

Chem. J. Chinese Universities ›› 2015, Vol. 36 ›› Issue (11): 2211.doi: 10.7503/cjcu20150620
• Physical Chemistry • Previous Articles Next Articles
WANG Shuangshuang1,2, LIU Peng2, CAI Wensheng1, SHAO Xueguang1,2,*( )
)
Received:2015-08-06
Online:2015-11-10
Published:2015-10-10
Contact:
SHAO Xueguang
E-mail:xshao@nankai.edu.cn
Supported by:CLC Number:
TrendMD:
WANG Shuangshuang, LIU Peng, CAI Wensheng, SHAO Xueguang. Effect of Hydrophobicity of Threads on the Solvent-controlled Shuttling in Rotaxanes†[J]. Chem. J. Chinese Universities, 2015, 36(11): 2211.
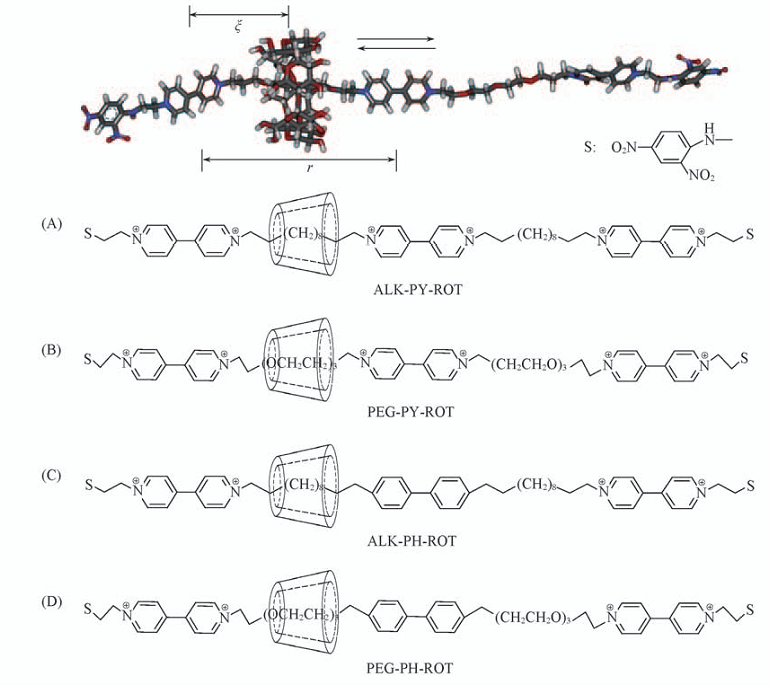
Fig.1 Structures of rotaxanes formed by α-CD and 2,4-dinitrophenyl moieties (A) 4,4'-Bipyridinium moieties and decametrylene moieties; (B) 4,4'-bipyridinium moieties and triethylene glycol moieties; (C) 4,4'-biphenyl moiety, 4,4'-bipyridinium moieties and decametrylene moieties; (D) 4,4'-biphenyl moiety, 4,4'-bipyridinium moieties and triethylene glycol moieties. ξ is the model reaction coordinate, r is the distance between the center of the left bipyridi-nium moieties and that of the middle bipyridinium or biphenyl moiety.
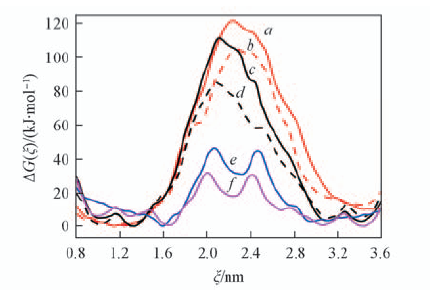
Fig.4 Free-energy profiles of rotaxanes A and B delineating the shuttling process along ξ in water and DMSO(a—d) and rotaxanes C and D delineating the shuttling process along ξ in water(e, f) a. A in water; b. A in DMSO; c. B in water; d. B in DMSO; e. C in water; f. C in DMSO.
