

Chem. J. Chinese Universities ›› 2016, Vol. 37 ›› Issue (8): 1499.doi: 10.7503/cjcu20160156
• Physical Chemistry • Previous Articles Next Articles
GUO Rui, ZHANG Chaojie*( ), ZHANG Geng, ZHOU Qi
), ZHANG Geng, ZHOU Qi
Received:2016-03-18
Online:2016-07-19
Published:2016-07-19
Contact:
ZHANG Chaojie
E-mail:myrazh@tongji.edu.cn
Supported by:CLC Number:
TrendMD:
GUO Rui,ZHANG Chaojie,ZHANG Geng,ZHOU Qi. Degradation of Perfluorooctanoic Acid by UV/Chloride Process†[J]. Chem. J. Chinese Universities, 2016, 37(8): 1499.
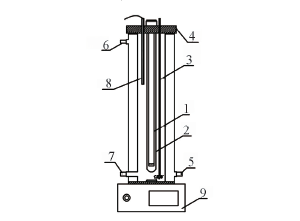
Fig.1 Equipment diagram 1. UV light; 2. quartz tube; 3. aerator pipe; 4. seal cover; 5. circulating water inlet valve; 6. circulating water outlet valve; 7. sampling place; 8. temperature probe; 9. magnetic stirring apparatus.
| System | cNaCl/(mmol·L-1) | Time(He)/min | pH | UV185 |
|---|---|---|---|---|
| PFOA+He+NaCl+UV185 | 0.3 | 30 | 10.0 | Irradiate |
| PFOA+He+UV185 | 0 | 30 | 10.0 | Irradiate |
| PFOA+He+NaCl | 0.3 | 30 | 10.0 | None |
Table 1 Reaction conditions of three systems*
| System | cNaCl/(mmol·L-1) | Time(He)/min | pH | UV185 |
|---|---|---|---|---|
| PFOA+He+NaCl+UV185 | 0.3 | 30 | 10.0 | Irradiate |
| PFOA+He+UV185 | 0 | 30 | 10.0 | Irradiate |
| PFOA+He+NaCl | 0.3 | 30 | 10.0 | None |
| System | Reaction kinetic | 103kobs/min-1 | Half-life/h | Reference |
|---|---|---|---|---|
| PFOA+NaCl+He+UV185 | First-order | 6.3 | 1.77 | This study |
| PFOA+KI+N2+UV254 | First-order | 7.0 | 1.58 | [ |
| PFOA+K2S2O8+O2+UV185 | First-order | 1.4 | 8.19 | [ |
| PFOA+N2+UV185 | First-order | 1.9 | [ |
Table 2 Degradations of PFOA in different reaction systems
| System | Reaction kinetic | 103kobs/min-1 | Half-life/h | Reference |
|---|---|---|---|---|
| PFOA+NaCl+He+UV185 | First-order | 6.3 | 1.77 | This study |
| PFOA+KI+N2+UV254 | First-order | 7.0 | 1.58 | [ |
| PFOA+K2S2O8+O2+UV185 | First-order | 1.4 | 8.19 | [ |
| PFOA+N2+UV185 | First-order | 1.9 | [ |
| [1] | Sagiv S., K. , Rifas-Shiman S., L. , Webster T., F. , Mora A., M. , Harris M., H. , Calafat A., M. , Ye X., Y. , Gillman M., W. , Oken, E. , Environl. Sci. Technol., 2015, 49( 19), 11849- 11858 |
| [2] |
Miller, A. , Elliott J., E. , Elliott K., H. , Lee, S. , Cyr, F. , Environ. Toxicol. Chem., 2015, 34( 8), 1799- 1808
doi: 10.1002/etc.2992 URL pmid: 25989421 |
| [3] |
朱鹏, 段雪梅, 刘靖尧. 高等学校化学学报, 2016, 37( 1), 79- 87
doi: 10.7503/cjcu20150568 |
|
Zhu, P. , Duan X., M. , Liu J., Y. , Chem. J. Chinese Universities, 2016, 37( 1), 79- 87
doi: 10.7503/cjcu20150568 |
|
| [4] |
Arvaniti O., S. , Stasinakis A., S. , Sci. Total. Environ., 2015, 524, 81- 92
doi: 10.1016/j.scitotenv.2015.04.023 URL pmid: 25889547 |
| [5] |
Wang T., Y. , Wang, P. , Meng, J. , Liu S., J. , Lu Y., L. , Khim J., S. , Giesy J., P. , Chemosphere, 2015, 129, 87- 99
doi: 10.1016/j.chemosphere.2014.09.021 URL pmid: 25262946 |
| [6] |
Armitage J., M. , Macleod, M. , Cousins I., T. , Environl. Sci. Technol., 2009, 43( 4), 1134- 1140
doi: 10.1021/es901832b URL pmid: 19320170 |
