

Chem. J. Chinese Universities ›› 2018, Vol. 39 ›› Issue (4): 735.doi: 10.7503/cjcu20170591
• Physical Chemistry • Previous Articles Next Articles
ZHOU Lie1,2, WU Qingyun2, XU Ying2,*( ), WANG Chenguang2, MA Longlong2, LI Wenzhi1, CHEN Peili2
), WANG Chenguang2, MA Longlong2, LI Wenzhi1, CHEN Peili2
Received:2017-08-31
Online:2018-04-10
Published:2018-03-27
Contact:
XU Ying
E-mail:xuying@ms.giec.ac.cn
Supported by:CLC Number:
TrendMD:
ZHOU Lie, WU Qingyun, XU Ying, WANG Chenguang, MA Longlong, LI Wenzhi, CHEN Peili. Depolymerization of the Alkali Lignin for Aromatic Compounds over Ni/SiO2-Al2O3 Solid Acid Catalysts†[J]. Chem. J. Chinese Universities, 2018, 39(4): 735.
| Catalyst | Acid content/(μmol·g-1) | Catalyst | Acid content/(μmol·g-1) |
|---|---|---|---|
| SiO2-Al2O3 | 628.54 | 15%Ni/SiO2-Al2O3 | 756.17 |
| 5%Ni/SiO2-Al2O3 | 673.95 | 20%Ni/SiO2-Al2O3 | 783.20 |
| 10%Ni/SiO2-Al2O3 | 693.08 |
Table 1 Results of catalysts acidity obtained by NH3-TPD
| Catalyst | Acid content/(μmol·g-1) | Catalyst | Acid content/(μmol·g-1) |
|---|---|---|---|
| SiO2-Al2O3 | 628.54 | 15%Ni/SiO2-Al2O3 | 756.17 |
| 5%Ni/SiO2-Al2O3 | 673.95 | 20%Ni/SiO2-Al2O3 | 783.20 |
| 10%Ni/SiO2-Al2O3 | 693.08 |
| Catalyst | SBET /(m2·g-1) | Vp/(cm3·g-1) | Dp/nm |
|---|---|---|---|
| SiO2-Al2O3 | 459.44 | 0.637 | 4.346 |
| 5%Ni/SiO2-Al2O3 | 432.85 | 0.628 | 4.319 |
| 10%Ni/SiO2-Al2O3 | 402.88 | 0.561 | 3.847 |
| 15%Ni/SiO2-Al2O3 | 390.14 | 0.468 | 3.424 |
| 20%Ni/SiO2-Al2O3 | 353.01 | 0.334 | 3.421 |
Table 2 Textural properties of different catalysts*
| Catalyst | SBET /(m2·g-1) | Vp/(cm3·g-1) | Dp/nm |
|---|---|---|---|
| SiO2-Al2O3 | 459.44 | 0.637 | 4.346 |
