

Chem. J. Chinese Universities ›› 2018, Vol. 39 ›› Issue (12): 2611.doi: 10.7503/cjcu20180009
• Inorganic Chemistry • Previous Articles Next Articles
ZHANG Dechun1,2, XU Qiwei2, LI Xia2,*( )
)
Received:2018-01-03
Online:2018-09-10
Published:2018-09-10
Contact:
LI Xia
E-mail:xiali@cnu.edu.cn
Supported by:CLC Number:
TrendMD:
ZHANG Dechun,XU Qiwei,LI Xia. Lanthanide Complexes Constructed by 2,2'-Oxybis(benzoic acid) and 1H-Imidazo[4,5-f][1,10]-phenanthroline: Fluorescence and Fluorescent Sensing for NH3†[J]. Chem. J. Chinese Universities, 2018, 39(12): 2611.
| Complex | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Empirical formula | C110H70N8O30Eu2 | C110H70N8O30Tb2 | C55H32N4O15Eu2 | C55H32N4O15Tb2 | C66H38N6O16Eu2 |
| Formula weight | 2287.66 | 2301.58 | 1292.77 | 1306.69 | 1474.94 |
| Crystal system | Triclinic | Triclinic | Triclinic | Triclinic | Monoclinic, |
| Space group | P | P | P | P | C2/c |
| a/nm | 1.15131(5) | 1.14885(5) | 1.31535(12) | 1.28091(10) | 2.84806(10) |
| b/nm | 1.38342(6) | 1.38288(6) | 1.39415(13) | 1.42179(11) | 1.40143(5) |
| c/nm | 1.70498(8) | 1.70231(8) | 1.45396(13) | 1.49624(11) | 2.97567(10) |
| α/(°) | 113.4990(10) | 113.4240(10) | 73.313(2) | 74.132(2) | 90 |
| β/(°) | 93.4370(10) | 93.4800(10) | 72.018(3) | 67.660(2) | 108.9160(10) |
| γ/(°) | 93.1820(10) | 92.9840(10) | 84.328(3) | 81.947(2) | 90 |
| V/nm3 | 2.47653(19) | 2.46845(19) | 2.4291(4) | 2.4224(3) | 11.2355(7) |
| Z | 1 | 1 | 2 | 2 | 8 |
| Dc/(Mg·m-3) | 1.534 | 1.548 | 1.767 | 1.791 | 1.744 |
| μ/mm-1 | 1.342 | 1.509 | 2.636 | 2.974 | 2.294 |
| F(000) | 1152 | 1156 | 1272 | 1280 | 5840 |
| Crystal size/mm3 | 0.234×0.139× 0.132 | 0.211×0.107× 0.067 | 0.391×0.232× 0.063 | 0.227×0.126× 0.101 | 0.300×0.204× 0.152 |
| θ Range for data collection/(°) | 2.95—25.01 | 2.95—25.01 | 2.97—25.01 | 2.90—25.01 | 2.91—27.55 |
| Limiting indices | -13≤h≤13, -16≤k≤16, -20≤l≤20 | -13≤h≤13, -16≤k≤16, -20≤l≤20 | -15≤h≤15, -16≤k≤16, -17≤l≤17 | -15≤h≤15, -16≤k≤16, -17≤l≤17 | -36≤h≤36, -17≤k≤18, -38≤l≤38 |
