

Chem. J. Chinese Universities ›› 2015, Vol. 36 ›› Issue (7): 1298.doi: 10.7503/cjcu20150192
• Organic Chemistry • Previous Articles Next Articles
XUE Fei, MA Rong, SUN Yadong, ABDUKADERA Ablimit, ZHANG Yonghong, LIU Chenjiang*( )
)
Received:2015-03-11
Online:2015-07-10
Published:2015-06-17
Contact:
LIU Chenjiang
E-mail:pxylcj@126.com
Supported by:CLC Number:
TrendMD:
XUE Fei, MA Rong, SUN Yadong, ABDUKADERA Ablimit, ZHANG Yonghong, LIU Chenjiang. Syntheses of Carboxyl Functionalized Benzotriazol-based Ionic Liquids and Their Application in Extraction-oxidative Desulfurization†[J]. Chem. J. Chinese Universities, 2015, 36(7): 1298.
| IL | Appearance | ESI-MS, m/z | IR(KBr), |
|---|---|---|---|
| 2a | Colourless transparency liquid | 206.1[M]+, 279.9[M]- | 3553, 3111, 3006, 1751, 1611, 1505, 1430, 1351, 1200, 1136, 1056, 753, 616 |
| 2b | Colourless transparency liquid | 234.1[M]+, 279.9[M]- | 3549, 3110, 2967, 2879, 1744, 1610, 1505, 1470, 1351, 1196, 1136, 1057, 753, 616 |
| 2c | Colourless transparency liquid | 290.2[M]+, 279.9[M]- | 3108, 3006, 2929, 2857, 1745, 1504, 1468, 1350, 1195, 1137, 1058, 753, 616, 600 |
| 2d | Colourless transparency liquid | 346.3[M]+, 279.9[M]- | 3108, 3002, 2920, 2851, 1732, 1504, 1467, 1337, 1192, 1137, 1058, 753, 612, 598 |
Table 1 Appearance, ESI-MS and IR data for ionic liquids 2a—2d
| IL | Appearance | ESI-MS, m/z | IR(KBr), |
|---|---|---|---|
| 2a | Colourless transparency liquid | 206.1[M]+, 279.9[M]- | 3553, 3111, 3006, 1751, 1611, 1505, 1430, 1351, 1200, 1136, 1056, 753, 616 |
| 2b | Colourless transparency liquid | 234.1[M]+, 279.9[M]- | 3549, 3110, 2967, 2879, 1744, 1610, 1505, 1470, 1351, 1196, 1136, 1057, 753, 616 |
| 2c | Colourless transparency liquid | 290.2[M]+, 279.9[M]- | 3108, 3006, 2929, 2857, 1745, 1504, 1468, 1350, 1195, 1137, 1058, 753, 616, 600 |
| 2d | Colourless transparency liquid | 346.3[M]+, 279.9[M]- | 3108, 3002, 2920, 2851, 1732, 1504, 1467, 1337, 1192, 1137, 1058, 753, 612, 598 |
| IL | 1H NMR(400 MHz, DMSO), δ | 13C NMR(100 MHz, DMSO), δ | |