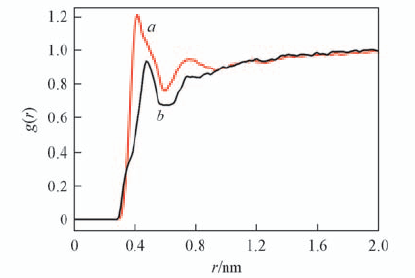
Fig.5 Radial distribution function of the water molecules along the radius from the center of mass of the central PY(PH) moiety a. PY in rotaxane B; b. PH in rotaxane D.
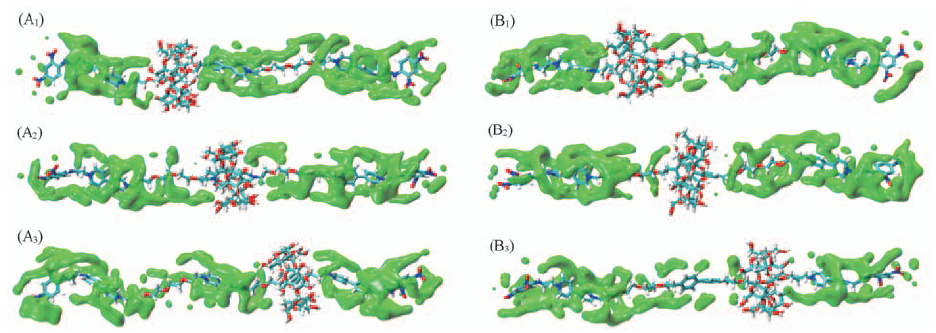
Fig.6 Volumetric map of the water density around rotaxane B(A1—A3) and rotaxane D(B1—B3) (A1—A3) Rotaxane B in its left free-energy minimum, energy barrier, and right free-energy minimum, respectively;(B1—B3) rotaxane D in its left free-energy minimum, energy barrier, and right free-energy minimum, respectively.
| Condition | ΔGforward/(kJ·mol-1) | Δ | ΔG≠/(kJ·mol-1) | Tshuttling/s | |
|---|---|---|---|---|---|
| 303 K | 373 K | ||||
| Rotaxane A in water | 121.4 | 111.0 | 116.2 | 1.3×107 | |
| Rotaxane B in water | 111.4 | 108.0 | 109.7 | 1.4×106 | |
| Rotaxane A in DMSO | 104.7 | 92.1 | 98.4 | 2.7×102 | 0.502 |
| Rotaxane B in DMSO | 85.4 | 80.0 | 82.7 | 61.6 | 0.105 |
| Rotaxane C in water | 46.5 | 42.3 | 44.4 | 7.5×10-6 | |
| Rotaxane D in water | 31.8 | 31.0 | 31.4 | 4.3×10-8 | |
Table 1 Activation free energies and shuttling time throw-over the barriers at 303 K and 373 K*
| Condition | ΔGforward/(kJ·mol-1) | Δ | ΔG≠/(kJ·mol-1) | Tshuttling/s | |
|---|---|---|---|---|---|
| 303 K | 373 K | ||||
| Rotaxane A in water | 121.4 | 111.0 | 116.2 | 1.3×107 | |
| Rotaxane B in water | 111.4 | 108.0 | 109.7 | 1.4×106 | |
| Rotaxane A in DMSO | 104.7 | 92.1 | 98.4 | 2.7×102 | 0.502 |
| Rotaxane B in DMSO | 85.4 | 80.0 | 82.7 | 61.6 | 0.105 |
| Rotaxane C in water | 46.5 | 42.3 | 44.4 | 7.5×10-6 | |
| Rotaxane D in water | 31.8 | 31.0 | 31.4 | 4.3×10-8 | |
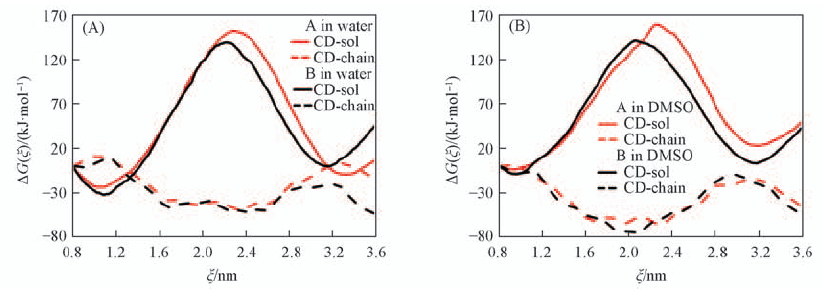
Fig.7 Decomposition of the total free-energy profile into CD-chain and CD-sol contributions for the shuttling process along ξ: rotaxanes A and B in water(A) and in DMSO(B)
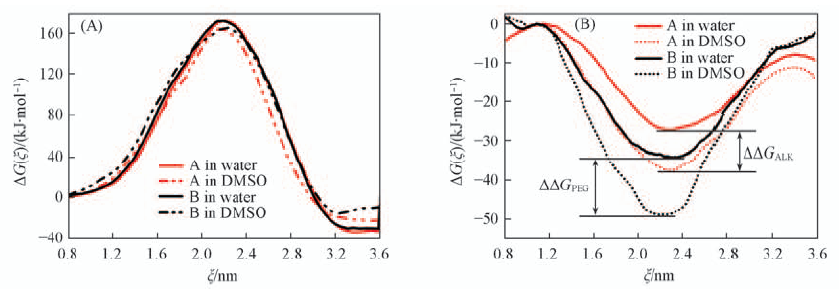
Fig.8 Interaction of the central bipyridinium moiety with the solvent(BIPY-sol)(A) and interaction of the chain-like moiety with the solvent(ALK/PEG-sol)(B)
| [1] | Zhai C. X., Huang F. H., Sci. China, Ser. B: Chem., 2009, 39(4), 315—328 |
| (翟春熙, 黄飞鹤. 中国科学B辑: 化学, 2009, 39(4), 315—328) | |