| Reflections collected/unique | 44087/8720 [R(int)=0.0650] | 43696/8696 [R(int)=0.0822] | 40881/8573 [R(int)=0.0930] | 39331/8520 [R(int)=0.1195] | 85920/12923 [R(int)=0.0974] |
| Data/restraints/parameters | 8720/108/718 | 8696/127/675 | 8573/0/685 | 8520/0/685 | 12923/0/812 |
| Largest difference peak and hole/(e·nm-3) | 1356 and -883 | 1353 and -779 | 1416 and -1360 | 1527 and -1000 | 1568 and -883 |
| Goodness-of-fit on F2 | 1.045 | 1.082 | 1.066 | 1.069 | 1.043 |
| Final R indices[I>2σ(I)] | R1=0.0447, wR2=0.1190 | R1=0.0522, wR2=0.1210 | R1=0.0370, wR2=0.0906 | R1=0.0462, wR2=0.0996 | R1=0.0415, wR2=0.0996 |
| R indices(all data) | R1=0.0571, wR2=0.1321 | R1=0.0780, wR2=0.1385 | R1=0.0504, wR2=0.1073 | R1=0.0794, wR2=0.1178 | R1=0.0585, wR2=0.1066 |
| CCDC No. | 1587904 | 1587905 | 1587906 | 1587909 | 1587910 |
Table 1 Crystal data and structural refinement for complexes 1—5
| Complex | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Empirical formula | C110H70N8O30Eu2 | C110H70N8O30Tb2 | C55H32N4O15Eu2 | C55H32N4O15Tb2 | C66H38N6O16Eu2 |
| Formula weight | 2287.66 | 2301.58 | 1292.77 | 1306.69 | 1474.94 |
| Crystal system | Triclinic | Triclinic | Triclinic | Triclinic | Monoclinic, |
| Space group | P | P | P | P | C2/c |
| a/nm | 1.15131(5) | 1.14885(5) | 1.31535(12) | 1.28091(10) | 2.84806(10) |
| b/nm | 1.38342(6) | 1.38288(6) | 1.39415(13) | 1.42179(11) | 1.40143(5) |
| c/nm | 1.70498(8) | 1.70231(8) | 1.45396(13) | 1.49624(11) | 2.97567(10) |
| α/(°) | 113.4990(10) | 113.4240(10) | 73.313(2) | 74.132(2) | 90 |
| β/(°) | 93.4370(10) | 93.4800(10) | 72.018(3) | 67.660(2) | 108.9160(10) |
| γ/(°) | 93.1820(10) | 92.9840(10) | 84.328(3) | 81.947(2) | 90 |
| V/nm3 | 2.47653(19) | 2.46845(19) | 2.4291(4) | 2.4224(3) | 11.2355(7) |
| Z | 1 | 1 | 2 | 2 | 8 |
| Dc/(Mg·m-3) | 1.534 | 1.548 | 1.767 | 1.791 | 1.744 |