|---|---|---|---|
| 2a | 1.67(t, J=4.0 Hz, 3H, CH3 ), 5.08(q, J=7.2 Hz, 2H, CH2), 5.99(s, 2H, CH2), 7.99—8.44(m, 4H, ArH) | 13.55, 47.05, 52.37, 113.94, 114.14, 119.37(q, 1JCF=320 Hz, 2C, CF3SO3 ), 130.73, 131.21, 134.07, 135.05, 166.41 | |
| 2b | 0.94(t, J=7.2 Hz, 3H, CH3 ), 1.30—1.40(m, 2H, CH2), 1.99—2.06(m, 2H, CH2), 5.09(t, J=7.2 Hz, 2H, CH2), 5.93(s, 2H, CH2), 7.99—8.47(m, 4H, ArH) | 13.08, 18.80, 30.27, 51.09, 52.66, 113.86, 114.22, 119.37(q, 1JCF=320 Hz, 2C, CF3SO3 ), 130.82, 131.14, 134.23, 135.00, 166.28 | |
| 2c | 0.84(t, J=7.2 Hz, 3H, CH3 ), 1.23—1.32(m, 10H, 5×CH2), 2.05(t, J=7.2 Hz, 2H, CH2), 5.07(t, J=7.2 Hz, 2H, CH2), 5.92(s, 2H, CH2), 7.99—8.45(m, 4H, ArH) | 13.70, 21.88, 25.43, 28.12, 28.28, 30.98, 51.32, 52.72, 113.83, 114.23, 119.38(q, 1JCF=320 Hz, 2C, CF3SO3 ), 130.78, 131.10, 134.22, 135.00, 166.29 | |
| 2d | 0.85(t,J=6.8 Hz, 3H, CH3 ), 1.22—1.31(m, 18H, 9×CH2), 2.03(t, J=6.8 Hz, 2H, CH2), 5.06(t, J=6.8 Hz, 2H, CH2), 5.92(s, 2H, CH2), 8.02—8.45(m, 4H, ArH) | 13.82, 21.98, 25.44, 28.17, 28.29, 28.59, 28.65, 28.77, 28.87, 31.18, 51.31, 52.64, 113.88, 114.24, 119.37(q,1JCF=320 Hz, 2C, CF3SO3 ), 130.81, 131.14, 134.22, 134.99, 166.16 | |
Table 2 1H NMR and 13C NMR data for ionic liquids 2a—2d
| IL | 1H NMR(400 MHz, DMSO), δ | 13C NMR(100 MHz, DMSO), δ | |
|---|---|---|---|
| 2a | 1.67(t, J=4.0 Hz, 3H, CH3 ), 5.08(q, J=7.2 Hz, 2H, CH2), 5.99(s, 2H, CH2), 7.99—8.44(m, 4H, ArH) | 13.55, 47.05, 52.37, 113.94, 114.14, 119.37(q, 1JCF=320 Hz, 2C, CF3SO3 ), 130.73, 131.21, 134.07, 135.05, 166.41 | |
| 2b | 0.94(t, J=7.2 Hz, 3H, CH3 ), 1.30—1.40(m, 2H, CH2), 1.99—2.06(m, 2H, CH2), 5.09(t, J=7.2 Hz, 2H, CH2), 5.93(s, 2H, CH2), 7.99—8.47(m, 4H, ArH) | 13.08, 18.80, 30.27, 51.09, 52.66, 113.86, 114.22, 119.37(q, 1JCF=320 Hz, 2C, CF3SO3 ), 130.82, 131.14, 134.23, 135.00, 166.28 | |
| 2c | 0.84(t, J=7.2 Hz, 3H, CH3 ), 1.23—1.32(m, 10H, 5×CH2), 2.05(t, J=7.2 Hz, 2H, CH2), 5.07(t, J=7.2 Hz, 2H, CH2), 5.92(s, 2H, CH2), 7.99—8.45(m, 4H, ArH) | 13.70, 21.88, 25.43, 28.12, 28.28, 30.98, 51.32, 52.72, 113.83, 114.23, 119.38(q, 1JCF=320 Hz, 2C, CF3SO3 ), 130.78, 131.10, 134.22, 135.00, 166.29 | |