| [7] |
Prevedouros, K. , Cousins I., T. , Buck R., C. , Korzeniowski S., H. , Environl. Sci. Technol., 2006, 40( 1), 32- 44
doi: 10.1021/es0614870 URL pmid: 17154003 |
| [8] |
Domingo J., L. , Environ. Int., 2012, 40, 187- 195
doi: 10.1016/j.envint.2011.08.001 URL pmid: 21864910 |
| [9] |
李飞, 曾庆玲, 沈春花, 赵志领, 刘淑坡. 中国环境科学, 2012, (9), 1602- 1612
doi: 10.3969/j.issn.1000-6923.2012.09.010 URL |
|
Li, F. , Zeng Q. L., Shen C. H., Zhao Z. L., Liu S. P., China Environ. Sci. , 2012, (9), 1602- 1612
doi: 10.3969/j.issn.1000-6923.2012.09.010 URL |
|
| [10] |
Wang, P. , Lu Y., L. , Wang T., Y. , Fu Y., N. , Zhu Z., Y. , Liu S., J. , Xie S., W. , Xiao, Y. , Giesy J., P. , Environ. Pollut., 2014, 190, 115- 122
doi: 10.1016/j.envpol.2014.03.030 URL pmid: 24747105 |
| [11] |
崔瑞娜, 张亚婷, 王建设, 戴家银. 环境化学, 2013, (7), 1318- 1327
doi: 10.7524/j.issn.0254-6108.2013.07.027 URL |
|
Cui R., N. , Zhang Y. T., Wang J. S., Dai J. Y., Environ. Chem. , 2013, (7), 1318- 1327
doi: 10.7524/j.issn.0254-6108.2013.07.027 URL |
|
| [12] |
Xiao, F. , Simcik M., F. , Halbach T., R. , Gulliver J., S. , Water Res., 2015, 72, 64- 74
doi: 10.1016/j.watres.2014.09.052 URL pmid: 25455741 |
| [13] |
Yao Y., M. , Zhu H., H. , Li, B. , Hu H., W. , Zhang, T. , Yamazaki, E. , Taniyasu, S. , Yamashita, N. , Sun H., W. , Ecotoxicol. Environ. Saf., 2014, 108, 318- 328
doi: 10.1016/j.ecoenv.2014.07.021 URL pmid: 25108512 |
| [14] | Guo C., S. , Zhang, Y. , Zhao, X. , Du, P. , Liu S., S. , Lv J., P. , Xu F., X. , Meng, W. , Xu, J. , Chemosphere, 2015, 127, 201- 207 |
| [15] |
Liu W., X. , He, W. , Qin, N. , Kong X., Z. , He Q., S. , Yang, B. , Yang, C. , Jorgensen S., E. , Xu F., L. , Environ. Pollut., 2015, 200, 24- 34
doi: 10.1016/j.envpol.2015.01.028 URL pmid: 25686885 |
| [16] | 陈舒, 焦杏春, 盖楠, 殷效彩, 朴海涛, 路国慧, 李小洁, 饶竹, 杨永亮. 岩矿测试, 2015, 34, 579- 585 |
| Chen, S. , Jiao X., C. , Gai, N. , Yin X., C. , Piao H., T. , Lu G., H. , Li X., J. , Rao, Z. , Yang Y., L. , Rock and Mineral Analysis, 2015, 34, 579- 585 | |
| [17] |
Vaalgamaa, S. , Vahatalo A., V. , Perkola, N. , Huhtala, S. , Sci. Total. Environ., 2011, 409( 16), 3043- 3048
doi: 10.1016/j.scitotenv.2011.04.036 URL pmid: 21592543 |
| [18] |
Senevirathna, S. , Tanaka, S. , Fujii, S. , Kunacheva, C. , Harada, H. , Shivakoti B., R. , Okamoto, R. , Chemosphere, 2010, 80( 6), 647- 651
doi: 10.1016/j.chemosphere.2010.04.053 |
| [19] |
Steinle D., E. , Reinhard, M. , Environ. Sci. Technol., 2008, 42( 14), 5292- 5297
doi: 10.1021/es703207s URL pmid: 18754383 |
| [20] | 宋洲. 紫外光化学氧化/还原处理全氟辛酸的研究, 武汉: 华中科技大学, 2014) |
| Song, Z. , Photochemical Oxidation or Reduction Degradation of Perfluorooctanoic Acid Using UV Irradiation, Huazhong University of Science and Technology, Wuhan, 2014( | |
| [21] |
李星星, 黄在银, 范高超, 吴烨楠, 谭学才. 高等学校化学学报, 2014, 35( 7), 1480- 1483
doi: 10.7503/cjcu20140176 |
|
Li X., X. , Huang Z., Y. , Fan G., C. , Wu Y., N. , Tan X., C. , Chem. J. Chinese Universities, 2014, 35( 7), 1480- 1483
doi: 10.7503/cjcu20140176 |
|
| [22] |
