), 7.54(d, J=8.8 Hz, 2H, PhH), 3.12(s, 3H, CH3), 1.35[s, 9H, C(CH3)3] | 154.39, 153.01, 151.68, 151.08, 146.29, 133.99, 131.73, 130.53, 128.37, 126.98, 126.30, 117.53, 116.83, 44.55, 34.98, 31.58 |
| Compd. | 1H NMR(400 MHz, DMSO-d6), δ | 13C NMR(101 MHz, DMSO-d6), δ |
| 12a | 11.27(s, 1H, NH), 9.05(s, 1H, NH), 8.05(s, 1H, pyrimidine H), 7.97(dd, J=8.7 Hz, 4H, PhH), 7.52(d, J=8.9 Hz, 2H, PhH), 6.84(d, J=8.9 Hz, 2H, PhH), 3.11—2.92[m, 4H, N(CH2)2], 1.73—1.57(m, 4H, CH2), 1.57—1.40(m, 2H, CH2) | 155.76, 152.36, 150.90, 147.12, 137.20, 135.32, 133.55, 128.18, 127.86, 127.06, 126.42, 126.38, 125.87, 123.17, 120.06, 117.07, 115.04, 51.18, 25.95, 24.35 |
| 12b | 11.28(s, 1H, NH), 9.09(s, 1H, NH), 8.05(s, 1H, pyrimidine H), 7.98(dd, J=8.7 Hz, 4H, PhH), 7.56(d, J=9.0 Hz, 2H, PhH), 6.85(d, J=8.9 Hz, 2H, PhH), 3.75—3.71[m, 4H, N(CH2)2], 3.02—2.96[m, 4H, O(CH2)2] | 155.70, 152.37, 150.91, 146.17, 137.19, 135.29, 134.05, 127.06, 126.43, 126.39, 119.99, 116.17, 116.01, 115.11, 66.65, 49.91 |
| 12c | 11.27(s, 1H, NH), 9.06(s, 1H, NH), 8.05(s, 1H, pyrimidine H), 7.98(dd, J=8.6 Hz, 4H, PhH), 7.53(d, J=8.8 Hz, 2H, PhH), 6.83(d, J=8.7 Hz, 2H, PhH), 3.06—3.01[m, 4H, N(CH2)2], 2.40—2.34(q, J=7.2 Hz, 2H, CH2), 1.03(t, J=7.2 Hz, 3H, CH3) | 155.74, 152.37, 150.91, 146.25, 135.31, 133.71, 127.05, 126.42, 126.39, 121.17, 120.05, 116.37, 116.20, 115.08, 52.90, 52.11, 49.62, 12.46 |
| 12d | 11.32(s, 1H, NH), 9.22(s, 1H, pyrimidineH), 8.08(s, 1H, pyrimidine H), 7.97(dd, J=8.9 Hz, 4H, PhH), 7.60(d, J=8.5 Hz, 2H, PhH), 7.09(d, J=8.5 Hz, 2H, PhH), 2.81(Hept, J=6.9 Hz, 1H, CH), 1.17[d, J=6.9 Hz, 6H, (CH3)2] | 155.50, 152.39, 150.91, 141.24, 139.13, 137.13, 135.21, 127.16, 126.51, 126.44, 126.40, 118.89, 115.42, 33.22, 24.55 |
| 12e | 11.24(s, 1H, NH), 8.95(s, 1H, NH), 8.03(s, 1H, pyrimidine H), 8.01—7.92(dd, J=8.7 Hz, 4H, PhH, NH), 7.48(d, J=9.0 Hz, 2H, PhH), 6.67(d, J=9.0 Hz, 2H, PhH), 2.81[s, 6H, N(CH3)2] | 155.96, 152.36, 150.90, 146.34, 137.22, 135.41, 131.57, 127.02, 126.40, 120.68, 114.86, 113.51, 99.99, 41.32 |
| 12f | 11.51(s, 1H, NH), 9.96(s, 1H, NH), 8.19(s, 1H, pyrimidine H), 7.97(m, 6H, PhH), 7.76(d, J=8.8 Hz, 2H, PhH), 3.13(s, 3H, CH3) | 154.34, 152.47, 150.98, 146.12, 136.94, 134.81, 131.89, 128.46, 127.34, 126.58, 117.55, 116.78, 44.53 |
| Compd. | IC50/(μmol·L-1) | |||
|---|---|---|---|---|
| K562 | PC-3 | MDA-MB-231 | HCT116 | |
| 8c | 3.68±0.08 | 11.46±0.67 | 18.16±1.64 | 4.48±0.12 |
| 10b | 9.13±2.35 | 4.43±0.19 | 11.79±0.08 | 4.41±0.12 |
| 10c | 2.23±0.26 | 6.17±1.49 | 11.67±4.02 | 12.24±2.50 |
| 12c | 3.25±0.85 | 5.07±0.34 | 3.35±0.27 | 1.15±0.09 |
| R-Roscovitine | 35.34±0.51 | 17.20±0.63 | 28.61±4.63 | 12.04±1.75 |
Table 5 Cytotoxicity of representative compounds in vitro*
| Compd. | IC50/(μmol·L-1) | |||
|---|---|---|---|---|
| K562 | PC-3 | MDA-MB-231 | HCT116 | |
| 8c | 3.68±0.08 | 11.46±0.67 | 18.16±1.64 | 4.48±0.12 |
| 10b | 9.13±2.35 | 4.43±0.19 | 11.79±0.08 | 4.41±0.12 |