| 2d | 0.85(t,J=6.8 Hz, 3H, CH3 ), 1.22—1.31(m, 18H, 9×CH2), 2.03(t, J=6.8 Hz, 2H, CH2), 5.06(t, J=6.8 Hz, 2H, CH2), 5.92(s, 2H, CH2), 8.02—8.45(m, 4H, ArH) | 13.82, 21.98, 25.44, 28.17, 28.29, 28.59, 28.65, 28.77, 28.87, 31.18, 51.31, 52.64, 113.88, 114.24, 119.37(q,1JCF=320 Hz, 2C, CF3SO3 ), 130.81, 131.14, 134.22, 134.99, 166.16 | |
| Entry | Desulfurization system | Sulfur-removal ratio(%) | Entry | Desulfurization system | Sulfur-removal ratio(%) |
|---|---|---|---|---|---|
| 1 | [C2O2BBTA][Cl] | 0.2 | 7 | [C2O2BBTA][Cl] + H2O2 | 4.9 |
| 2 | [C2O2EBTA][NTf2] | 8.5 | 8 | [C2O2EBTA][NTf2] + H2O2 | 96.7 |
| 3 | [C2O2BBTA][NTf2] | 12.4 | 9 | [C2O2BBTA][NTf2] + H2O2 | 98.3 |
| 4 | [C2O2OBTA][NTf2] | 16.8 | 10 | [C2O2OBTA][NTf2] + H2O2 | 97.5 |
| 5 | [C2O2DBTA][NTf2] | 17.2 | 11 | [C2O2DBTA][NTf2] + H2O2 | 98.4 |
| 6 | H2O2 | 0.1 |
Table 3 Effect of different desulfurization systems on DBT removal
| Entry | Desulfurization system | Sulfur-removal ratio(%) | Entry | Desulfurization system | Sulfur-removal ratio(%) |
|---|---|---|---|---|---|
| 1 | [C2O2BBTA][Cl] | 0.2 | 7 | [C2O2BBTA][Cl] + H2O2 | 4.9 |
| 2 | [C2O2EBTA][NTf2] | 8.5 | 8 | [C2O2EBTA][NTf2] + H2O2 | 96.7 |
| 3 | [C2O2BBTA][NTf2] | 12.4 | 9 | [C2O2BBTA][NTf2] + H2O2 | 98.3 |
| 4 | [C2O2OBTA][NTf2] | 16.8 | 10 | [C2O2OBTA][NTf2] + H2O2 | 97.5 |
| 5 | [C2O2DBTA][NTf2] | 17.2 | 11 | [C2O2DBTA][NTf2] + H2O2 | 98.4 |
| 6 | H2O2 | 0.1 |
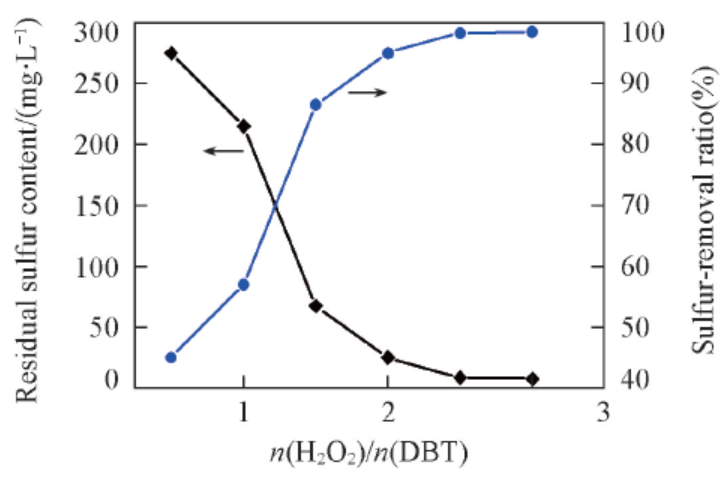
Fig.1 Effect of H2O2/DBT molar ratio on DBT removalReaction conditions: 75 ℃, m(Model oil)∶m(Ionic liquid)=5∶1, 1 h, IL: [C2O2BBTA][NTf2], DBT(S: 500 mg/L) in n-octane.
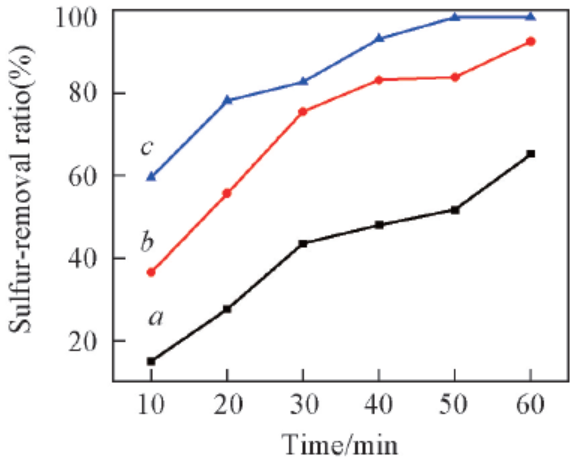
Fig.2 Effect of reaction temperature and time on DBT removalReaction conditions: n(H2O2)∶n(DBT)=2.5∶1, m(Model oil)∶m(Ionic liquid)=5∶1, IL: [C2O2BBTA][NTf2], DBT(S: 500 mg/L) in n-octane. a. 55 ℃ ; b. 65 ℃ ; c. 75 ℃.