| μ/mm-1 | 1.342 | 1.509 | 2.636 | 2.974 | 2.294 |
| F(000) | 1152 | 1156 | 1272 | 1280 | 5840 |
| Crystal size/mm3 | 0.234×0.139× 0.132 | 0.211×0.107× 0.067 | 0.391×0.232× 0.063 | 0.227×0.126× 0.101 | 0.300×0.204× 0.152 |
| θ Range for data collection/(°) | 2.95—25.01 | 2.95—25.01 | 2.97—25.01 | 2.90—25.01 | 2.91—27.55 |
| Limiting indices | -13≤h≤13, -16≤k≤16, -20≤l≤20 | -13≤h≤13, -16≤k≤16, -20≤l≤20 | -15≤h≤15, -16≤k≤16, -17≤l≤17 | -15≤h≤15, -16≤k≤16, -17≤l≤17 | -36≤h≤36, -17≤k≤18, -38≤l≤38 |
| Reflections collected/unique | 44087/8720 [R(int)=0.0650] | 43696/8696 [R(int)=0.0822] | 40881/8573 [R(int)=0.0930] | 39331/8520 [R(int)=0.1195] | 85920/12923 [R(int)=0.0974] |
| Data/restraints/parameters | 8720/108/718 | 8696/127/675 | 8573/0/685 | 8520/0/685 | 12923/0/812 |
| Largest difference peak and hole/(e·nm-3) | 1356 and -883 | 1353 and -779 | 1416 and -1360 | 1527 and -1000 | 1568 and -883 |
| Goodness-of-fit on F2 | 1.045 | 1.082 | 1.066 | 1.069 | 1.043 |
| Final R indices[I>2σ(I)] | R1=0.0447, wR2=0.1190 | R1=0.0522, wR2=0.1210 | R1=0.0370, wR2=0.0906 | R1=0.0462, wR2=0.0996 | R1=0.0415, wR2=0.0996 |
| R indices(all data) | R1=0.0571, wR2=0.1321 | R1=0.0780, wR2=0.1385 | R1=0.0504, wR2=0.1073 | R1=0.0794, wR2=0.1178 | R1=0.0585, wR2=0.1066 |
| CCDC No. | 1587904 | 1587905 | 1587906 | 1587909 | 1587910 |
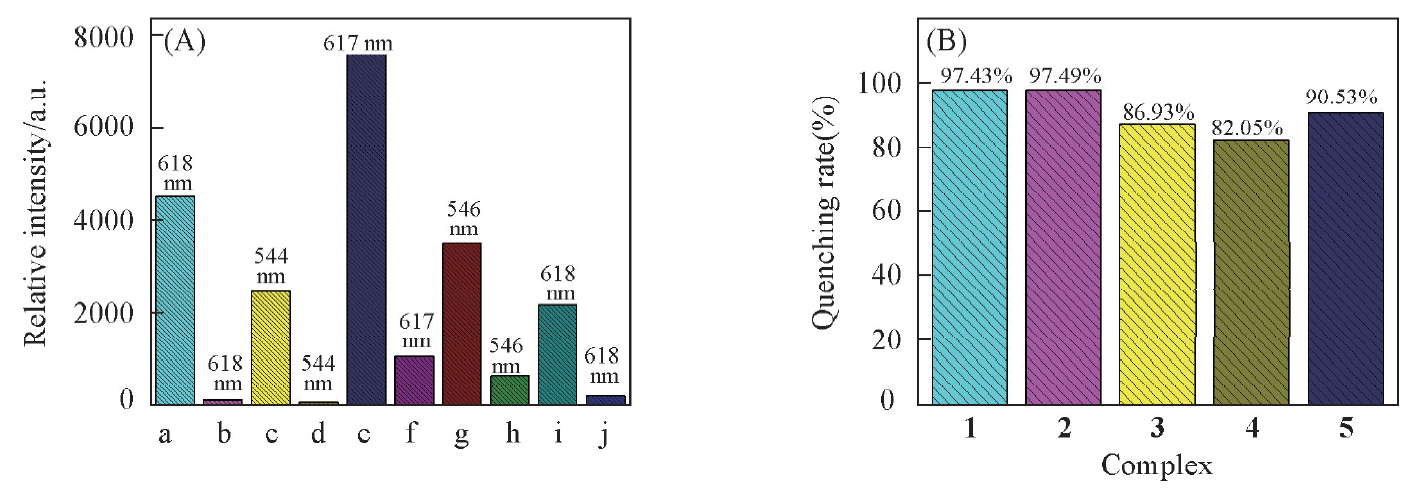
Fig.6 Fluorescence intensity(5D0→7F2 or 5D4→7F5) changes(A) and the quenching rate(B) of complexes 1—5 upon addition of NH3 at room temperaturec(NH3)=10 mmol/L, m(complex)=10 mg, V(NH3)=10 mL. (A) a. complex 1; b. complex 1+NH3; c. complex 2; d. complex 2+NH3; e. complex 3; f. compound 3+NH3; g. complex 4; h. complex 4+NH3; i. complex 5; j. complex 5+NH3.
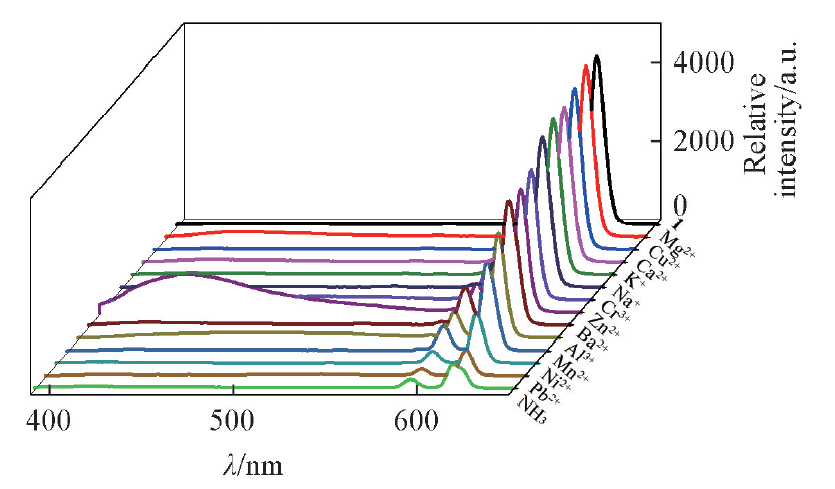
Fig.7 Fluorescence spectra changes of complex 1 upon addition of NH3 and mental ions at room temperaturec(NH3)=c(metal ion)=5 mmol/L, V=10 mL, m(complex 1)=10 mg. λex=368 nm, λem=618 nm.