| [2] | Ma X., Tian H., Acc. Chem. Res., 2014, 47(7), 1971—1981 |
| [3] | Sun S., Shi J. B., Dong Y. P., Hu X. Y., Wang L. Y., Prog. Chem., 2014, 26(8), 1409—1426 |
| (孙书, 石建兵, 董宇平, 胡晓玉, 王乐勇. 化学进展, 2014, 26(8), 1409—1426) | |
| [4] | Yang N. W., Chen T., Fu J. J., Chem. J. Chinese Universities, 2014, 35(5), 971—975 |
| (杨年旺, 陈涛, 傅佳骏. 高等学校化学学报, 2014, 35(5), 971—975) | |
| [5] | Liu P., Shao X. G., Cai W. S., Prog. Chem., 2013, 25(5), 692—697 |
| (刘鹏, 邵学广, 蔡文生. 化学进展, 2013, 25(5), 692—697) | |
| [6] | Gao P., Wang P. J., Geng X., Ye L., Zhang A. Y., Feng Z. G., Acta Chim. Sinica, 2013, 71(3), 347—350 |
| (高鹏, 王培境, 耿雪, 叶霖, 张爱英, 冯增国. 化学学报, 2013, 71(3), 347—350) | |
| [7] | Han B., Liao X. L., Yang B., Prog. Chem., 2014, 26(6), 1039—1049 |
| (韩彬, 廖霞俐, 杨波. 化学进展, 2014, 26(6), 1039—1049) | |
| [8] | Ma X., Tian H., Chem. Soc. Rev., 2010, 39, 70—80 |
| [9] | Murakami H., Kawabuchi A., Matsumoto R., Ido T., Nakashima N., J. Am. Chem. Soc., 2005, 127(45), 15891—15899 |
| [10] | Wenz G., Han B. H., Müller A., Chem. Rev., 2006, 106(3), 782—817 |
| [11] | Kawaguchi Y., Harada A., Org. Lett., 2000, 2(10), 1353—1356 |
| [12] | Liu P., Chipot C., Shao X. G., Cai W. S., J. Phys. Chem. C, 2012, 116(7), 4471—4476 |
| [13] | Hénin J., Chipot C., J. Chem. Phys., 2004, 121(7), 2904—2914 |
| [14] | Chipot C., Hénin J., J. Chem. Phys., 2005, 123(24), 244906 |
| [15] | Rodriguez-Gomez D., Darve E., Pohorille A., J. Chem. Phys., 2004, 120(8), 3563—3578 |
| [16] | Comer J., Roux B., Chipot C., Mol. Simulat., 2014, 40(1—3), 218—228 |
| [17] | Comer J., Gumbart J. C., Hénin J., Lelièvre T., Pohorille A., Chipot C., J. Phys. Chem. B, 2015, 119(3), 1129—1151 |
| [18] | Phillips J. C., Braun R., Wang W., Gumbart J., Tajkhorshid E., Villa E., Chipot C., Skeel R. D., Kalé L., Schulten K., J. Comput. Chem., 2005, 26(16), 1781—1802 |
| [19] | Kuttel M., Brady J. W., Naidoo K. J., J. Comput. Chem., 2002, 23(13), 1236—1243 |
| [20] | Best R. B., Zhu X., Shim J. Y., Lopes P. E. M., Mittal J., Feig M., Mackerell A. D., J. Chem. Theory Comput., 2012, 8(9), 3257—3273 |
| [21] | Liu P., Cai W. S., Chipot C., Shao X. G., J. Phys. Chem. Lett., 2010, 1(12), 1776—1780 |