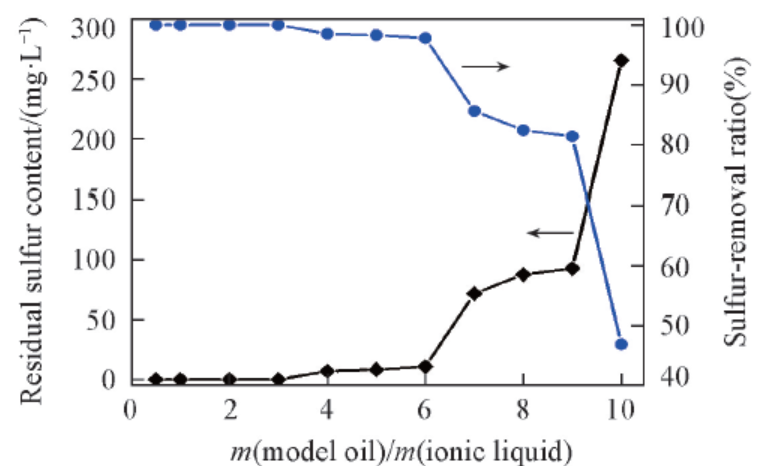
Fig.3 Effect of mass ratio of model oil to ionic li-quid on DBT removalReaction conditions: 75 ℃, n(H2O2)∶n(DBT)=2.5∶1, 1 h, IL: [C2O2BBTA][NTf2], DBT(S: 500 mg/L) in n-octane.
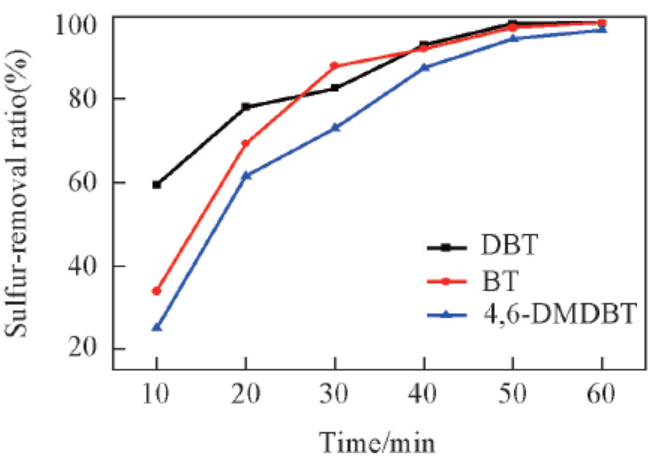
Fig.4 Oxidation of different sulfur-containing compoundsReaction conditions: 75 ℃, n(H2O2)∶n(S)=2.5∶1, m(Model oil)∶m(Ionic liquid)=5∶1, 1 h, IL: [C2O2BBTA][NTf2].
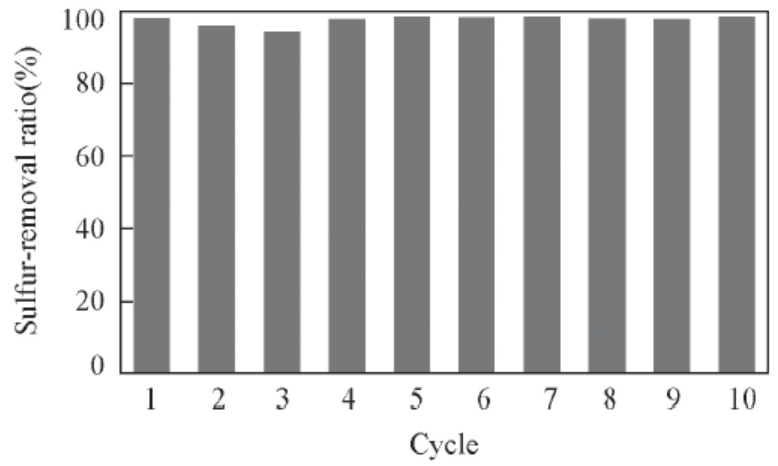
Fig.5 Recycling of IL on removal of DBT in model oilReaction conditions: 75 ℃, n(H2O2)∶n(DBT)=2.5∶1, m(Model oil)∶m(Ionic liquid)=5∶1, 1 h, IL: [C2O2BBTA][NTf2], DBT(S: 500 mg/L) in n-octane.
