

Chem. J. Chinese Universities ›› 2014, Vol. 35 ›› Issue (5): 1029.doi: 10.7503/cjcu20131208
• Physical Chemistry • Previous Articles Next Articles
XU Li1,2, PAN Guoshun1,2,*( ), LIANG Xiaolu1,2, LUO Guihai1,2, Zou Chunli1,2, CHEN Gaopan1,2
), LIANG Xiaolu1,2, LUO Guihai1,2, Zou Chunli1,2, CHEN Gaopan1,2
Received:2013-12-10
Online:2014-05-10
Published:2014-01-23
Contact:
PAN Guoshun
E-mail:pangs@tsinghua.edu.cn
Supported by:CLC Number:
TrendMD:
XU Li, PAN Guoshun, LIANG Xiaolu, LUO Guihai, Zou Chunli, CHEN Gaopan. N/S Co-doped Non-precious Metal as Non-platinum Cathode Catalyst for Alkaline Membrane Fuel Cells†[J]. Chem. J. Chinese Universities, 2014, 35(5): 1029.
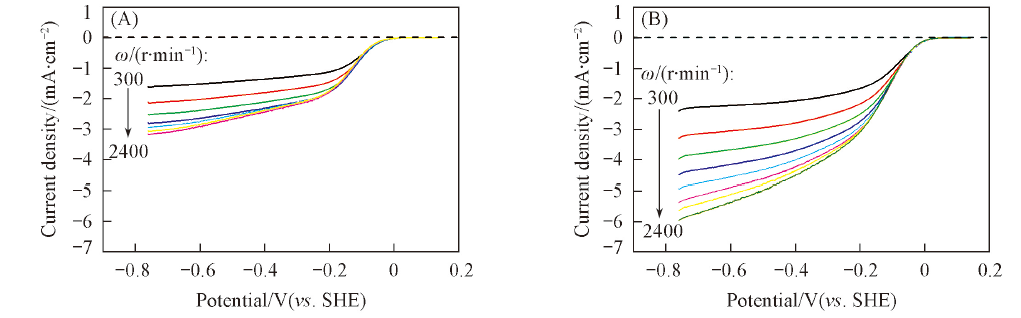
Fig.2 Polarization curves for ORR on Fe-N/C-600(A) and Fe-N/C-TsOH-600(B) catalyst measured in O2-saturated 0.1 mol/L KOH electrolytes at various rotation rates^ω/(r·min-1) from top to bottom: 300, 600, 900, 1200, 1500, 1800, 2100, 2400.
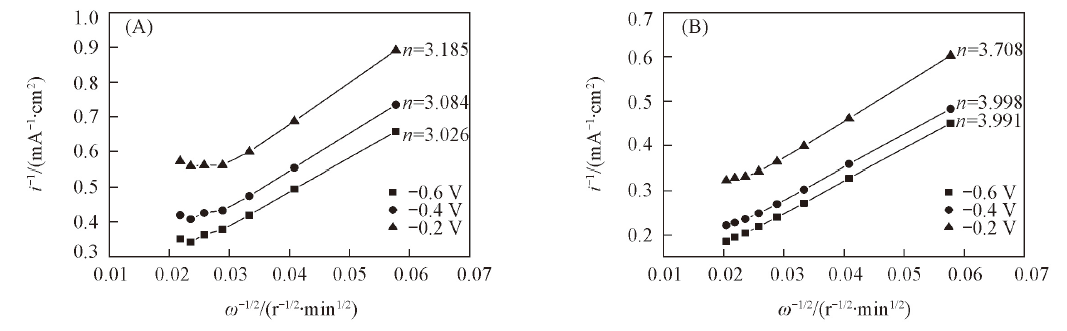
Fig.3 Koutecky-Levich plots for Fe-N/C-600(A) and Fe-N/C-TsOH-600(B) in O2-saturated 0.1 mol/L KOH solution at the potential of -0.6, -0.4 and -0.2 V, respectively
