

Chem. J. Chinese Universities ›› 2014, Vol. 35 ›› Issue (6): 1212.doi: 10.7503/cjcu20131009
• Organic Chemistry • Previous Articles Next Articles
MU Yangyang1, ZHEN Qiannan1, WANG Mengfan2,*( ), QI Wei1,*(
), QI Wei1,*( ), SU Rongxin1, HE Zhimin1
), SU Rongxin1, HE Zhimin1
Received:2013-10-15
Online:2014-06-10
Published:2013-11-25
Contact:
WANG Mengfan,QI Wei
E-mail:mwang@tju.edu.cn;qiwei@tju.edu.cn
Supported by:CLC Number:
TrendMD:
MU Yangyang, ZHEN Qiannan, WANG Mengfan, QI Wei, SU Rongxin, HE Zhimin. Preparation and Thermal Kinetic Deactivation of Cross-linked Enzyme Aggregates of Penicillin Acylase†[J]. Chem. J. Chinese Universities, 2014, 35(6): 1212.
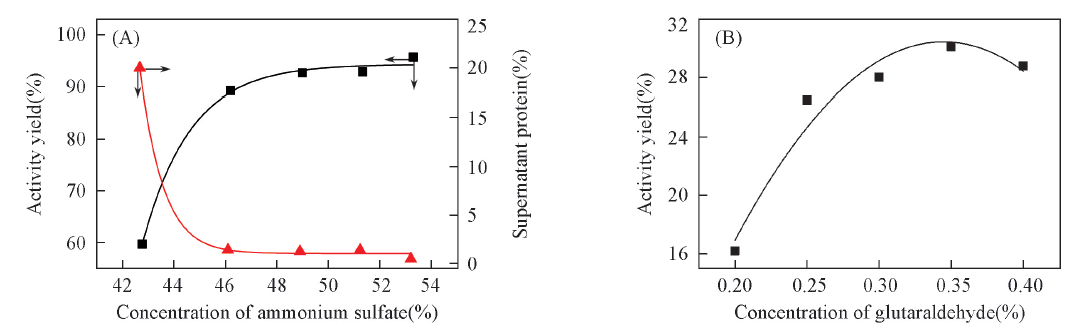
Fig.1 Effects of ammonium sulfate(A) and glutaraldehyde(B) concentration on the activity yieldActivity yield(%)=Ai/A0×100%; Ai is the activity of enzyme aggregates or CLEAs; A0 is the activity of free enzyme.
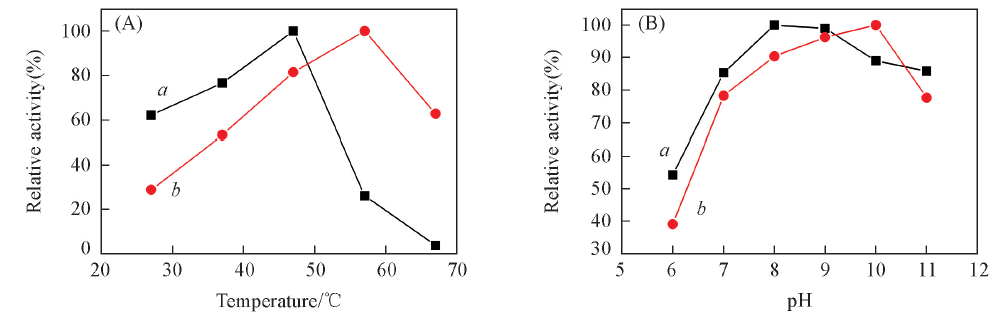
Fig.2 Optimal temperatures(A) and pH(B) of free penicillin acylase(a) and its CLEAs(b) Relative activity(%) was calculated by assuming the activity at optimal temperature or pH as 100%.