| 5%Ni/SiO2-Al2O3 | 432.85 | 0.628 | 4.319 |
| 10%Ni/SiO2-Al2O3 | 402.88 | 0.561 | 3.847 |
| 15%Ni/SiO2-Al2O3 | 390.14 | 0.468 | 3.424 |
| 20%Ni/SiO2-Al2O3 | 353.01 | 0.334 | 3.421 |
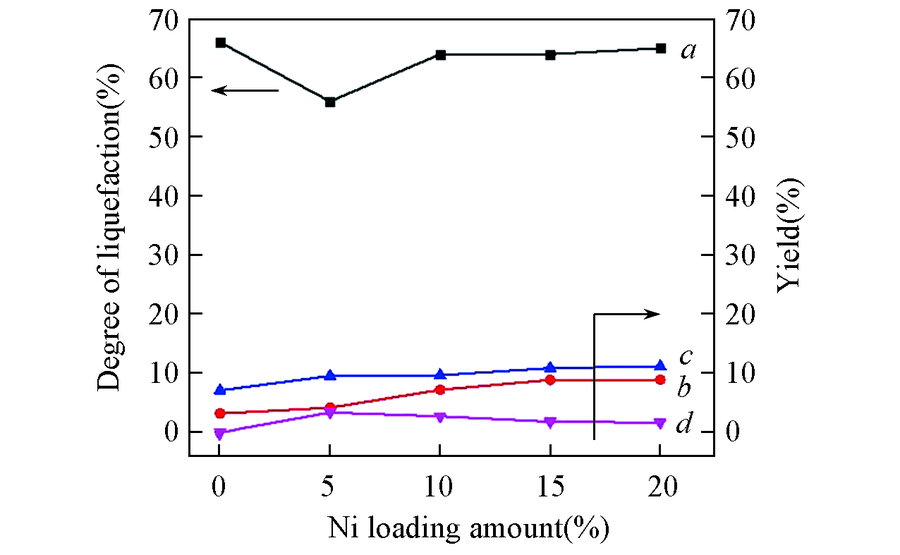
Fig.2 Liquefaction and depolymerized products over different catalystsa. Degree of liquefaction; b. yield of arenes; c. yield of phenols; d. yield of benzene-alcohol. Condition: 0.5 g of alkali lignin, 0.2 g of catalysts, 40 mL of ethanol, 4 h, 280 ℃, 2 MPa H2.
| Product | Yield of product(%) | ||
|---|---|---|---|
| SiO2-Al2O3 | 5%Ni/SiO2-Al2O3 | 20%Ni/SiO2-Al2O3 | |
| Arenes | 3.17 | 4.18 | 8.91 |
| Toluene | - | 0.04 | 0.03 |
| Ethylbenzene | - | 0.18 | - |
| p-Xylene | 0.01 | 0.01 | 0.08 |
| o-Xylene | 2.26 | 2.82 | 5.96 |
| Benzene, 1-ethyl-2-methyl- | 0.02 | 0.05 | 0.09 |
| Benzene, 1,2,3-trimethyl- | 0.09 | 0.13 | 0.34 |
| Benzene, 1-ethyl-3,5-dimethyl- | 0.05 | 0.08 | 0.20 |
| Benzene, 1-methyl-3-(1-methylethyl)- | 0.06 | 0.04 | 0.13 |
| Benzene, 2-ethyl-1,4-dimethyl- | 0.36 | 0.40 | 1.01 |
| Benzene, 1,3-diethyl-5-methyl- | 0.02 | 0.01 | 0.09 |
| Benzene, 2,4-diethyl-1-methyl- | 0.08 | 0.12 | 0.23 |
| Benzene, 1-ethyl-2,4,5-trimethyl- | 0.03 | 0.05 | - |
| Benzene, 1,3,5-triethyl- | 0.02 | 0.02 | 0.06 |
| Benzene, 1,2-diethyl-3,4-dimethyl- | 0.12 | 0.08 | 0.39 |
| Naphthalene, 1-methyl- | 0.03 | 0.04 | 0.11 |
| Naphthalene, 2,7-dimethyl- | 0.01 | 0.02 | 0.06 |
| Naphthalene, 1,4-dimethyl- | 0.01 | 0.01 | 0.04 |
| Naphthalene, 2-(1-methylethyl)- | - | 0.01 | - |
| Phenanthrene, 9-ethyl- | 0.02 | 0.07 | 0.09 |
| Phenolic compound | 7.08 | 9.55 | 14.92 |
| 6-Methyl-4-indanol | 3.28 | 2.45 | 6.02 |
| Phenol, 2-ethyl-5-methyl- | 0.21 | 0.46 | 0.42 |