| 10c | 2.23±0.26 | 6.17±1.49 | 11.67±4.02 | 12.24±2.50 |
| 12c | 3.25±0.85 | 5.07±0.34 | 3.35±0.27 | 1.15±0.09 |
| R-Roscovitine | 35.34±0.51 | 17.20±0.63 | 28.61±4.63 | 12.04±1.75 |
| [1] | Di V. F., Adinolfi E., Oncogene,2017, 36(3), 293-303 |
| [2] | Di V. F., Cancer Res., 2012, 72(21), 5441-5447 |
| [3] | Legraverend M., Grierson D. S., Bioorg. Med. Chem.,2006, 14(12), 3987-4006 |
| [4] | Sun L., Vasilevich N. I., Fuselier J. A., Hocart S. J., Coy D. H., Bioorg. Med. Chem. Lett.,2004, 14(9), 2041-2046 |
| [5] | Mulamoottil V. A., Nayak A., Jeong L. S., Asian J. Org. Chem.,2015, 45(37), 748-761 |
| [6] | Trávnícek Z., Štarha P., Vanco J., Šilha T., Hošek J., Pavel Suchý J., Pražanová G., J. Med. Chem.,2012, 55(10), 4568-4579 |
| [7] | Nivsarkar M., Thavaselvam D., Prasanna S., Sharma M., Kaushik M. P., Bioorg. Med. Chem. Lett.,2005, 15(5), 1371-1373 |
| [8] | Wu F., Li P., Hu D., Song B., Res. Chem. Intermediat.,2016, 42(9), 1-16 |
| [9] | Niu H. Y., Bai S. X., Wu S., Qu G. R., Guo H. M., Asian J. Org. Chem.,2012, 1(3), 238-244 |
| [10] | Guo J., Tan B., Ye Q., Liang G., Yi M., Jiang R., Chem. Res. Chinese Universities,2017, 33(4), 581-586 |
| [11] | Pan Y., Wang K., Liu Y., Qin R., Cao L., Wang J., Zhou G., Zhang A., Chem. Res. Chinese Universities,2017, 33(3), 388-391 |
| [12] | Pui C. H., Jeha S., Kirkpatrick P., Nat. Rev. Drug Discov.,2005, 4(5), S12-S13 |
| [13] | Gandhi V., Keating M. J., Bate G., Kirkpatrick P., Nat. Rev. Drug Discov.,2006, 5(1), 17-18 |
| [14] | Keating M. J., Kantarjian H., Talpaz M., Redman J., Koller C., Barlogie B., Velasquez W., Plunkett W., Freireich E. J., Mccredie K. B., Blood,1989, 74(1), 19-25 |
| [15] | Newlands E. S., Stevens M. F., Wedge S. R., Wheelhouse R. T., Brock C., Cancer Treat. Rev.,1997, 23(1), 35-61 |
| [16] | Sanford D. S., Wierda W. G., Burger J. A., Keating M. J., O'Brien S. M., Cl. Lymph. Myelom. Leuk.,2015, 15(7), 385-391 |
| [17] | Asghar U., Witkiewicz A. K., Turner N. C., Knudsen E. S., Nat. Rev. Drug Discov.,2015, 14(2), 130-146 |
| [18] | Zhang Z. P., Yang X. Y., Fang H., Chinese J. Org. Chem.,2015, 37(6), 1479-1486 |
| (张自鹏, 杨新颖, 方浩. 有机化学, 2017, 37(6), 1479-1486) | |
| [19] | Yang J., Wang L. J., Liu J. J., Zhong L., Zheng R. L., Xu Y., Ji P., Zhang C. H., Wang W. J., Lin X. D., J. Med. Chem.,2012, 55(23), 10685-10699 |
| [20] | Lu X., Li X., Yang J., Huang B., Kang D., Zhao F., Zhou Z., Clercq E. D., Daelemans D., Pannecouque C., Bioorg. Med. Chem.,2016, 24(18), 4424-4433 |
| [21] | Liu Z., Tang L., Zhu H., Xu T., Qiu C., Zheng S., Gu Y., Feng J., Zhang Y., Liang G., J. Med. Chem.,2016, 59(10), 4637-4650 |
| [1] | CAO Shujie, LI Hongjun, GUAN Wenli, REN Mengtian, ZHOU Chuanzheng. Progress on the Stereocontrolled Synthesis of Phosphorothioate Oligonucleotides [J]. Chem. J. Chinese Universities, 2022, 43(Album-4): 20220304. |