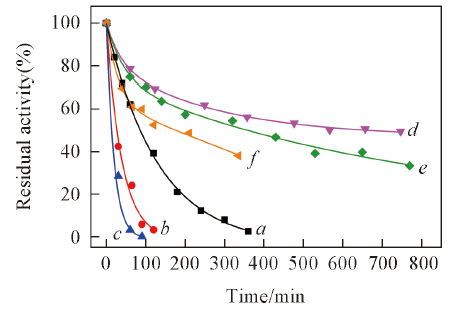
Fig.3 Thermal stability of free penicillin acylase(a—c) and its CLEAs(d—f)Residual activity(%) was calculated by assuming the initial activity of enzyme samples as 100%. Temperature/K: a, d: 329.15; b, e: 331.15; c, f: 333.15.
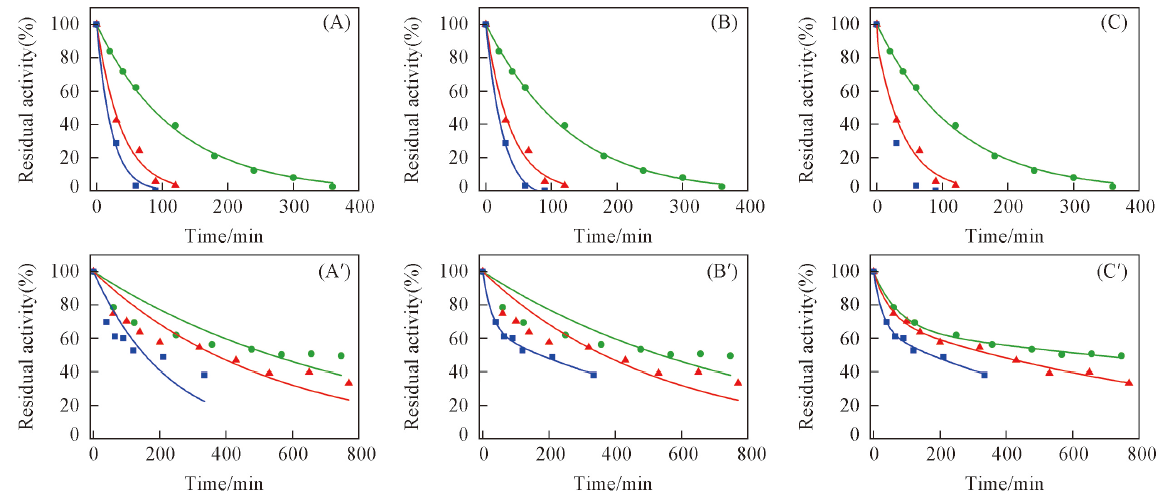
Fig.4 Thermal deactivation kinetic curves of free enzyme(A—C) and its CLEAs(A'—C')(A, A') One step model; (B, B') parallel model; (C, C') serial model. ● 329.15 K; ▲ 331.15 K; ■ 333.15 K.