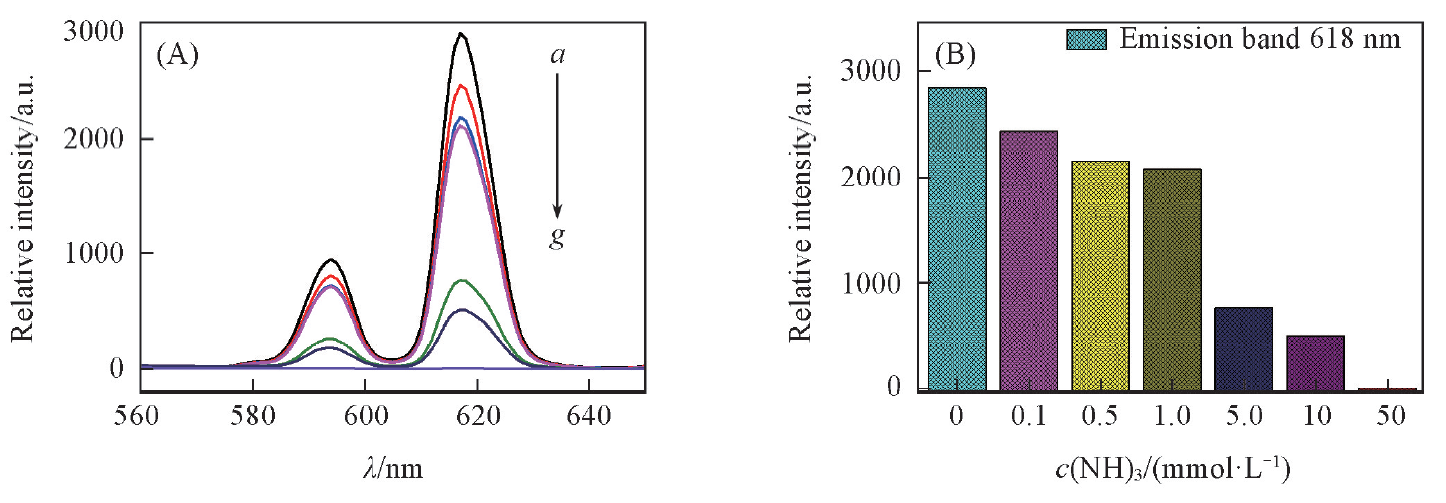
Fig.8 Effect of NH3 on the fluorescence spectra of complex 1(A), histogram of fluorescence intensity and NH3 concentration(B)c(NH3)=0, 0.1, 0.5, 1.0, 5.0, 10, 50 mmol/L(a—g), V(NH3)=10 mL, m(complex 1)=10 mg, T=25 ℃, λex=368 nm, λem=618 nm.
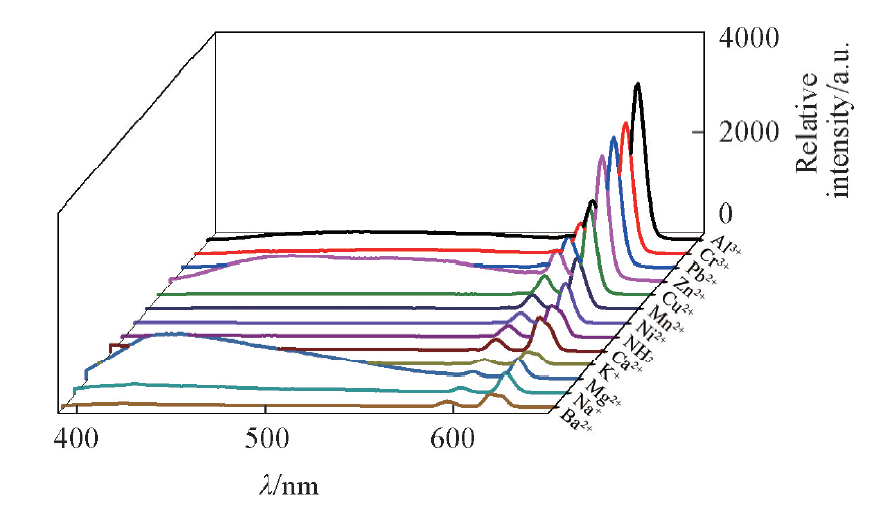
Fig.10 Emission spectra of complex 1-NH3 in the presence of different metal ionsc(NH3)=c(metal ion)=5 mmol/L, V=10 mL, m(complex 1)=10 mg, T=25 ℃, λex=368 nm, λem=618 nm.