Vecitis C., D. , Park, H. , Cheng, J. , Mader B., T. , Hoffmann M., R. , Front Environ. Sci. Eng., 2009, 3( 2), 129- 151
doi: 10.1007/s11783-009-0022-7 URL |
| [23] |
Hori, H. , Nagaoka, Y. , Yamamoto, A. , Sano, T. , Yamashita, N. , Taniyasu, S. , Kutsuna, S. , Osaka, I. , Arakawa, R. , Environ Sci. Tech-nol., 2006, 40( 3), 1049- 1054
doi: 10.1021/es0517419 URL pmid: 16509356 |
| [24] |
Arvaniti O., S. , Hwang, Y. , Andersen H., R. , Stasinakis A., S. , Thomaidis N., S. , Aloupi, M. , Chem. Eng. J., 2015, 262, 133- 139
doi: 10.1016/j.cej.2014.09.079 URL |
| [25] |
Zhang L., H. , Zhu, D. , Nathanson G., M. , Hamers R., J. , Angew. Chem. Int. Ed., 2014, 53( 37), 9746- 9751
doi: 10.1002/ange.201404328 URL pmid: 25044766 |
| [26] | Sauer M., C. , Crowell R., A. , Shkrob I., A. , J. Phy. Chem., 2004, 108( 25), 5490- 5502 |
| [27] |
Chen, J. , Zhang P., Y. , Liu, J. , J. Environ. Sci., 2007, 19( 4), 387- 390
doi: 10.1016/S1001-0742(07)60064-3 URL pmid: 17915698 |
| [28] |
Thomsen C., L. , Madsen, D. , Keiding S., R. , J. Phy. Chem., 1999, 110( 7), 3453- 3462
doi: 10.1063/1.478212 URL |
| [29] |
Lehr, L. , Zanni M., T. , Frischkorn, C. , Science, 1999, 284( 5414), 635- 638
doi: 10.1126/science.284.5414.635 URL pmid: 10213684 |
| [30] |
Qu, Y. , Zhang C., J. , Li, F. , Chen, J. , Zhou, Q. , Water Res., 2010, 44( 9), 2939- 2947
doi: 10.1016/j.watres.2010.02.019 URL pmid: 20227745 |
| [31] |
Chen, J. , Zhang P., Y. , Water Sci. Technol., 2006, 54( 11/12), 317- 325
doi: 10.2166/wst.2006.731 URL pmid: 17302335 |
| [32] |
Feng Y., G. , Smith D., W. , Bolton J., R. , Water Environ. Res., 2010, 82( 4), 328- 334
doi: 10.2175/106143009X447920 URL pmid: 20432650 |
| [33] |
马福军. 青海师范大学民族师范学院学报, 2007, 18, 65- 66
doi: 10.3969/j.issn.1671-7473.2007.01.021 URL |
|
Ma F., J. , Journal of Minorities Teachers College of Qinghai Teachers University, 2007, 18, 65- 66
doi: 10.3969/j.issn.1671-7473.2007.01.021 URL |
|
| [34] |
Ding G., H. , Fromel, T. , Brandhof E., J. , Environ. Toxicol. Chem., 2012, 31( 3), 605- 610
doi: 10.1002/etc.1713 URL pmid: 22170568 |
| [35] | 杨佘维. 真空紫外耦合高频超声对水中全氟辛基磺酸钾(PFOS)的脱氟研究, 广州: 华南理工大学, 2013 ) |
| Yang S., W. , Defluorination of Aqueous Perfluorooctanesulfonate(PFOS ) by Combined Process of Vacuum Ultraviolet and High-frequency Ultrasound, South China University of Technology, Guangzhou, 2013( | |
| [36] |
Thogersen, J. , Jensen S., K. , Christiansen, O. , Keiding S., R. , J. Phy. Chem., 2004, 108( 37), 7483- 7489
doi: 10.1136/jmg.2003.010447 URL |
| [37] |
陈静, 张彭义, 刘剑. 环境科学, 2007, 28, 772- 776
doi: 10.3321/j.issn:0250-3301.2007.04.014 URL |
|
Chen, J. , Zhang P., Y. , Liu, J. , Environ. Sci., 2007, 28, 772- 776
doi: 10.3321/j.issn:0250-3301.2007.04.014 URL |
| [1] | CHANG Yunfei, LIAO Mingyi, WEN Jiaming. Reduction Performance and Mechanism of Liquid Terminated-carboxyl Fluoroelastomers Using NaBH4/MCl x Reduction System [J]. Chem. J. Chinese Universities, 2022, 43(6): 20210835. |