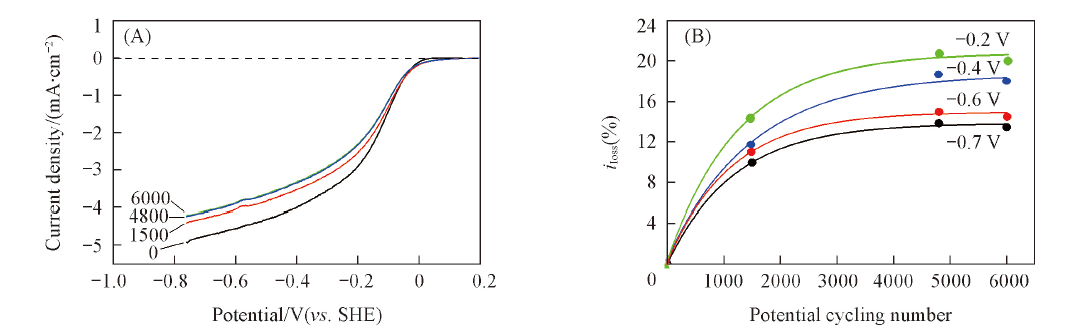
Fig.4 Polarization curves for the oxygen reduction in O2-saturated 0.1 mol/L KOH on Fe-N/C-TsOH-600 before and after the potential cycling stability test(A) and iloss at different potential for the ORR in O2-saturated 0.1 mol/L KOH on Fe-N/C-TsOH-600 as a function of the number of potential cycling(B)^(A) Electrode rotating rate: 1500 r/min. Potential cycling was performed in N2-saturated 0.1 mol/L KOH with a scan rate of 50 mV/s between -0.76 and 0.14 V(vs. SHE) for 0, 1500, 4800 and 6000 cycles.
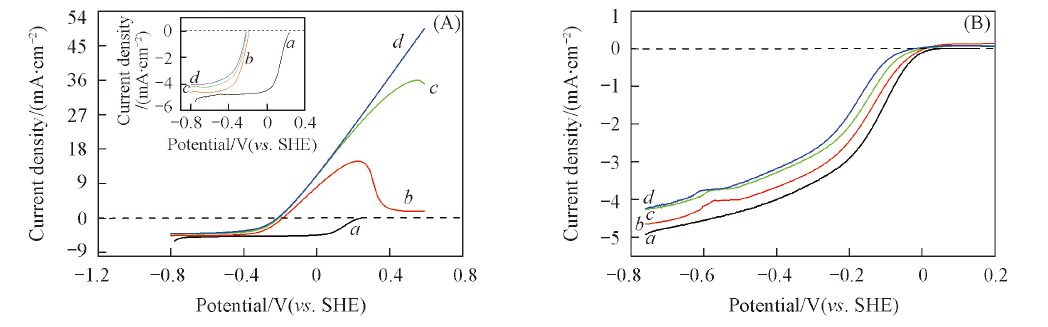
Fig.5 Polarization curves for O2 reduction on Pt/C electrode(A) and Fe-N/C-TsOH-600(B) in O2-saturated 0.1 mol/L KOH electrolyte without and with different concentrations of methanol solution^Potential scan rate: 5 mV/s. Electrode rotating rate: 1500 r/min. c(CH3OH)/(mol·L-1): a. 0; b. 0.5; c. 1.0; d. 5.0.
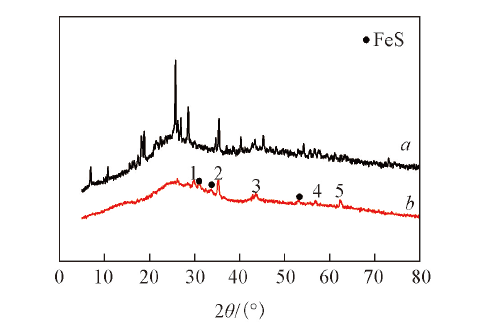
Fig.6 XRD patterns of Fe-N/C-TsOH-600(a) and Fe-N/C-TsOH(b) catalysts^Peaks 1—5: Fe3O4(022); Fe3O4(113); Fe(011), FeC(013); Fe3O4(115) and Fe3O4(044).