| [22] | Jorgensen W. L., Chandrasekhar J., Madura J. D., Impey R. W., Klein M. L., J. Chem. Phys., 1983, 79(2), 926—935 |
| [23] | Strader M. L., Feller S. E., J. Phys. Chem. A, 2002, 106(6), 1074—1080 |
| [24] | Feller S. E., Zhang Y. H., Pastor R. W., Brooks B. R., J. Chem. Phys., 1995, 103(11), 4613—4621 |
| [25] | Ryckaert J. P., Ciccotti G., Berendsen H. J. C., J. Chem. Phys., 1977, 23(3), 327—341 |
| [26] | Andersen H. C., J. Chem. Phys., 1983, 52(1), 24—34 |
| [27] | Miyamoto S., Kollman P. A., J. Comput. Chem., 1992, 13(8), 952—962 |
| [28] | Darden T., York D., Pedersen L., J. Chem. Phys., 1993, 98(12), 10089—10092 |
| [29] | Tuckerman M., Berne B. J., Martyna G. J., J. Chem. Phys., 1992, 97(3), 1990—2001 |
| [30] | Humphrey W., Dalke A., Schulten K., J. Mol. Graph., 1996, 14(1), 33—38 |
| [31] | Hénin J., Fiorin G., Chipot C., Klein M. L., J. Chem. Theory Comput., 2010, 6(1), 35—47 |
| [32] | Eyring H., J. Chem. Phys., 1935, 3, 107—115 |
| [33] | Collet O., Chipot C., J. Am. Chem. Soc., 2003, 125(21), 6573—6580 |
| [1] | WANG Ruijie, JIAO Xiaoyu, PAN Yu, WANG Xunchun, YANG Yang, CHENG Zhongjun. Preparation of Transparent Antistatic Multifunctional Superhydrophobic Film [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210703. |
| [2] | CUI Shaoli, ZHANG Weijia, SHAO Xueguang, CAI Wensheng. Revealing the Effect of Threonine on the Binding Ability of Antifreeze Proteins with Ice Crystals by Free-energy Calculations [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210838. |
| [3] | ZHANG Renli, WANG Yao, YU Zhiquan, SUN Zhichao, WANG Anjie, LIU Yingya. Molybdenum Peroxide Anchored on Fluoronated UiO-66 as Catalyst in the Oxidation of Sulfur Containing Compounds [J]. Chem. J. Chinese Universities, 2021, 42(6): 1914. |
| [4] | MIAO Mengyao, GUO Yichang, SHAO Xueguang, CAI Wensheng. Mechanism of Ion Transport Across Membranes Assisted by Molecular Shuttles [J]. Chem. J. Chinese Universities, 2021, 42(10): 3116. |
| [5] | AN Yue, HUANG Hanxiong. Condensate Microdrop Dynamic Behavior on Injection-compression Molded Bionic Polypropylene Nanosurfaces [J]. Chem. J. Chinese Universities, 2020, 41(8): 1888. |