| Phenol, 2-ethyl- | 0.28 | 0.19 | 0.26 |
| Phenol, 2-ethyl-4,5-dimethyl- | 0.25 | 2.12 | 1.26 |
| Propofol | 0.16 | 0.66 | 0.26 |
| Phenol, 2-ethyl-4-methyl- | 0.46 | 0.71 | 0.70 |
| Phenol, 4-ethyl- | 0.76 | 1.70 | - |
| Phenol, 2-methyl-5-(1-methylethyl)- | - | 0.27 | 0.27 |
| Product | Yield of product(%) | ||
| SiO2-Al2O3 | 5%Ni/SiO2-Al2O3 | 20%Ni/SiO2-Al2O3 | |
| Phenol, 3,5-bis(1,1-dimethylethyl)- | - | 0.17 | 0.21 |
| Phenol, 2-ethyl-4,5-dimethyl- | 0.22 | 0.17 | 0.21 |
| Phenol, 3,5-diethyl- | 0.22 | 0.60 | 0.57 |
| Ethanone, 1-(2-hydroxy-5-methylphenyl)- | 0.47 | - | 0.89 |
| Phenol, 2,4-bis(1-methylethyl)- | 0.46 | - | - |
| Benzyl alcohol compound | - | 3.37 | 1.67 |
| Benzenemethanol, 4-(1,1-dimethylethyl)- | - | 3.37 | 1.67 |
Table 3 Analysis of depolymerized products over different catalysts
| Product | Yield of product(%) | ||
|---|---|---|---|
| SiO2-Al2O3 | 5%Ni/SiO2-Al2O3 | 20%Ni/SiO2-Al2O3 | |
| Arenes | 3.17 | 4.18 | 8.91 |
| Toluene | - | 0.04 | 0.03 |
| Ethylbenzene | - | 0.18 | - |
| p-Xylene | 0.01 | 0.01 | 0.08 |
| o-Xylene | 2.26 | 2.82 | 5.96 |
| Benzene, 1-ethyl-2-methyl- | 0.02 | 0.05 | 0.09 |
| Benzene, 1,2,3-trimethyl- | 0.09 | 0.13 | 0.34 |
| Benzene, 1-ethyl-3,5-dimethyl- | 0.05 | 0.08 | 0.20 |
| Benzene, 1-methyl-3-(1-methylethyl)- | 0.06 | 0.04 | 0.13 |
| Benzene, 2-ethyl-1,4-dimethyl- | 0.36 | 0.40 | 1.01 |
| Benzene, 1,3-diethyl-5-methyl- | 0.02 | 0.01 | 0.09 |
| Benzene, 2,4-diethyl-1-methyl- | 0.08 | 0.12 | 0.23 |
| Benzene, 1-ethyl-2,4,5-trimethyl- | 0.03 | 0.05 | - |
| Benzene, 1,3,5-triethyl- | 0.02 | 0.02 | 0.06 |
| Benzene, 1,2-diethyl-3,4-dimethyl- | 0.12 | 0.08 | 0.39 |