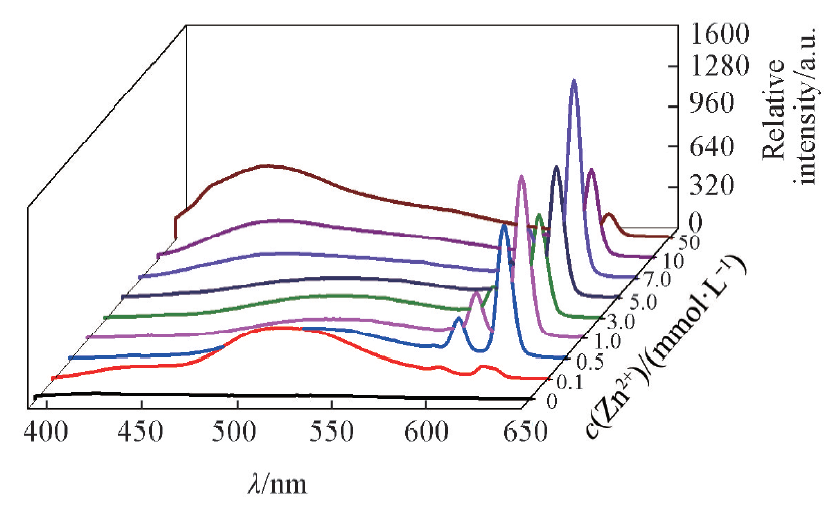
Fig.11 Effect of Zn2+ on the fluorescence spectra of complex 1-NH3c(Zn2+)=0—50 mmol/L, c(NH3)=20 mmol/L, V(Zn2+)=10 mL, m(complex 1)=10 mg, T=25 ℃, λex=368 nm, λem=618 nm.
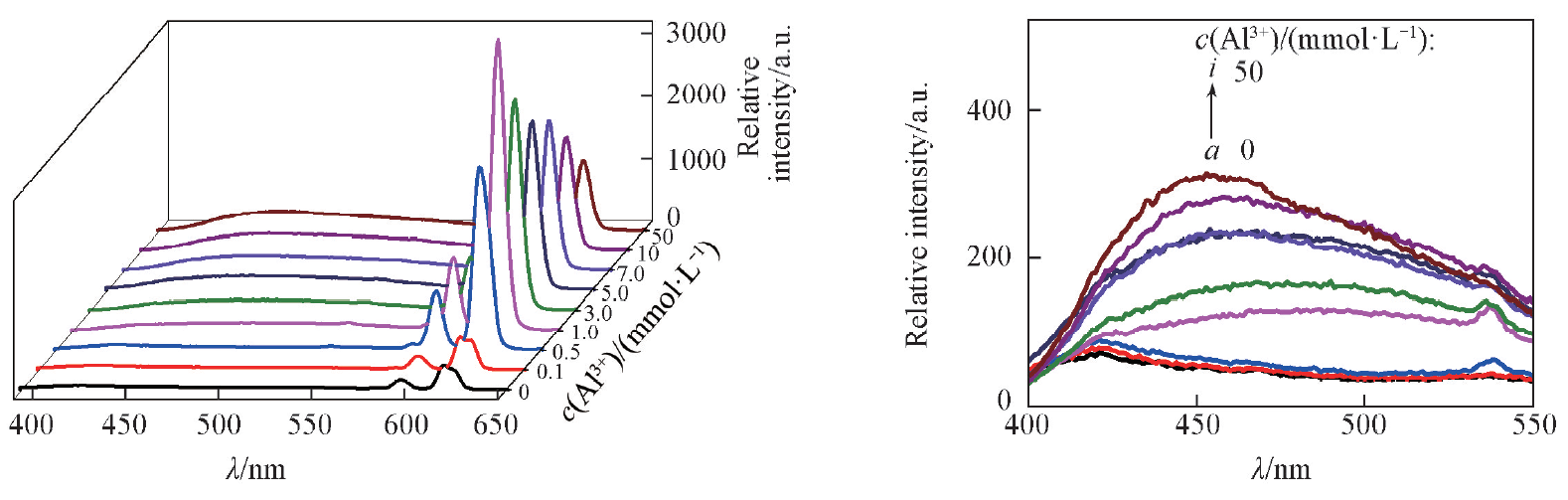
Fig.12 Effect of Al3+ on the fluorescence spectra of complex 1-NH3 c(Al3+)=0—50 mmol/L, c(NH3)=5 mmol/L, V=10 mL, m(complex 1)=10 mg, T=25 ℃, λex=368 nm, λem=618 nm.