| Free enzyme | CLEAs | |||
|---|---|---|---|---|
| T/K | kd/min-1 | R2 | kd/min-1 | R2 |
| 329.15 | 0.0083 | 0.9985 | 0.0012 | 0.6480 |
| 331.15 | 0.0264 | 0.9904 | 0.0018 | 0.7587 |
| 333.15 | 0.0343 | 0.9966 | 0.0044 | 0.6548 |
Table 1 One-step deactivation parameters of free penicillin acylase and its CLEAs
| Free enzyme | CLEAs | |||
|---|---|---|---|---|
| T/K | kd/min-1 | R2 | kd/min-1 | R2 |
| 329.15 | 0.0083 | 0.9985 | 0.0012 | 0.6480 |
| 331.15 | 0.0264 | 0.9904 | 0.0018 | 0.7587 |
| 333.15 | 0.0343 | 0.9966 | 0.0044 | 0.6548 |
| Free enzyme | CLEAs | |||||||
|---|---|---|---|---|---|---|---|---|
| T/K | α | kd1/min-1 | kd2/min-1 | R2 | α | kd1/min-1 | kd2/min-1 | R2 |
| 329.15 | -28.55 | 0.0109 | 0.0110 | 0.9985 | 0.0372 | 0.0012 | 0.0012 | 0.5308 |
| 331.15 | 12.45 | 0.0265 | 0.0265 | 0.9808 | 1.320×10-5 | 0.0019 | 0.0019 | 0.6897 |
| 333.15 | -33.91 | 0.0270 | 0.0267 | 0.9976 | 0.6542 | 0.0372 | 0.0016 | 0.9919 |
Table 2 Parallel deactivation parameters of free penicillin acylase and its CLEAs
| Free enzyme | CLEAs | |||||||
|---|---|---|---|---|---|---|---|---|
| T/K | α | kd1/min-1 | kd2/min-1 | R2 | α | kd1/min-1 | kd2/min-1 | R2 |
| 329.15 | -28.55 | 0.0109 | 0.0110 | 0.9985 | 0.0372 | 0.0012 | 0.0012 | 0.5308 |
| 331.15 | 12.45 | 0.0265 | 0.0265 | 0.9808 | 1.320×10-5 | 0.0019 | 0.0019 | 0.6897 |
| 333.15 | -33.91 | 0.0270 | 0.0267 | 0.9976 | 0.6542 | 0.0372 | 0.0016 | 0.9919 |
| Free enzyme | CLEAs | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T/K | α | β | kd1/min-1 | kd2/min-1 | R2 | α | β | kd1/min-1 | kd2/min-1 | R2 |
| 329.15 | 0.5017 | 0.5017 | 0.0083 | 0.0083 | 0.9972 | 0.6531 | 0.3446 | 4.024×10-4 | 0.0132 | 0.9935 |
| 331.15 | 0.1048 | 0.8952 | 1.1743 | 0.0240 | 0.9653 | 0.7156 | 0.2837 | 9.871×10-4 | 0.0205 | 0.9887 |
| 333.15 | 0.6542 | 0.3461 | 0.0016 | 0.0372 | 0.9892 | |||||
Table 3 Serial deactivation parameters of free penicillin acylase and its CLEAs
| Free enzyme | CLEAs | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T/K | α | β | kd1/min-1 | kd2/min-1 | R2 | α | β | kd1/min-1 | kd2/min-1 | R2 |
| 329.15 | 0.5017 | 0.5017 | 0.0083 | 0.0083 | 0.9972 | 0.6531 | 0.3446 | 4.024×10-4 | 0.0132 | 0.9935 |
| 331.15 | 0.1048 | 0.8952 | 1.1743 | 0.0240 | 0.9653 | 0.7156 | 0.2837 | 9.871×10-4 | 0.0205 | 0.9887 |
| 333.15 | 0.6542 | 0.3461 | 0.0016 | 0.0372 | 0.9892 | |||||
| [1] | Zhang Y., Jin W., Yang M. C., Zhang T. Q., Zhou C., Xie F., Song Q., Ren H., Jin Q. H., Mu Y., Chem. Res. Chinese Universities, 2012, 28(5), 792—796 |
| [2] | Mohy E. M. S., Enshasy H. A. E., Hassan M. E., Haroun B., Hassan E. A., J. Appl. Polym. Sci., 2012, 125(5), 3820—3828 |
| [3] | Gotti R., Fiori J., Calleri E., Temporini C., Lubda D., Massolini G., J. Chromatogr. A, 2012, 1234, 45—49 |