| Naphthalene, 1-methyl- | 0.03 | 0.04 | 0.11 |
| Naphthalene, 2,7-dimethyl- | 0.01 | 0.02 | 0.06 |
| Naphthalene, 1,4-dimethyl- | 0.01 | 0.01 | 0.04 |
| Naphthalene, 2-(1-methylethyl)- | - | 0.01 | - |
| Phenanthrene, 9-ethyl- | 0.02 | 0.07 | 0.09 |
| Phenolic compound | 7.08 | 9.55 | 14.92 |
| 6-Methyl-4-indanol | 3.28 | 2.45 | 6.02 |
| Phenol, 2-ethyl-5-methyl- | 0.21 | 0.46 | 0.42 |
| Phenol, 2-ethyl- | 0.28 | 0.19 | 0.26 |
| Phenol, 2-ethyl-4,5-dimethyl- | 0.25 | 2.12 | 1.26 |
| Propofol | 0.16 | 0.66 | 0.26 |
| Phenol, 2-ethyl-4-methyl- | 0.46 | 0.71 | 0.70 |
| Phenol, 4-ethyl- | 0.76 | 1.70 | - |
| Phenol, 2-methyl-5-(1-methylethyl)- | - | 0.27 | 0.27 |
| Product | Yield of product(%) | ||
| SiO2-Al2O3 | 5%Ni/SiO2-Al2O3 | 20%Ni/SiO2-Al2O3 | |
| Phenol, 3,5-bis(1,1-dimethylethyl)- | - | 0.17 | 0.21 |
| Phenol, 2-ethyl-4,5-dimethyl- | 0.22 | 0.17 | 0.21 |
| Phenol, 3,5-diethyl- | 0.22 | 0.60 | 0.57 |
| Ethanone, 1-(2-hydroxy-5-methylphenyl)- | 0.47 | - | 0.89 |
| Phenol, 2,4-bis(1-methylethyl)- | 0.46 | - | - |
| Benzyl alcohol compound | - | 3.37 | 1.67 |
| Benzenemethanol, 4-(1,1-dimethylethyl)- | - | 3.37 | 1.67 |
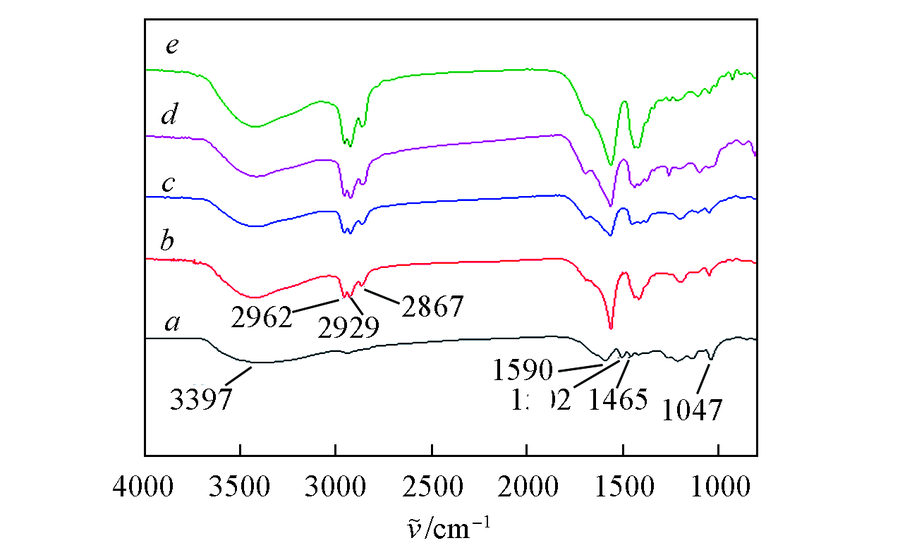
Fig.3 FTIR spectra of alkali lignin and depolymerized oligomersa. Alkali lignin; b. oligomers of 5%Ni/SiO2-Al2O3; c. oligomers of 10%Ni/SiO2-Al2O3; d. oligomers of 15%Ni/SiO2-Al2O3; e. oligomers of 20%Ni/SiO2-Al2O3.
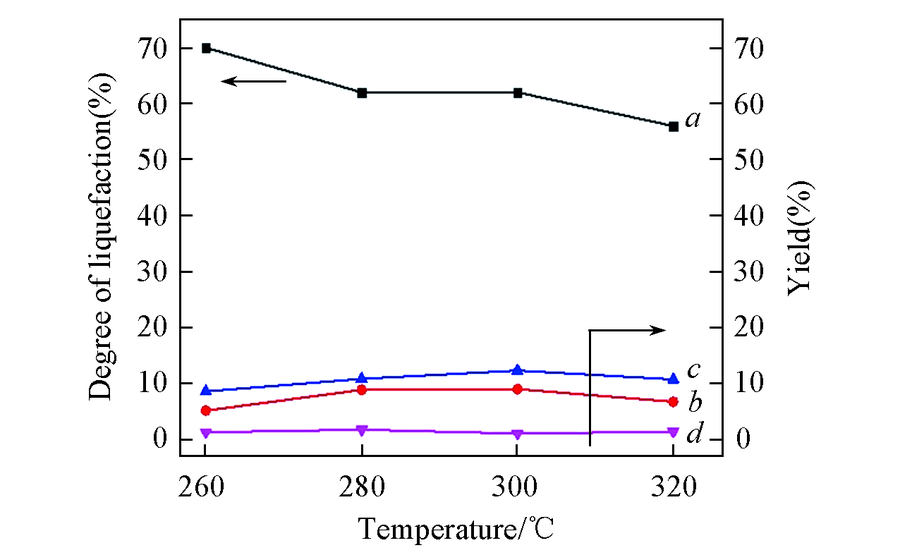
Fig.4 Liquefaction and depolymerized products at different temperaturesa. Degree of liquefaction; b. yield of arenes; c. yield of phenols; d. yield of benzene-alcohol. Condition: 0.5 g of alkali lignin, 0.2 g of catalysts, 40 mL of ethanol, 4 h, 2 MPa H2.