| [1] | Qiao J., Xu L., Liu Y., Xu P., Shi J., Liu S., Tian B., Electrochim. Acta, 2013, 96, 298—305 |
| [2] | Dillon R., Srinivasan S., Arico A. S., Antonucci V., J. Power Sources, 2004, 127(1/2), 112—126 |
| [3] | Xia D., Liu S., Wang Z., Chen G., Zhang L., Zhang L., Hui S., Zhang J., J. Power Sources, 2008, 177(2), 296—302 |
| [4] | Wang C., Hsu H., Cheng S. T., Du H. Y., Chen C. P., Wu J. C. S., Shih H. C., Chen L. C., Chen K. H., J. Mater. Chem., 2010, 20(35), 7551—7557 |
| [5] | Yu J. S., Kim M. S., Kim J. H., Phys. Chem. Chem. Phys., 2010, 12(46), 15274—15281 |
| [6] | Xu L., Ding L., Dai X. F., Zhang J., Tian B. L., Liu S. Y., Qiao J. L., Chem. J. Chinese Universities, 2013, 34(1), 149—154 |
| (徐莉, 丁蕾, 戴先逢, 张璟, 田丙伦, 刘师尧, 乔锦丽.高等学校化学学报, 2013,34(1), 149—154) | |
| [7] | Merzougui B., Hachimi A., Akinpelu A., Bukola S., Shao M., Electrochim. Acta, 2013, 107, 126—132 |
| [8] | Wang C. H., Chang S. T., Hsu H. C., Du H. Y., Wu J. C. S., Chen L. C., Chen K. H., Diamond Relat. Mater., 2011, 20(3), 322—329 |
| [9] | Jaouen F., Goellner V., Lefevre M., Herranz J., Proietti E., Dodelet J. P., Electrochim. Acta, 2013, 87, 619—628 |
| [10] | Holze R., Vogel I., Vielstich W., J. Electroanal. Chem., 1986, 210(2), 277—286 |
| [11] | Li X. G., Popov B. N., Kawahara T., Yanagi H., J. Power Sources, 2011, 196(4), 1717—1722 |
| [12] | Li X. G., Liu G., Popov B. N., J. Power Sources, 2010, 195(19), 6373—6378 |
| [13] | Liu G., Li X. G., Ganesan P., Popov B. N., Electrochim. Acta, 2010, 55(8), 2853—2858 |
| [14] | Liu G., Li X. G., Ganesan P., Popov B. N., Appl. Catal. B: Environ., 2009, 93(1/2), 156—165 |
| [15] | Sheng Z. H., Shao L., Chen J. J., Bao W. J., Wang F. B., Xia X. H., ACS Nano, 2011, 5(6), 4350—4358 |
| [16] | Maiyalagan T Viswanathan B., Varadaraju U., Electrochem. Commun., 2005, 7(9), 905—912 |
| [17] | Wang C. H., Shih H. C., Tsai Y. T., Du H. Y., Chen L. C., Chen K. H., Electrochim. Acta, 2006, 52(4), 1612—1617 |
| [18] | Choi B., Yoon H., Park I. S., Jang J., Sung Y. E., Carbon, 2007, 45(13), 2496—2501 |
| [19] | Kondo T., Suzuki T., Nakamura J., J. Phys. Chem. Lett., 2011, 2(6), 577—580 |
| [20] | Wu G., Li D., Dai C., Wang D., Li N., Langmuir, 2008, 24(7), 3566—3575 |
| [21] | Shrestha S., Mustain W. E., ECS Trans., 2010, 33(1), 293—302 |
| [22] | Ma Y., Jiang S., Jian G., Tao H., Yu L., Wang X., Zhu J., Hu Z., Chen Y., Energ. Environ. Sci., 2009, 2(2), 224—229 |
| [23] | Su F. B., Tian A. Q., Poh C. K., Wang Z., Lim S. H., Liu Z. L., Lin J. Y., Chem. Mater., 2010, 22(3), 832—839 |
| [24] | Biddinger E. J., Deak D. V., Ozkan U. S., Top. Catal., 2009, 52(11), 1566—1574 |
| [25] | Yang L., Jiang S., Zhao Y., Zhu L., Chen S., Wang X., Wu Q., Ma J., Ma Y., Hu Z., Angew. Chem. Int. Ed., 2011, 50(31), 7132—7135 |
| [26] | Sheng Z. H., Gao H. L., Bao W. J., Wang F. B., Xia X. H., J. Mater. Chem., 2012, 22(2), 390—395 |
| [27] | Byeon A., Lee J. W., J. Phys. Chem. C, 2013, 117(46), 24167—24173 |
