

Chem. J. Chinese Universities ›› 2018, Vol. 39 ›› Issue (1): 148.doi: 10.7503/cjcu20170198
• Physical Chemistry • Previous Articles Next Articles
LUO Man, JIANG Wenquan*( ), HAN Xue, GUO Ronggui, LI Tao, YU Limin
), HAN Xue, GUO Ronggui, LI Tao, YU Limin
Received:2017-03-31
Online:2018-01-10
Published:2017-12-19
Contact:
JIANG Wenquan
E-mail:jiangwenquan@grinm.com
CLC Number:
TrendMD:
LUO Man, JIANG Wenquan, HAN Xue, GUO Ronggui, LI Tao, YU Limin. Synthesis and Characterization of Full Concentration-gradient LiNi0.643Co0.055Mn0.302O2 Cathode Material for Lithium-ion Batteries[J]. Chem. J. Chinese Universities, 2018, 39(1): 148.
| Sample | Tap-density/(g·cm-3) | Sample | Tap-density/(g·cm-3) |
|---|---|---|---|
| 811 | 1.911 | Li-811 | 1.996 |
| FCG 811-505 | 1.966 | FCG Li-811-505 | 2.029 |
| 505 | 2.180 | Li-505 | 2.235 |
Table 1 Tap-density of precursors and corresponding lithiated oxides
| Sample | Tap-density/(g·cm-3) | Sample | Tap-density/(g·cm-3) |
|---|---|---|---|
| 811 | 1.911 | Li-811 | 1.996 |
| FCG 811-505 | 1.966 | FCG Li-811-505 | 2.029 |
| 505 | 2.180 | Li-505 | 2.235 |
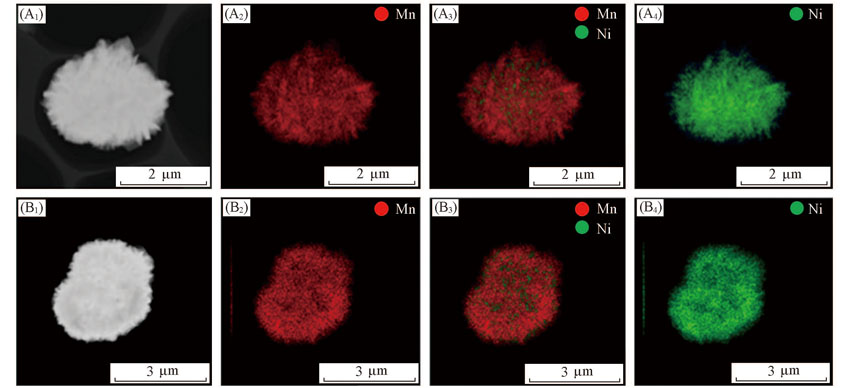
Fig.3 TEM images(A1, B1) and element mapping(A2—A4, B2—B4) of FCG 811-505(A1—A4) and FCG Li-811-505(B1—B4)(A2), (B2) element Mn; (A3), (B3) elements Mn and Ni; (A4), (B4) element Ni.