| [1] | Sydbom A., Blomberg A., Pamia S., Stenfors S., Sandström T., Dahlén S. E., Eur. Respir. J., 2001, 17, 733—746 |
| [2] | Stanislaus A., Marafl A., Rana M. S., Catal. Today, 2010, 153, 1—68 |
| [3] | Gao H. S., Li Y. G., Wu Y., Luo M. F., Li Q., Xing J. M., Liu H. Z., Energy Fuels, 2009, 23, 2690—2694 |
| [4] | Li C. Y., Tan R., Zhao J. F., Yin D. H., Chem. J. Chinese Universities, 2014, 35(2), 297—302 |
| (黎成勇, 谭蓉, 赵江峰, 银董红. 高等学校化学学报,2014, 35(2), 297—302) | |
| [5] | Jia X. D., Han S. Y., Duan H. F., Lin Y. J., Cao J. G., Liang D. P., Wu M. C., Chem. Res. Chinese Universities, 2013, 29(1), 82—86 |
| [6] | Hallett J. P., Welton T., Chem. Rev., 2011, 111, 3508—3576 |
| [7] | Wang Y. L., Luo J., Zhi H. Z., Chem. Res. Chinese Universities, 2013, 29(5), 879—883 |
| [8] | Zhang Q. H., Shreeve J. M., Chem. Rev., 2014, 114, 10527—10574 |
| [9] | Lo W. H., Yang H. Y., Wei G. T., Green Chem., 2003, 5, 639—642 |
| [10] | Chen X. C., Yuan S., Abdeltawab A. A., Al-Deyab S. S., Zhang J. W., Yu L., Yu G. R., Sep. Purif. Technol., 2014, 133, 187—193 |
| [11] | Rodríguez-Cabo B., Rodríguez H., Rodil E., Arce A., Soto A., Fuel, 2014, 117, 882—889 |
| [12] | Zhang H., He J. X., Yang C. R., Chen Z. W., J. Northwest Univ., (Nat. Sci. Edn.), 2013, 43(1), 60—63 |
| (张航, 贺建勋, 杨彩茸, 陈志伟.西北大学学报(自然科学版), 2013, 43(1), 60—63) | |
| [13] | Dharaskar S. A., Wasewar K. L., Varma M. N., Shende D. Z., Yoo C. K., Ind. Eng. Chem. Res., 2014, 53, 19845—19854 |
| [14] | Liang W. D., Zhang S., Li H. F., Zhang G. D., Fuel Process Technol., 2013, 109, 27—31 |
| [15] | Nie Y., Dong Y. X., Bai L., Dong H. F., Zhang X. P., Fuel, 2013, 103, 997—1002 |
| [16] | Jiang W., Zhu W. S., Chang Y. H., Chao Y. H., Yin S., Liu H., Zhu F. X., Li H. M., Chem. Eng. J., 2014, 250, 48—54 |
| [17] | Wang B., Liu C. J., Wang J. D., Lei Z. K., Hu D. L., Chem. J. Chinese Universities, 2012, 33(1), 76—81 |
| (王斌, 刘晨江, 王吉德, 雷振凯, 胡东林. 高等学校化学学报,2012, 33(1), 76—81) | |
| [18] | Li H., Liu C. J., Zhang Y. H., Sun Y. D., Wang B., Liu W. B., Org. Lett., 2015, 17, 932—935 |
| [19] | Zhang S. M., Hou Y. W., Huang W. G., Shan Y. K., Electrochim. Acta, 2005, 50, 4097—4103 |
| [20] | Otsuki S., Nonaka T., Takashima N., Qian W. H., Ishihara A., Imai T., Kabe T., Energy Fuels, 2000, 14, 1232—1239 |
| [1] | CUI Wei, ZHAO Deyin, BAI Wenxuan, ZHANG Xiaodong, YU Jiang. CO2 Absorption in Composite of Aprotic Solvent and Iron-based Ionic Liquid [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220120. |
| [2] | PENG Kuilin, LI Guilin, JIANG Chongyang, ZENG Shaojuan, ZHANG Xiangping. Research Progress for the Role of Electrolytes in the CO2 Electrochemical Reduction [J]. Chem. J. Chinese Universities, 2022, 43(7): 20220238. |
| [3] | JI Shuangqi, JIN Zhao, GUAN Wenna, PAN Xiangyu, GUAN Tong. Preparation and Chromatographic Performance of Mixed-mode Silica Stationary Phase Modified by Double Cationic Ionic Liquid and Octadecyl Group [J]. Chem. J. Chinese Universities, 2022, 43(6): 20220008. |