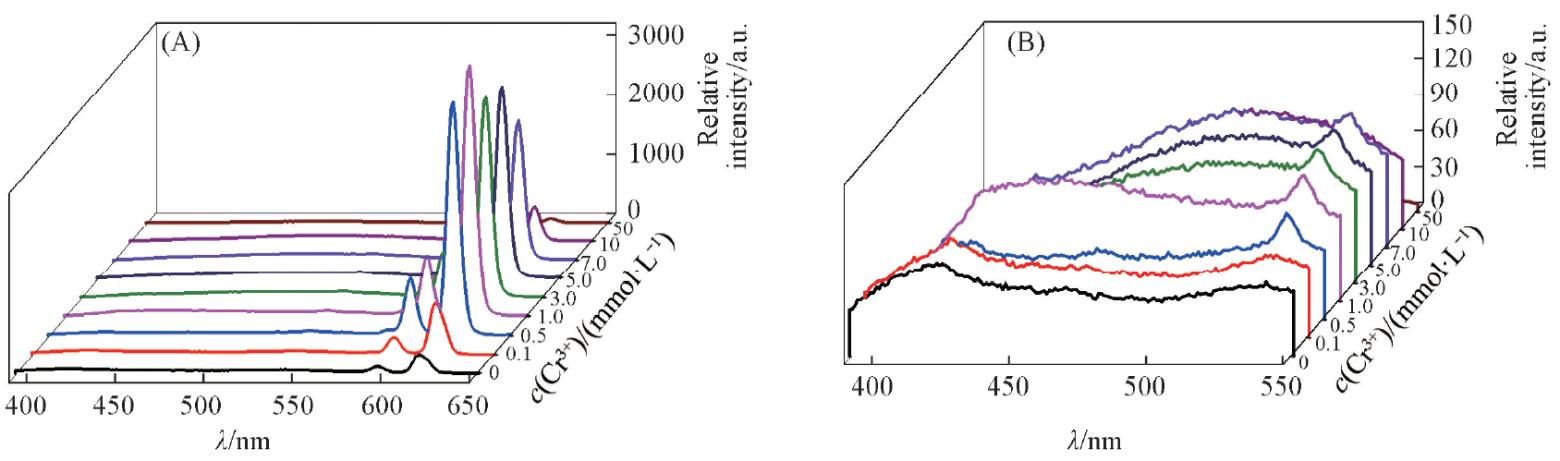
Fig.13 Effect of Cr3+ on the fluorescence spectra of complex 1-NH3c(Cr3+)=0—50 mmol/L, c(NH3)=5 mmol/L, V=10 mL, m(complex 1)=10 mg, T=25 ℃, λex=368 nm, λem=618 nm.
| [1] | Rush C.M., Lyda S.D., Mycopathologia,1982, 79, 147—152 |
| [2] | Li R.J., Liang J.X., Lv B.W., Mo J.Q., Zhao D.M., Contemp.Med.,2014, 20(15), 41—42 |
| (李荣杰, 梁俊雄, 吕博文, 莫俊强, 赵德明. 当代医学, 2014, 20(15), 41—42) | |
| [3] | Li X.G., Yu Y.G., Wang L.Y., Guo X., Wang L.M., Int. J. Lab. Med.,2012, 33(11), 1340—1342 |
| (李晓光, 于永光, 王丽艳, 郭欣, 王连明. 国际检验医学杂志, 2012, 33(11), 1340—1342) | |
| [4] | Yang Y.L., Chin. J. Med., 2006, 41(3), 18—21 |
| (杨艳玲. 中国医刊, 2006, 41#(3), 18—21) | |
| [5] | Sarıoğlan A., Durak-Çetin Y., Okutan H., Akgün F., Chem. Eng. Sci.,2017, 171, 440—450 |
| [6] | Zhang Y., Luo X.G., He K., Huo D.Q., Liu J., Liu P., Shi X.J., Hou C.J., Water, Air, & Soil Poll.,2012, 223, 2969—2977 |
| [7] | Luo X.G., Liu P., Hou C.J., Huo D.Q., Dong J.L., Fa H.B., Yang M., Rev. Sci. Instrum.,2010, 81, 105—113 |
| [8] | Ling T.L., Ahmad M., Yook H.L., Anal. Methods,2013, 5 , 6709—6714 |
| [9] | Shingaya Y., Kubo H., Ito M., Surf. Sci.,1999, 427/428, 173—178 |
| [10] | Oudenhoven J. F.M., Knoben W., van Schaijk R., Proced. Eng.,2015, 120, 983—986 |
| [11] | Katz M.J., Ramnial T., Yu H.Z., Leznoff D.B., J. Am. Chem. Soc.,2008, 130, 10662—10673 |
| [12] | Kim S.J., Hwang I.S., Kang Y.C., Lee J.H., Sensors,2011, 11, 10603—10614 |
| [13] | Shen C.Y., Huang H.C., Hwang R.C., Sensor. Actuat. A: Physical,2008, 147, 464—469 |
| [14] | Chen W.H., Chen S.M., Hung C.I., Sci. Total Environ.,2013, 444, 336—346 |
| [15] | Alvi M.A., Al-Ghamdi A.A., Khan S.A., Appl. Phys. A Mater.,2017, 123(3), 1—5 |
| [16] | Huang W., Besar K., LeCover R, Rule A. M., Breysse P.N., Katz H.E., J. Am. Chem. Soc.,2012, 134, 14650—14653 |
| [17] | Sun Y.Q., Liu Q., Zhou L.L., Chen Y.P., Cryst. Eng. Comm.,2014, 16(19), 3986—3993 |
| [18] | Wang L., Zhu X., Tang X., Wu C., Zhou Z., Sun C., Deng S.L., Ai H., Gao J., Chem. Commun.,2015, 51, 4390—4393 |
| [19] | Edelmann F.T., Chem. Soc. Rev., 2012, 41, 7657—7672 |
| [20] | Yan L., Ye Z., Peng C., Zhang S., Tetrahedron,2012, 68, 2725—2727 |
| [21] | Liu D., Lang J.P., Abrahams B.F., J. Am. Chem. Soc.,2011, 133(29), 11042—11045 |
| [22] | Liu Z., He W., Guo Z., Chem. Soc. Rev.,2013, 42, 1568—1600 |