| [28] | Liu Z., Peng F., Wang H., Yu H., Tan J., Zhu L., Catal. Commun., 2011, 16(1), 35—38 |
| [29] | Deak D., Biddinger E. J., Luthman K. A., Ozkan U. S., Carbon, 2010, 48(12), 3637—3659 |
| [30] | Liu Z., Shi Q., Peng F., Wang H., Hao Y., Li J., Wei X., Catal. Commun., 2012, 22, 34—38 |
| [31] | Liu Z., Shi Q., Peng F., Wang H., Zhang R., Yu H., Electrochem. Commun., 2012, 16(1), 73—76 |
| [32] | Zhang J., Liu X., Blume R., Zhang A. H., Schlogl R., Su D. S., Science, 2008, 322(5898), 73—77 |
| [33] | Wang H., Bo X., Zhang Y., Guo L., Electrochim. Acta, 2013, 108, 404—411 |
| [34] | Ahmadi R., Amini M. K., Bennett J. C., J. Catal., 2012, 292, 81—89 |
| [35] | Wang S., Zhang L., Xia Z., Roy A., Chang D. W., Baek J., Dai L., Angew. Chem. Int. Ed., 2012, 51(17), 4209—4212 |
| [36] | Papageorgopoulos D. C., Liu F., Conrad O., Electrochim. Acta, 2007, 52(15), 4982—4986 |
| [37] | Gochi-Ponce C., Alonso-Nunez G., Alonso-Vante N., Electrochem. Commun., 2006, 8(9), 1487—1491 |
| [38] | Kramm U. I., Herrmann I., Fiechter S., Zehl G., Zizak I., Abs-Wurmbach I., Radnik J., Dorbandt I., Bogdanoff P., ECS Trans., 2009, 25(1), 659—670 |
| [39] | Herrmann I., Kramm U. I., Radnik J., Fiechter S., Bogdanoff P., J. Electrochem. Soc., 2009, 156(10), 1283—1292 |
| [40] | Mattery P. H., Zhang L., Ozkan U. S., J. Catal., 2006, 239(1), 83—96 |
| [41] | Zhang H. J., Jiang Q. Z., Sun L., Yuan X., Ma Z. F., Electrochim Acta, 2010, 55(3), 1107—1112 |
| [42] | Wu G., Chen Z., Artyushkova K., Garzo F. H., Zelenay P., ECS Trans., 2008, 16(2), 159—170 |
| [43] | Cline K. K., McDermott M. T., McCreery R. L., J. Phys. Chem., 1994, 98(20), 5314—5319 |
| [44] | Matter P. H., Wang E., Ozkan U. S., J. Catal., 2006, 243(2), 395—403 |
| [45] | Matter P. H., Zhang U. S., Ozkan U. S., J. Catal., 2006, 239(1), 83—96 |
| [46] | Zheng B., Wang J., Wang F. B., Xia X. H., Electrochem. Commun., 2013, 28, 24—26 |
| [47] | Yang Z., Nie H., Chen X., Chen X., Huang S., J. Power Source, 2013, 236, 238—249 |
| [48] | Wang H., Maiyalagan T., Wang X., ACS Catal., 2012, 2(5), 781—794 |
| [49] | Su Y., Zhang Y., Zhuang X., Li S., Wu D., Zhang F., Feng X., Carbon, 2013, 62, 296—301 |
| [50] | Wang H., Bo X., Zhang Y., Guo L., Electrochim. Acta, 2013, 108, 404—411 |
| [51] | Grabke H. J., Moszynski D., Muller-Lorenz E. M., Schneider A., Surf. Interface Anal., 2002, 34(1), 369—374 |
| [1] | CHENG Qian, YANG Bolong, WU Wenyi, XIANG Zhonghua. S-doped Fe-N-C as Catalysts for Highly Reactive Oxygen Reduction Reactions [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220341. |
| [2] | CHU Yuyi, LAN Chang, LUO Ergui, LIU Changpeng, GE Junjie, XING Wei. Single-atom Cerium Sites Designed for Durable Oxygen Reduction Reaction Catalyst with Weak Fenton Effect [J]. Chem. J. Chinese Universities, 2022, 43(9): 20220294. |