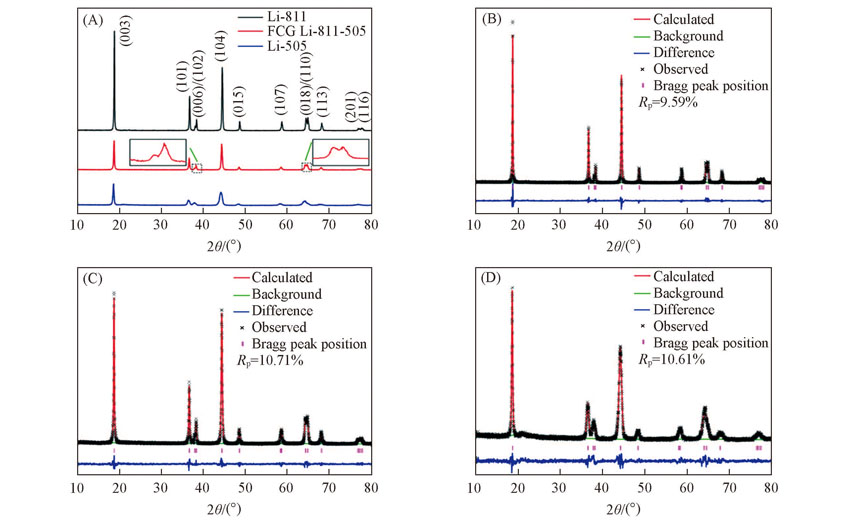
Fig.5 XRD patterns of the cathode materials Li-811, FCG Li-811-505 and Li-505(A) Comparison of three cathode materials’ XRD patterns; (B)—(D) the Rietveld refinement results of XRD patterns for Li-811, FCG Li-811-505 and Li-505, respectively.
| Sample | a/nm | c/nm | V/nm | c/a | I(003)/I(104) |
|---|---|---|---|---|---|
| Li-811 | 0.2869(32) | 1.4187(15) | 0.101110(19) | 4.945 | 1.328 |
| FCG Li-811-505 | 0.2877(5) | 1.4242(24) | 0.102088(31) | 4.950 | 1.216 |
| Li-505 | 0.2888(17) | 1.4309(9) | 0.103343(11) | 4.955 | 0.879 |
Table 2 Lattice parameters obtained from Rietveld refinements of Li-811, FCG Li-811-505 and Li-505
| Sample | a/nm | c/nm | V/nm | c/a | I(003)/I(104) |
|---|---|---|---|---|---|
| Li-811 | 0.2869(32) | 1.4187(15) | 0.101110(19) | 4.945 | 1.328 |
| FCG Li-811-505 | 0.2877(5) | 1.4242(24) | 0.102088(31) | 4.950 | 1.216 |
| Li-505 | 0.2888(17) | 1.4309(9) | 0.103343(11) | 4.955 | 0.879 |
| Atom | Site | x | y | z | g |
|---|---|---|---|---|---|
| Li1 | 3a | 0 | 0 | 0.5 | 2.6464 |
| Ni2 | 3a | 0 | 0 | 0.5 | 0.1079 |
| Li2 | 3b | 0 | 0 | 0 | -1.2765 |
| Ni1 | 3b | 0 | 0 | 0 | 0.8459 |
| Co | 3b | 0 | 0 | 0 | 0.1108 |
| Mn | 3b | 0 | 0 | 0 | 0.1000 |
| O | 6c | 0 | 0 | 0.2585 | 1.0000 |
Table 3 Detailed Rietveld refinement data of XRD for FCG Li-811-505*
| Atom | Site | x | y | z | g |
|---|---|---|---|---|---|
| Li1 | 3a | 0 | 0 | 0.5 | 2.6464 |
| Ni2 | 3a | 0 | 0 | 0.5 | 0.1079 |
| Li2 | 3b | 0 | 0 | 0 | -1.2765 |
| Ni1 | 3b | 0 | 0 | 0 | 0.8459 |
| Co | 3b | 0 | 0 | 0 | 0.1108 |
| Mn | 3b | 0 | 0 | 0 | 0.1000 |
| O | 6c | 0 | 0 | 0.2585 | 1.0000 |
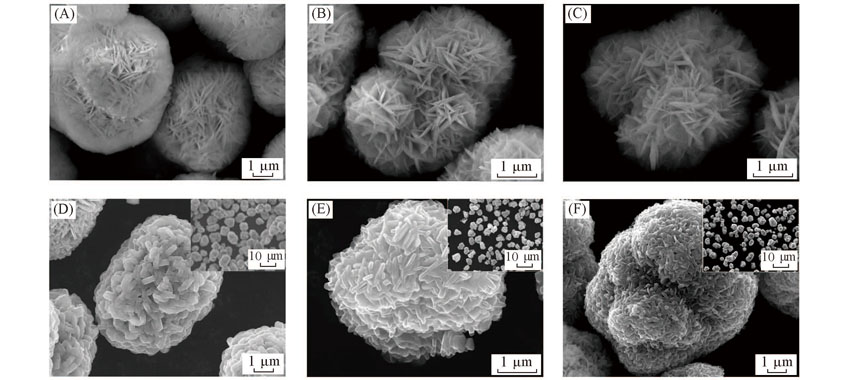
Fig.6 FESEM images of precursors 811(A), FCG 811-505(B), 505(C) and their lithiated oxides Li-811(D), FCG Li-811-505(E) and Li-505(F) Inset are the corresponding FESEM images at low magnification.