| [23] | Zhao H.Q., Yang S.P., Ding N.N., Qin L., Qiu G.H., Chen J.X., Zhang W.H., Chen W.H., Hor T. S.A., Dalton Trans.,2016, 45, 5092—5100 |
| [24] | Qin J.S., Du D. Y., Li W. L., Zhang J. P., Li S. L., Su Z. M., Wang X. L., Xu Q., Shao K. Z., Lan Y. Q., Chem. Sci., 2012, 3, 2114—2118 |
| [25] | Wang H.N., Meng X., Yang G.S., Wang X.L., Shao K.Z., Su Z.M., Wang C.G., Chem. Commun.,2011, 47, 7128—7130 |
| [26] | Zhang D.C., Ma D., Li X., J. Coord. Chem.,2017, 70(18), 3233—3251 |
| [27] | Zhang D.C., Zhou X., Li X., Spectrosc. Spect. Anal.,2016, 36(9), 2841—2845 |
| (张德春, 周鑫, 李夏. 光谱学与光谱分析, 2016, 36(9), 2841—2845) | |
| [28] | Sun Y.Q., Wan F., Li X.X., Lin J., Wu T., Zheng S.T., Bu X., Chem. Commun.,2016, 52, 10125—10128 |
| [29] | Dong G.Y., Li R., Fan T.T., Li J.J., Li X., Chem. J. Chinese Universities,2016, 37(8), 1421—1429 |
| (董高云, 李睿, 樊婷婷, 李佳佳, 李夏. 高等学校化学学报, 2016, 37(8), 1421—1429) | |
| [30] | Chen H., Zhao X., Gao H., Zhu H.D., Jiang L.H., Ling Q.D., Chem. J. Chinese Universities,2015, 36(1), 41—47 |
| (陈鸿, 赵璇, 高慧, 朱海娣, 姜丽红, 凌启淡. 高等学校化学学报, 2015, 36(1), 41—47) | |
| [31] | Sheldrick G.M., SHELXS-97, Program for Crystal Structure Refinement, University of Götingen,Götingen, 1997 |
| [32] | Sheldrick G.M., SHELXL-97, Program for Crystal Straeture Solution, University of Göttingen, Götingen, 1997 |
| [1] | WANG Junyang, LIU Zheng, ZHANG Qian, SUN Chunyan, LI Hongxia. Application of DNA Silver Nanoclusters in the Fluorescence Biosensors based on Functional Nucleic Acids [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220010. |
| [2] | LU Cong, LI Zhenhua, LIU Jinlu, HUA Jia, LI Guanghua, SHI Zhan, FENG Shouhua. Synthesis, Structure and Fluorescence Detection Properties of a New Lanthanide Metal-Organic Framework Material [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220037. |
| [3] | LI Qiao, ZHAO Yang, WANG Enju. Moisture Absorption Reaction and Fluorescence Property of Highly Active Michael System Based on Arylidenemalononitrile [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210690. |
| [4] | LI Hua, YANG Ke, HUANG Junfeng, CHEN Fengjuan. Design and Construction of UiO-66-NH2/wood Composite for Efficient Removal of Trace Heavy Metal Ions from Water [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210701. |
| [5] | TIAN Xueqin, MO Zheng, DING Xin, WU Pengyan, WANG Yu, WANG Jian. A Squaramide-containing Luminescent Metal-organic Framework as a High Selective Sensor for Histidine [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210589. |