| [2] | LI Haibo, XIAO Changfa, JIANG Long, HUANG Yun, DAN Yi. Copolymerization of Methyl Acrylate and 1-Octene Catalyzed by the Loaded Aluminum Chloride on MCM-41 Molecular Sieve [J]. Chem. J. Chinese Universities, 2021, 42(9): 2974. |
| [3] | ZHU Qichen, XIONG Ming, TAO Siyu, TANG Siwei, REN Qizhi. Effect of Light Source on the Photocatalytic Performance of Dihydroxynaphthalene by Water-soluble Sulfonated Porphyrins [J]. Chem. J. Chinese Universities, 2021, 42(6): 1933. |
| [4] | ZHANG Hui, ZHANG Chenjie, XU Minmin, YUAN Yaxian, YAO Jianlin. Investigation on the Reaction of o-Aminothiophenol and 2-Iodobenzoyl Chloride Monitored by SERS-HPLC Technique [J]. Chem. J. Chinese Universities, 2020, 41(11): 2496. |
| [5] | ZHENG Qiuguang,LIU Hailiang,XIAO Changfa. Preparation and Performance of Poly(vinylidene chloride-co-vinyl chloride) Porous Membranes via Thermally Induced Phase Separation† [J]. Chem. J. Chinese Universities, 2019, 40(4): 841. |
| [6] | JIANG Tao, WANG Ning, PENG Shuming, LI Mei, HAN Wei, CHEN Yitung. Electrochemical Behaviour of Gd(Ⅲ) on Bi Electrode and Thermodynamic Data of BixGdy Intermetallic Compounds in LiCl-KCl Molten Salts† [J]. Chem. J. Chinese Universities, 2018, 39(8): 1759. |
| [7] | LI Yuan, WANG Tingting, LI Mei, CHENG Han. Determination of Dopamine Based on RO/Gold Nanoparticles-poly(dienedimethylammonium chloride) Modified Carbon Fiber Microelectrode† [J]. Chem. J. Chinese Universities, 2018, 39(8): 1656. |
| [8] | YANG Zhaojie, LEI Bei, DU Wenhao, ZHANG Xi. Structure and Properties of Starch/Polybutylene Succinate Blends Modified by Magnesium Chloride/1-Butyl-3-methylimidazolium Chloride† [J]. Chem. J. Chinese Universities, 2018, 39(7): 1611. |
| [9] | YU Xiaodan, LIN Xinchen, FENG Wei, LI Weiguang. One-step Preparation and UV-Fenton Properties of Fe3O4/TiO2@Bio-carbon Composities† [J]. Chem. J. Chinese Universities, 2018, 39(11): 2500. |
| [10] | WANG Shaojun, LUO Ting, ZHANG Xiaomin, SHU You, ZHU Jin, SU Shengpei. Manipulation of Native Cellulose Eletrospinning from LiCl-DMAc System [J]. Chem. J. Chinese Universities, 2017, 38(6): 990. |
| [11] | HAN Linhuan, LI Kaifeng, CHEN Yanwei. Growth of Gold Nanorods and Their Interaction with Ferric Chloride† [J]. Chem. J. Chinese Universities, 2017, 38(5): 706. |
| [12] | GAO Zhongzheng, YANG Liguo, BAI Dong, CHEN Lixia, TAO Zhu, XIAO Xin. Interaction of Inverted Cucurbit[7]uril with N,N'-dibenzyl -4,4'-pyridine Chloride† [J]. Chem. J. Chinese Universities, 2017, 38(2): 212. |
| [13] | LI Wei, WU Suhua, Ren Xinxin. Effect of Co-stabilizers on the Properties of Ba/Zn System [J]. Chem. J. Chinese Universities, 2017, 38(11): 2089. |
| [14] | CHEN Xiaolin, WU Hao, LIU Lifen, GAO Congjie. Synthesis and Characterization of Trimesoylamidoamine† [J]. Chem. J. Chinese Universities, 2016, 37(5): 983. |
| [15] | XUN Yan, CAO Zhong, SONG Tianming, LÜ Chaozhi, LIU Feng, HE Jinglin, YANG Ronghua. MWCNTs-rGO/PDDA-AuNPs Nanocomposite Modified Electrode for Sensitive Detection of Ractopamine† [J]. Chem. J. Chinese Universities, 2016, 37(5): 835. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||