| [2] | WANG Ruyue, WEI Hehe, HUANG Kai, WU Hui. Freezing Synthesis for Single Atom Materials [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220428. |
| [3] | YAO Qing, YU Zhiyong, HUANG Xiaoqing. Progress in Synthesis and Energy-related Electrocatalysis of Single-atom Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220323. |
| [4] | WEI Chunhong, JIANG Qian, WANG Panpan, JIANG Chengfa, LIU Yuefeng. Atomic Scale Investigation of Pt Atoms/clusters Promoted Co-catalyzed Fischer-Tropsch Synthesis [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220074. |
| [5] | JIN Ruiming, MU Xiaoqing, XU Yan. Bio-chemical Synthesis of Melanin Precursor—— 5,6-Dihydroxyindole(DHI) [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220134. |
| [6] | ZHANG Xinxin, XU Di, WANG Yanqiu, HONG Xinlin, LIU Guoliang, YANG Hengquan. Effect of Mn Promoter on CuFe-based Catalysts for CO2 Hydrogenation to Higher Alcohols [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220187. |
| [7] | ZHOU Zixuan, YANG Haiyan, SUN Yuhan, GAO Peng. Recent Progress in Heterogeneous Catalysts for the Hydrogenation of Carbon Dioxide to Methanol [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220235. |
| [8] | LI Yidi, TIAN Xiaochun, LI Junpeng, CHEN Lixiang, ZHAO Feng. Electron Transfer on the Semiconductor-microbe Interface and Its Environmental Application [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220089. |
| [9] | LU Cong, LI Zhenhua, LIU Jinlu, HUA Jia, LI Guanghua, SHI Zhan, FENG Shouhua. Synthesis, Structure and Fluorescence Detection Properties of a New Lanthanide Metal-Organic Framework Material [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220037. |
| [10] | ZHUANG Jiahao, WANG Dingsheng. Current Advances and Future Challenges of Single-atom Catalysis [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220043. |
| [11] | FENG Li, SHAO Lanxing, LI Sijun, QUAN Wenxuan, ZHUANG Jinliang. Synthesis of Ultrathin Sm-MOF Nanosheets and Their Visible-light Induced Photodegradation of Mustard Simulant [J]. Chem. J. Chinese Universities, 2022, 43(4): 20210867. |
| [12] | ZHANG Zhinan, CHENG Haiming, TENG Shiyong, ZHANG Ying. Synthesis and Optical Properties of RbPb2Cl5 [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220418. |
| [13] | XING Peiqi, LU Tong, LI Guanghua, WANG Liyan. Controllable Syntheses of Two Cd(II) Metal-organic Frameworks Possessing Related Structures [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220218. |
| [14] | TAN Yuling, YANG Ling, YU Hong, NI Chunyan, LANG Jianping. Progress of the Synthesis of High-Nuclearity Ag/S Nanoclusters [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210476. |
| [15] | WANG Jie, HUO Haiyan, WANG Yang, ZHANG Zhong, LIU Shuxia. General Strategy for In situ Synthesis of NENU-n Series Polyoxometalate-based MOFs on Copper Foil [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210557. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||