| [6] | REN Wen, ZHANG Guoli, YAN Han, HU Xinghua, LI Kun, WANG Jingfeng, LI Ruiqi. Preparation of Superhydrophobic Polyaniline/Polytetrafluoroethylenethylene Composite Membrane and Its Separation Ability for Oil-Water Emulsion † [J]. Chem. J. Chinese Universities, 2020, 41(4): 846. |
| [7] | SHEN Wenjie. Molecular-fence Catalysts for Low-temperature Oxidation of Methane to Methanol [J]. Chem. J. Chinese Universities, 2020, 41(3): 375. |
| [8] | LIU Shuaizhuo,ZHANG Qian,LIU Ning,XIAO Wenyan,FAN Leiyi,ZHOU Ying. One-step Synergistic Hydrophobic Modification of Melamine Sponge and Its Application † [J]. Chem. J. Chinese Universities, 2020, 41(3): 521. |
| [9] | WANG Wu, LAI Hua, CHENG Zhongjun, LIU Yuyan. Reversible Regulation of Droplet Directional/anti-directional Rolling on Superhydrophobic Shape Memory Microarray Surface [J]. Chem. J. Chinese Universities, 2020, 41(11): 2538. |
| [10] | DU Yifan, AI Chaoqian, ZHANG Yaoyao, WANG Wei. Preparation and Quasi-superhydrophobic Properties of the Surface of Mullite Whiskers/Cordierite † [J]. Chem. J. Chinese Universities, 2019, 40(9): 1955. |
| [11] | LI Dan, WU Zhonghan, ZHOU Dan, JIANG Ding, LU Xinhuan, XIA Qinghua. Controllable Synthesis and Catalytic Applications of Hydrophobic Hybrid Zeolites† [J]. Chem. J. Chinese Universities, 2019, 40(7): 1359. |
| [12] | TIAN Yao,ZHANG Chunquan,WANG Wenzhe,ZHOU Yingfang,LU Yitong,ZHANG Peng,JIA Zhenfu,ZHOU Chengyu,CHEN Shilan. Preparation of Polyrotaxane Cross-linking Agent with “Pulley” Effect and Its Potential Application in Swelling Grain Used as Profile Control and Water Plugging Agent† [J]. Chem. J. Chinese Universities, 2018, 39(9): 2098. |
| [13] | ZHANG Hong, CAI Wensheng, SHAO Xueguang. Effect of Different Force Fields on B-DNA to A-DNA Conversion† [J]. Chem. J. Chinese Universities, 2018, 39(6): 1205. |
| [14] | HUANG Weiwei, REN Jiawang, FANG Qianrong, Valentin VALTCHEV. Synthesis of Zeolite β@IISERP-COF2 Core-shell Hybrid Materials† [J]. Chem. J. Chinese Universities, 2018, 39(6): 1127. |
| [15] | YI Junming,SONG Sen,ZHANG Sheng,ZHANG Shaowei,TIAN Mengkui,NI Xinlong. Effects of Terminal Groups of Guest on the Pseudorotaxane Assembly of Cucurbit[7]uril† [J]. Chem. J. Chinese Universities, 2018, 39(5): 911. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||