| [4] | MENG Xianglong, YANG Ge, GUO Hailing, LIU Chenguang, CHAI Yongming, WANG Chunzheng, GUO Yongmei. Synthesis of Nano-zeolite and Its Adsorption Performance for Hydrogen Sulfide [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210687. |
| [5] | CHANG Sihui, CHEN Tao, ZHAO Liming, QIU Yongjun. Thermal Degradation Mechanism of Bio-based Polybutylactam Plasticized by Ionic Liquids [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220353. |
| [6] | ZHANG Renli, WANG Yao, YU Zhiquan, SUN Zhichao, WANG Anjie, LIU Yingya. Molybdenum Peroxide Anchored on Fluoronated UiO-66 as Catalyst in the Oxidation of Sulfur Containing Compounds [J]. Chem. J. Chinese Universities, 2021, 42(6): 1914. |
| [7] | WANG Man, WANG Xin, ZHOU Jing, GAO Guohua. Efficient Synthesis of Dimethyl Carbonate via Transesterification of Methanol and Ethylene Carbonate Catalyzed by Poly(ionic liquid)s [J]. Chem. J. Chinese Universities, 2021, 42(12): 3701. |
| [8] | WAN Ren, SONG Fan, PENG Changjun, LIU Honglai. Group Contribution Method for Infinite Dilution Molar Conductivity of Unconventional Ions in Water [J]. Chem. J. Chinese Universities, 2021, 42(12): 3672. |
| [9] | ZHOU Molin, JIANG Xin, YI Ting, YANG Xiangguang, ZHANG Yibo. Improvement of Interface Stability Between Sulfide Solid Electrolyte Li10GeP2S12 and Lithium Metal [J]. Chem. J. Chinese Universities, 2020, 41(8): 1810. |
| [10] | CHENG Shifu,HU Hao,CHEN Bihua,WU Haihong,GAO Guohua,HE Mingyuan. Preparation and Electrochemical Performance of Porous Carbons Prepared from Binary Ionic Liquids † [J]. Chem. J. Chinese Universities, 2020, 41(5): 1048. |
| [11] | GAO Chong,YU Fengli,XIE Congxia,YU Shitao. Baeyer-Villiger Oxidation of Cyclic Ketones Catalyzed by Amino Alcohol Heteropoly Acid Ionic Liquid † [J]. Chem. J. Chinese Universities, 2020, 41(5): 1101. |
| [12] | GAO Naiwei, MA Qiang, HE Yonglin, WANG Yapei. Green Electronic Devices Based on Ionic Liquids † [J]. Chem. J. Chinese Universities, 2020, 41(5): 901. |
| [13] | WANG Xiao,JIN Biao,WANG Yubin,XU Zhuoyue,ZHANG Lu,ZHANG Xiaoting,YANG Liushuan. Interaction Mechanism of Anions in Hydrothermal Crystallization of Desulfurization Gypsum Whiskers † [J]. Chem. J. Chinese Universities, 2020, 41(3): 473. |
| [14] | PIAO Huilan,MA Pinyi,QIN Zucheng,JIANG Yanxiao,SUN Ying,WANG Xinghua,SONG Daqian. Determination of Triazine Herbicides from Fruit Juice Samples Using Effervescence Assisted Microextraction Method Based on Acidic Ionic Liquid Packed Syringe [J]. Chem. J. Chinese Universities, 2020, 41(2): 228. |
| [15] | ZHANG Li,QIAN Mingchao,LIU Xueke,Gao Shuaitao,YU Jiang,XIE Haishen,WANG Hongbin,SUN Fengjiang,SU Xianghong. Dynamic Study of Oxidative Desulfurization by Iron-based Ionic Liquids/NHD † [J]. Chem. J. Chinese Universities, 2020, 41(2): 317. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||