| [3] | ZHENG Anni, JIN Lei, YANG Jiaqiang, WANG Zhaoyun, LI Weiqing, YANG Fangzu, ZHAN Dongping, TIAN Zhongqun. Effects of 5,5-Dimethylhydantoin on Electroless Copper Plating [J]. Chem. J. Chinese Universities, 2022, 43(8): 20220191. |
| [4] | GU Yu, XI Baojuan, LI Jiangxiao, XIONG Shenglin. Structure Regulation of Single-atom Catalysts in Oxygen Reduction Reactions [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220036. |
| [5] | WANG Hongning, HUANG Li, QING Jiang, MA Tengzhou, JIANG Wei, HUANG Weiqiu, CHEN Ruoyu. Activation of Biochar from Cattail and the VOCs Adsorption Application [J]. Chem. J. Chinese Universities, 2022, 43(4): 20210824. |
| [6] | ZHANG Xiaoyu, XUE Dongping, DU Yu, JIANG Su, WEI Yifan, YAN Wenfu, XIA Huicong, ZHANG Jianan. MOF-derived Carbon-based Electrocatalysts Confinement Catalyst on O2 Reduction and CO2 Reduction Reactions [J]. Chem. J. Chinese Universities, 2022, 43(3): 20210689. |
| [7] | LI Weihui, LI Haobo, ZENG Cheng, LIANG Haoyue, CHEN Jiajun, LI Junyong, LI Huiqiao. Hot-pressed PVDF-based Difunctional Protective Layer for Lithium Metal Anodes [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210629. |
| [8] | HE Yujing, LI Jiale, WANG Dongyang, WANG Fuling, XIAO Zuoxu, CHEN Yanli. Zinc-based Activated Fe/Co/N Doped Biomass Carbon Electrocatalysts with High Oxygen Reduction Activity [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220475. |
| [9] | CHANG Sihui, CHEN Tao, ZHAO Liming, QIU Yongjun. Thermal Degradation Mechanism of Bio-based Polybutylactam Plasticized by Ionic Liquids [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220353. |
| [10] | YUE Shengli, WU Guangbao, LI Xing, LI Kang, HUANG Gaosheng, TANG Yi, ZHOU Huiqiong. Research Progress of Quasi-two-dimensional Perovskite Solar Cells [J]. Chem. J. Chinese Universities, 2021, 42(6): 1648. |
| [11] | WANG Hongning, HUANG Li, SONG Fujiao, ZHU Ting, HUANG Weiqiu, ZHONG Jing, CHEN Ruoyu. Synthesis and VOCs Adsorption Properties of Hollow Carbon Nanospheres [J]. Chem. J. Chinese Universities, 2021, 42(6): 1704. |
| [12] | WANG Kunhua, YAO Jisong, YANG Junnan, SONG Yonghui, LIU Yuying, YAO Hongbin. Synthesis and Device Optimization of Highly Efficient Metal Halide Perovskite Light-emitting Diodes [J]. Chem. J. Chinese Universities, 2021, 42(5): 1464. |
| [13] | MA Jun, ZHONG Yang, ZHANG Shanshan, HUANG Yijun, ZHANG Lipeng, LI Yaping, SUN Xiaoming, XIA Zhenhai. Design and Theoretical Calculation of Heteroatoms Doped Graphdiyne Towards Efficiently Catalyzing Oxygen Reduction and Evolution Reactions [J]. Chem. J. Chinese Universities, 2021, 42(2): 624. |
| [14] | LIU Yao, DENG Zhengtao. Fast Synthesis of Highly Luminescent Two-dimensional Tin-halide Perovskites by Anti-solvent Method [J]. Chem. J. Chinese Universities, 2021, 42(12): 3774. |
| [15] | ZHANG Jun, WANG Bin, PAN Li, MA Zhe, LI Yuesheng. Synthesis and Properties of Imidazolium-based Polyethylene Ionomer [J]. Chem. J. Chinese Universities, 2020, 41(9): 2070. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||