| [4] | Pereira S. C., Bussamara R., Marin G., Giordano R. L. C., Dupont J., Giordano R. C., Green Chem., 2012, 14, 3146—3156 |
| [5] | Xue Y. P., Jiang T., Liu X., Zheng Y. G., Biochem. Eng. J., 2013, 74, 88—94 |
| [6] | Li D. C., Cheng S. W., Wei D. Z., Biotechnol. Lett., 2007, 29(12), 1825—1830 |
| [7] | Cabrera Z., Lopez-Gallego F., Fernandez-Lorente G., Palomo J. M., Montes T., Grazu V., Guisán J. M., Fernández-Lafuente R., Enzyme Microb. Tech., 2007, 40(5), 997—1000 |
| [8] | Zhou H. C., Yang L. R., Shou Q. H., Xu P., Li W. S., Wang F. C., Yu P. H., Liu H. Z., Ind. Eng. Chem. Res., 2012, 51(12), 4582—4590 |
| [9] | Chen C. I., Ko Y. M., Lien W. L., Lin Y. H., Li I. T., Chen C. H., Shieh C. J., Liu Y. C., J. Membrane Sci., 2012, 401/402, 33—39 |
| [10] | Cao L., van Rantwijk F., Sheldon R. A., Org. Lett., 2000, 2(10), 1361—1364 |
| [11] | Hormigo D., García-Hidalgo J., Acebal C., Isabel de la M., Arroyo M., Bioresource Technol., 2012, 115, 117—182 |
| [12] | Sinirlioglu A. Z., Sinirlioglu D., Akbas F., Bioresource Technol., 2013, 146, 807—811 |
| [13] | Roessl U., Nahálka J., Nidetzky B., Biotechnol. Lett., 2010, 32, 341—350 |
| [14] | Jaiprakash G. S., Kamalesh K. K., Gangadhar R. A., Biotechnol. Tech., 1987, 1(1), 69—72 |
| [15] | Šulek F., Fernández D. P., Knez Z., Habulin M., Sheldon R. A., Process Biochem., 2011, 46(3), 765—769 |
| [16] | Schoevaart R., Wolbers M. W., Golubovic M., Ottens M., Kieboom A. P. G., van Rantwijk F., van der Wielen L. A. M., Sheldon R. A., Biotechnol. Bioeng., 2004, 87(6), 754—762 |
| [17] | Vafiadia C., Topakasa E., Alissandratosa A., Fauldsb C. B., Christakopoulos P., J. Biotechnol., 2008, 133(4), 497—504 |
| [18] | Barbosa O., Torres R., Ortiz C., Fernandez-Lafuente R., Process Biochem., 2012, 47(8), 1220—1227 |
| [19] | Barbosa O., Torres R., Ortiz C., Fernandez-Lafuente R., Process Biochem., 2012, 47(5), 766—774 |
| [20] | Yu C. Y., Li X. F., Lou W. Y., Zong M. H., J. Biotechnol., 2012, 166(1/2), 12—19 |
| [21] | Yan J. Y., Gui X. H., Wang G. L., Yan Y. J., Appl. Biochem. Biotechnol., 2012, 166(4), 925—932 |
| [22] | Wang M. F., Qi W., He Z. M., Chem. J. Chinese Universities, 2010, 31(9), 1774—1779 |
| (王梦凡, 齐崴, 何志敏.高等学校化学学报, 2010,31(9), 1774—1779) | |
| [23] | Hu Y., Yang J., Tang S. S., Chu X. M., Zou B., Huang H., Chem. J. Chinese Universities, 2013, 34(5), 1195—1202 |
| (胡燚, 杨姣, 唐苏苏, 初旭明, 邹彬, 黄和.高等学校化学学报, 2013,34(5), 1195—1202) | |
| [24] | Xie X.Y., Wang C. X., Wang Z. Y., Acta Chim. Sinica, 2006, 64, 2151—2156 |
| (谢修银, 汪存信, 王志勇. 化学学报, 2006, 64, 2151—5156) | |
| [25] | Ladero M., Ruiz G., Pessela B. C. C., Vian A., Santos A., Garcia-ohoa F., Biochem. Eng. J., 2006, 31, 14—24 |
| [26] | Ladero M., Santos A., Garcia-ohoa F., Enzyme Microb. Tech., 2006, 38(1/2), 1—9 |