| [1] | Nagaura T., Tozawa K., Prog. Batteries Solar Cells, 1990, 9, 209—217 |
| [2] | Whittingham M. S., MRS Bull., 2008, 33(4), 411—419 |
| [3] | Chung S. Y., Bloking J. T., Chiang Y. M., Nat. Mater., 2002, 1(2), 123—128 |
| [4] | Amine K., Belharouak I., Chen Z. H., Tran T., Yumoto H., Ota N., Myung S. T., Sun Y. K., Adv. Mater., 2010, 22(28), 3052—3057 |
| [5] | Lee Y. S., Shin W. K., Kannan A. G., Koo S. M., Kim D. W., Appl. Mater. Interfaces, 2015, 7(25), 13944—13951 |
| [6] | Sun Y. K., Yoon C. S., Myung S. T., Belharouak I., Amine K., J. Electrochem. Soc., 2009, 156(12), A1005—A1010 |
| [7] | Kim H., Kim M. G., Jeong H. Y., Nam H., Cho J., Nano Lett., 2015, 15(3), 2111—2119 |
| [8] | Sun Y. K., Myung S. T., Shin H. S., Bae Y. C., Yoon C. S., J. Phys. Chem. B, 2006, 110(13), 6810—6815 |
| [9] | Sun Y. K., Myung S. T., Kim M. H., Prakash J., Amine K., J. Am. Chem. Soc., 2005, 127(38), 13411—13418 |
| [10] | Sun Y. K., Myung S. T., Park B. C., Amine K., Chem. Mater., 2006, 18(22), 5159—5163 |
| [11] | Sun Y. K., Kim D. H., Yoon C. S., Myung S. T., Prakash J., Amine K., Adv. Funct. Mater., 2010, 20(3), 485—491 |
| [12] | Liao J. Y., Manthiram A., J. Power Sources, 2015, 282, 429—436 |
| [13] | Li J., Camardese J., Shunmugasundaram R., Glazier S., Lu Z., Dahn J. R., Chem. Mater., 2015, 27(9), 3366—3377 |
| [14] | Sun Y. K., Lee B. R., Noh H. J., Wu H., Myung S. T., Amine K., J. Mater. Chem., 2011, 21(27), 10108—10112 |
| [15] | Sun Y. K., Myung S. T., Park B. C., Prakash J., Belharouak I., Amine K., Nat. Mater., 2009, 8(4), 320—324 |
| [16] | Chen Z., Lee D. J., Sun Y. K., Amine K., MRS Bull., 2011, 36(7), 498—505 |
| [17] | Zhang Y., Cao H., Zhang J., Xia B., Solid State Ionics, 2006, 177(37), 3303—3307 |
| [18] | Zhang S., Deng C., Fu B. L., Yang S. Y., Ma L., Powder Technol., 2010, 198(3), 373—380 |
| [19] | Lee M. H., Kang Y. J., Myung S. T., Sun Y. K., Electrochim. Acta, 2004, 50(4), 939—948 |
| [20] | Yang S. T., Yue H. Y., Yin Y. H., Chem. J. Chinese Universities, 2006, 27(11), 2017—2021 |
| (杨书廷, 岳红云, 尹艳红.高等学校化学学报, 2006, 27(11), 2017—2021) | |
| [21] | Dou S., Wang W. J., Solid State Electrochem., 2011, 15(2), 399—404 |
| [22] | Lee K. S., Myung S. T., Moon J. S., Sun Y. K., Electrochim. Acta, 2008, 53(20), 6033—6037 |
| [23] | Lee K. S., Myung S. T., Amine K., Yashiro H., Sun Y. K., J. Electrochem. Soc., 2007, 154(10), A971—A977 |
| [24] | Hwang B. J., Tsai Y. W., Chen C. H., Santhanam R., J. Mater. Chem., 2003, 13(8), 1962—1968 |