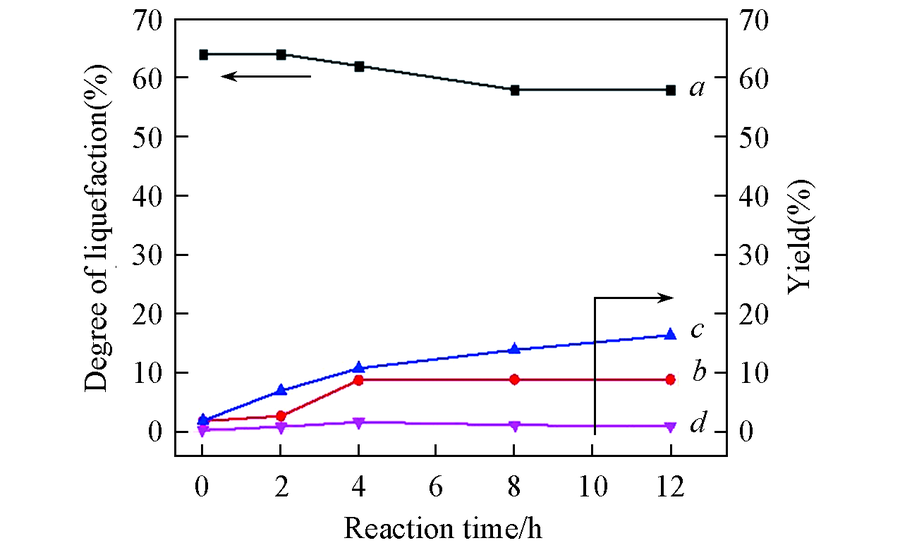
Fig.5 Liquefaction and depolymerized products with different reaction timea. Degree of liquefaction; b. yield of arenes; c. yield of phenols; d. yield of benzene-alcohol. Condition: 0.5 g of alkali lignin, 0.2 g of catalysts, 40 mL of ethanol, 300 ℃, 2 MPa H2.
| [1] | Shi N., Liu Q. Y., Zhang Q., Wang T. J., Ma L. L., Green Chem., 2013, 15, 1967-1974 |
| [2] | Yan L.F., Green Chemistry, University of Science and Technology of China Press, Hefei, 2007 |
| (闫立峰. 绿色化学. 合肥: 中国科学技术大学出版社, 2007) | |
| [3] | Liao Y. H., Liu Q. Y., Wang T. J., Long J. X., Ma L. L., Zhang Q., Green Chem., 2014, 16, 3305-3312 |
| [4] | Yang D., Wu X., Qiu X., Chang Y., Lou H., Bioresour. Technol., 2014, 155, 418-421 |
| [5] | Zhao Y., Xu Q., Pan T., Zuo Y., Y Fu., Guo Q. X., Appl. Catal. A: Gen., 2013, 467, 504-508 |
| [6] | Qin Y., Yang D., Guo W., Qiu X., Journal of Industrial and Engineering Chemistry, 2015, 27, 192-200 |
| [7] | Wang L., Geng J., Li L. F., Chang J. M., Chem. J. Chinese Universities, 2016, 37(4), 736-744 |
| (王亮, 耿晶, 李露菲, 常建民. 高等学校化学学报, 2016, 37(4), 736-744) | |
| [8] | Chao W., Yang X. M., Zhao Y., Zhu Y. C., Wang Z. C., Chem. J. Chinese Universities, 2017, 38(2), 312-317 |
| (晁威, 杨晓敏, 周玉, 朱燕超, 王子忱. 高等学校化学学报, 2017, 38(2), 312-317) | |
| [9] | Li X. F., Zuo Y., Zhang Y., Fu Y., Guo Q. X., Fuel,2013, 113, 435-422 |
| [10] | Wang J., Qian Y., Deng Y., Liu D., Li H., Qiu X., Appl. Surf. Sci., 2016, 390, 617-622 |
| [11] | Shao Y., Xia Q. E., Dong L., Liu X. H., Han X., Stewart F. P., Cheng Y. Q., Luke L. D., Anibal J. Ramirez-Cuesta, Yang S. H., Wang Y. Q., Nature Commun., 2017, 8, 16104-16112 |
| [12] | Yoshikawa T., Shinohara S., Yagi T., Ryumon N., Nakasaka Y., Tago T., Masuda T., Appl. Catal. B: Environ., 2014, 146, 289-297 |
| [13] | Ma Z., Troussard E., van Bokhoven J. A., Appl. Catal. A: Gen., 2012, 423/424, 130-136 |
| [14] | Li S., Masoud T. A., Ydna Q. S., Jeremy S. L., Science,2016, 9, 329-333 |
| [15] | Deepa A. K., Dhepe P. L., ACS Catalysis, 2015, 5, 365-379 |
| [16] | Liu Y., Chen L. G., Wang T. J., Zhang Q., Yan J. Y., Ma L. L., ACS Sustainable Chemistry & Engineering,2015, 3(8), 1745-1755 |