| [6] | WU Zexin, ZHU Yuanjie, WANG Hongzhong, WANG Junan, HE Ying. Methyl-modified Carbazole/Diphenyl Sulfone-based AIE-TADF Blue Emitter and Its OLEDs [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220371. |
| [7] | LIU Miao, LIU Ruibo, LIU Badi, QIAN Ying. Synthesis, Two-photon Fluorescence Imaging and Photodynamic Therapy of Lysosome-targeted Indole-BODIPY Photosensitizer [J]. Chem. J. Chinese Universities, 2022, 43(10): 20220326. |
| [8] | MA Jianxin, LIU Xiaodong, XU Na, LIU Guocheng, WANG Xiuli. A Multi-functional Zn(II) Coordination Polymer with Luminescence Sensing, Amperometric Sensing, and Dye Adsorption Performance [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210585. |
| [9] | WU Ji, ZHANG Hao, LUO Yuhui, GENG Wuyue, LAN Yaqian. A Microporous Cationic Ga(III)-MOF with Fluorescence Properties for Selective sensing Fe3+ Ion and Nitroaromatic Compounds [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210617. |
| [10] | HAN Zongsu, YU Xiaoyong, MIN Hui, SHI Wei, CHENG Peng. A Rare Earth Metal-Organic Framework with H6TTAB Ligand [J]. Chem. J. Chinese Universities, 2022, 43(1): 20210342. |
| [11] | LI Ran, ZHANG Xudong, MU Lidan, SUN Tong, AI Ganggang, SHA Yelong, ZHANG Yuqi, WANG Jijiang. Preparation and Application of Triplethiophene Derivative Functionalized SiO2 Inverse Opal Photonic Crystal Fluorescent Films [J]. Chem. J. Chinese Universities, 2021, 42(9): 2989. |
| [12] | YUAN Chunling, YAO Xiaotiao, XU Yuanjin, QIN Xiu, SHI Rui, CHENG Shiqi, WANG Yilin. Colorimetry/Ratio Fluorimetry Determination of Glucose with Bifunctional Carbon Dots [J]. Chem. J. Chinese Universities, 2021, 42(8): 2428. |
| [13] | ZHOU Jieqiong, HUANG Yan, ZHANG Zhiling, PANG Daiwen, TIAN Zhiquan. Water-soluble Ag2Te Quantum Dots with Emission in the Second Near-infrared Window [J]. Chem. J. Chinese Universities, 2021, 42(6): 2072. |
| [14] | ZHAO Kaiqing, WU Ruoyu, LUO Yifeng, SHI Chunhong, HU Jun. Construction of Active Sites in Porous Organic Polymers for Various Heavy Metal Ions Capture [J]. Chem. J. Chinese Universities, 2021, 42(3): 834. |
| [15] | CHEN Hongda, ZHANG Hua, WANG Zhenxin. Development of Small Animals in vivo Fluorescence-photothermal Dual Mode Imaging System [J]. Chem. J. Chinese Universities, 2021, 42(3): 725. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||