| [25] | Li J., Zheng J. M., Guo X. J., Chem. J. Chinese Universities, 2006, 27(7), 1311—1314 |
| (李劼, 郑建明, 郭晓健.高等学校化学学报, 2006, 27(7), 1311—1314) | |
| [26] | Fu C., Li G., Luo D., Li Q., Fan J., Li L., Appl. Mater. Interfaces, 2014, 6(18), 15822—15831 |
| [27] | Wu K., Wang F., Gao L., Li M., Xiao L., Zhao L., Hu S., Wang X., Xu Z., Wu Q., Electrochim. Acta, 2012, 75, 393—398 |
| [28] | Wu F., Wang M., Su Y., Bao L., Chen S., J. Power Sources, 2010, 195(8), 2362—2367 |
| [29] | Kang K., Ceder G., Phys. Rev. B, 2006, 74(9), 094105 |
| [30] | Koyama Y., Arai H., Tanaka I., Uchimoto Y., Ogumi Z., J. Mater. Chem. A, 2014, 2(29), 11235—11245 |
| [31] | Bi Y., Yang W., Du R., Zhou J., Liu M., Liu Y., Wang D., J. Power Sources, 2015, 283, 211—218 |
| [32] | Liu W., Oh P., Liu X., Lee M. J., Cho W., Chae S., Cho J., Angew. Chem. Int. Ed., 2015, 54(15), 4440—4457 |
| [33] | Cheng C., Tan L., Liu H., Huang X., Mater. Res. Bull., 2011, 46(11), 2032—2035 |
| [34] | Vu D. L., Lee J., Korean J. Chem. Eng., 2016, 33(2), 514—526 |
| [35] | Sclar H., Kovacheva D., Zhecheva E., Stoyanova R., Lavi R., Kimmel G., Zinigrad E., J. Electrochem. Soc., 2009, 156(11), A938—A948 |
| [36] | Deng C., Zhang S., Fu B. L., Yang S. Y., Ma L., J. Alloys Comp., 2010, 496(1), 521—527 |
| [37] | Lu Z., MacNeil D. D., Dahn J. R., Electrochem. Solid-State Lett., 2001, 4(11), A191—A194 |
| [1] | JIA Yanggang, SHAO Xia, CHENG Jie, WANG Pengpeng, MAO Aiqin. Preparation and Lithium Storage Performance of Pseudocapacitance-controlled Perovskite High-entropy Oxide La(Co0.2Cr0.2Fe0.2Mn0.2Ni0.2)O3 Anode Materials [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220157. |
| [2] | SUN Xuefeng, RENAGUL Abdurahman, YANG Tongsheng, YANG Qianting. Synthesis and Luminescence Properties of Cr,In Co-doped Small Size MgGa2O4 Near-infrared Persistent Luminescence Nanoparticles [J]. Chem. J. Chinese Universities, 2022, 43(4): 20210850. |
| [3] | ZHANG Shiyu, HE Runhe, LI Yongbing, WEI Shijun, ZHANG Xingxiang. Fabrication of Lithium-sulfur Battery Cathode with Radiation Crosslinked Low Molecular Weight of Polyacrylonitrile and the Mechanism of Sulfur Storage [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210632. |
| [4] | BAO Junquan, ZHENG Shibing, YUAN Xuming, SHI Jinqiang, SUN Tianjiang, LIANG Jing. An Organic Salt PTO(KPD)2 with Enhanced Performance as a Cathode Material in Lithium-ion Batteries [J]. Chem. J. Chinese Universities, 2021, 42(9): 2911. |