| [17] | Liu H., Jiang T., Han B., Liang S., Zhou Y., Science,2009, 326, 1250-1252 |
| [18] | Chia M., Dumesic J. A., Chem. Commun. (Camb), 2011, 47, 12233-12235 |
| [19] | Chen P. R., Zhang Q., Shu R. Y., Xu Y., Ma L. L., Wang T. J., Bioresour. Technol., 2017, 226, 125-131 |
| [20] | Singh S. K., Ekhe J. D., RSC Advances, 2014, 4, 27971-27978 |
| [21] | Custodis V. B., Karakoulia S. A., Triantafyllidis K. S., van Bokhoven J. A., ChemSusChem,2016, 9, 1134-1145 |
| [22] | Wu Q. Y., M L. L., Long J. X., Shu R. Y., Zhang Q., Wang T. J., Xu Y., Chinese J. Chem. Phys., 2016, 8, 474-480 |
| [23] | Zhang X. H., Zhang Q., Wang T. J., Ma L. L., Yu Y., Chen L., Bioresour Technol., 2013, 134, 73-80 |
| [24] | Song Q., Wang F., Cai J., Wang Y., Zhang J., Yu W., Xu J., Energy & Environmental Science, 2013, 6, 994-1007 |
| [25] | Toledano A., Serrano L., Balu A. M., Luque R., Pineda A., Labidi J., ChemSusChem,2013, 6, 529-536 |
| [26] | Zhang X. H., Zhang Q., Wang T. J., Ma L. L., Yu Y. X., Chen L. G., Bioresour. Technol., 2013, 134, 73-80 |
| [27] | Zhang J., Teo J., Chen X., Asakura H., Tanaka T., Teramura K., Yan N., ACS Catalysis, 2014, 4, 1574-1583 |
| [28] | Song Q., Wang F., Xu J., Chem. Commun. (Camb), 2012, 48, 7019-7021 |
| [29] | Upare D. P., Yoon S., Lee C. W., J. Porous Mater., 2013, 20, 1129-1136 |
| [30] | Laskar D. D., Tucker M. P., Chen X., Helms G. L., Yang B., Green Chemistry 2014, 16, 897-910 |
| [31] | Wang X., Rinaldi R., ChemSusChem,2012, 5, 1455-1466 |
| [32] | Shu R. Y., Xu Y., Ma L. L., Zhang Q., Wang T. J., Chen P. R., Wu Q., RSC Adv., 2016, 6, 88788-88796 |
| [33] | Pandey M. P., Kim C. S., Chemical Engineering & Technology, 2011, 34, 29-41 |
| [1] | CHEN Xiangyun, ZHU Benqiang, YUAN Bing, YU Fengli, XIE Congxia, YU Shitao. Hydrogenation of α-Pinene Catalyzed by Ru Nanoparticles Stabilized by Magnetic Alkali Lignin Amine [J]. Chem. J. Chinese Universities, 2020, 41(8): 1826. |
| [2] | ZHANG Rongbin, TONG Sai, YANG Jinmei, TANG Xiannong, HUANG Chuanqing, WANG Xuewen, FENG Gang, CAI Jianxin. Graphene Supported Nickel Catalyst for Methanation of Carbon Dioxide† [J]. Chem. J. Chinese Universities, 2017, 38(12): 2255. |