| [5] | GAO Xiaole, WANG Jiaxin, LI Zhifang, LI Yanchun, YANG Donghua. Synthesis of NiOx-ZSM-5 Composite Materials and Its Electrocatalytic Hydrogen Evolution Performance in Microbial Electrolysis Cell [J]. Chem. J. Chinese Universities, 2021, 42(9): 2886. |
| [6] | WU Zhuoyan, LI Zhi, ZHAO Xudong, WANG Qian, CHEN Shunpeng, CHANG Xinghua, LIU Zhiliang. A Highly Efficient One-step Preparation Method of Nano-silicon and Carbon Composite for High-performance Lithium Ion Batteries [J]. Chem. J. Chinese Universities, 2021, 42(8): 2500. |
| [7] | YI Conghua, SU Huajian, QIAN Yong, LI Qiong, YANG Dongjie. Preparation of Lignin Nanocarbon and Its Performance as a Negative Electrode for Lithium-ion Batteries [J]. Chem. J. Chinese Universities, 2021, 42(6): 1807. |
| [8] | WANG Yimeng, LIU Kai, WANG Baoguo. Coating Strategies of Ni-rich Layered Cathode in LIBs [J]. Chem. J. Chinese Universities, 2021, 42(5): 1514. |
| [9] | MAO Eryang, WANG Li, SUN Yongming. Advances in Alloy-based High-capacity Li-containing Anodes for Lithium-ion Batteries [J]. Chem. J. Chinese Universities, 2021, 42(5): 1552. |
| [10] | ZHANG Huishuang, GAO Yanxiao, WANG Qiuxian, LI Xiangnan, LIU Wenfeng, YANG Shuting. High-low Temperature Properties of Ni-rich LiNi0.6Co0.2Mn0.2O2 Cathode Material by Hydrothermal Synthesis with CTAB Assisted [J]. Chem. J. Chinese Universities, 2021, 42(3): 819. |
| [11] | SUN Quanhu, LU Tiantian, HE Jianjiang, HUANG Changshui. Advances in the Study of Heteratomic Graphdiyne Electrode Materials [J]. Chem. J. Chinese Universities, 2021, 42(2): 366. |
| [12] | ZHOU Zhan, MA Lufang, TAN Chaoliang. Preparation of Layered (NH4)2V6O16·H2O Nanosheets as an Anode for Li-ion Batteries [J]. Chem. J. Chinese Universities, 2021, 42(2): 662. |
| [13] | GONG Shanshan, WU Tong, WANG Guange, HUANG Qing, SU Yuefeng, WU Feng. Screening of Deep Eutectic Solvent Based on Efficient Recovery of Spent Lithium⁃ion Battery Cathode Materials [J]. Chem. J. Chinese Universities, 2021, 42(10): 3151. |
| [14] | XIANG Houzheng, XIE Hongxiang, LI Wenchao, LIU Xiaolei, MAO Aiqin, YU Haiyun. Synthesis and Electrochemical Performance of Spinel-type High-entropy Oxides [J]. Chem. J. Chinese Universities, 2020, 41(8): 1801. |
| [15] | LU Di,ZHENG Chunman,CHEN Yufang,LI Yujie,ZHANG Hongmei. Synthesis of Li-rich Layers/Spinel/Carbon Composite Cathode Materials with Phenol Formaldehyde Resin and Its Electrochemical Performance† [J]. Chem. J. Chinese Universities, 2020, 41(7): 1684. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||