| [3] | KONG Danni, JIANG Tao, ZHANG Yiying, CAO Fahai. Catalytic Performance of Activated Carbon Supported Pt-Ni Bimetallic Catalyst for Glycerol in situ Hydrogenolysis† [J]. Chem. J. Chinese Universities, 2016, 37(6): 1140. |
| [4] | WANG Wenliang, GENG Jing, LI Lufei, CHANG Jianmin. Catalytic Properties of Fast Pyrolysis Char Loaded with Cu-Zn on Alkali Lignin Pyrolysis for Monophenols† [J]. Chem. J. Chinese Universities, 2016, 37(4): 736. |
| [5] | YANG Jun , WANG Xue-Guang*, LI Lin, SHEN Kui, LU Xiong-Gang, DING Wei-Zhong*. Catalytic Conversion of 1-Methylnaphthalene as Tar Model Compound from Hot Coke Oven Gas over Ni/MgO/Al2O3 Catalysts [J]. Chem. J. Chinese Universities, 2010, 31(9): 1841. |
| [6] | JING Xiao-Yan*, LU Yi, SONG Da-Lei, ZHANG Mi-Lin. Reduction of Aromatics on the Surface of Mg-Li Alloy [J]. Chem. J. Chinese Universities, 2009, 30(2): 289. |
| [7] | WEI Jun-Mei, XU Bo-Qing, SUN Ke-Qiang, LI Jin-Lu, ZHU Qi-Ming . CO2 Reforming of CH4 over Ni Supported on Nano-ZrO2(Ⅱ)── Effect of Catalyst Composition and Reaction Conditions on Catalytic Reactivity [J]. Chem. J. Chinese Universities, 2002, 23(11): 2106. |
| [8] | WEI Jun-Mei, XU Bo-Qing, LI Jin-Lu, CHENG Zhen-Xing, WANG Ya-Quan, ZHU Qi-Ming . CO2 Reforming of CH4 over Ni Supported on Nano-ZrO2(Ⅰ) Comparison with Conventional Oxide Supported Nickel [J]. Chem. J. Chinese Universities, 2002, 23(1): 92. |
| [9] | ZHENG Fu-Ping, OU Yu-Xiang, CHEN Jiang-Tao, ZHOU Zhi-Ming, CHEN Bo-Ren, YE Lin, ZHAO Dao-Hui. The Preparation and Characterization of Nanosized Pd(OH)2and Its Application in the Catalytic Hydrogenolysis of HBIW [J]. Chem. J. Chinese Universities, 1999, 20(6): 843. |
| [10] | LANG Pei-Zhen, LU Guang-Hua . QSAR Study for the Toxicity of Nitroaromatics to the Fathead Minnow [J]. Chem. J. Chinese Universities, 1995, 16(7): 1083. |
| [11] | Ma Li, Wang Qi . The Technique of Temperature Programmed Continuous Flow Reactor and Its Application [J]. Chem. J. Chinese Universities, 1989, 10(3): 271. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||