| [27] | Grancic P., Illeova V., Polakovic M., Sefcik J., Chem. Eng. Sci., 2012, 70, 14—21 |
| [1] | ZHOU Ning, TANG Xiaohua, CAO Hong, ZHA Fei, LI Chun, XIE Chunyan, XU Mingping, SUN Yige. Preparation, Characterization and Degradation to BPA of Pomegranate-like Gel Microsphere Entrapmented Laccase [J]. Chem. J. Chinese Universities, 2022, 43(2): 20210705. |
| [2] | ZHU Haotian, JIN Meixiu, TANG Wensi, SU Fang, LI Yangguang. Properties of Transition Metal-biimidazole-Dawson-type Tungstophosphate Hybrid Compounds as Supports for Enzyme Immobilization [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220328. |
| [3] | WU Rong, DONG Qihui, SUN Yiyi, SU Erzheng. Efficient Enzyme Immobilization by Combining Adsorption and Cellulose Membrane Coating † [J]. Chem. J. Chinese Universities, 2019, 40(9): 1888. |
| [4] | HUANG Xiaolin,XIE Huan,CAO Hong,JIN Hongjie,LI Chun,WU Tinghua. Preparation, Characterization of Magnetic Core Gel Microspheres Loaded Ionic Liquids and Its Application in Cells Immobilization Technique† [J]. Chem. J. Chinese Universities, 2019, 40(4): 793. |
| [5] | DUAN Bingyi,WANG Yu,GUO Ningning,WANG Runwei,ZHANG Zongtao,QIU Shilun. Preparation of Yolk-shell Fe3O4@SiO2@PMO Magnetic Microspheres for Laccase Immobilization† [J]. Chem. J. Chinese Universities, 2019, 40(2): 210. |
| [6] | Xiaoyu FAN,Ke WANG,Shiyong SUN,Biaobiao MA,Rui LÜ. Construction and Catalytic Performances of Fe-aminoclay Nanostructured Lipase † [J]. Chem. J. Chinese Universities, 2019, 40(12): 2512. |
| [7] | GAO Fengqin,WANG Shan,WANG Yunfang,ZHAO Danlei,CUI Ru,JIANG Yucheng. Immobilization of Chloroperoxidase with Magnetic Graphene Oxide and Its Application of Decolorization of Acid Blue 45† [J]. Chem. J. Chinese Universities, 2018, 39(5): 904. |
| [8] | ZHOU Yamei, KONG Xiangzheng, HAN Hui, JIANG Xubao, ZHU Xiaoli. Easy Synthesis of Porous Polyurea and Its Application in Enzyme Immobilization and Kinetic Resolution of Racemic Phenylethanol† [J]. Chem. J. Chinese Universities, 2017, 38(3): 495. |
| [9] | XIA Ying, CAO Hao, ZHANG Yingjiu. Effect of Substrate Material on the Immobilization of Multifunctional Amylase† [J]. Chem. J. Chinese Universities, 2015, 36(2): 330. |
| [10] | LI Qiujin, ZHAO Zhiqi, YUAN Yamei, GONG Jixian, ZHANG Jianfei. Immobilization of Papain with Ionic Liquids-regenerated Cellulose Membrane for Wool Fabric Modification† [J]. Chem. J. Chinese Universities, 2015, 36(12): 2590. |
| [11] | SONG Min, ZHANG Linping, ZHONG Yi, XU Hong, MAO Zhiping. Catalytic Properties of Manganese Complex of Cyclic Polyamine Encapsulated in Ethyl Cellulose Microcapsules† [J]. Chem. J. Chinese Universities, 2014, 35(9): 1941. |
| [12] | HU Yi, YANG Jiao, TANG Su-Su, CHU Xu-Ming, ZOU Bin, HUANG He. Covalent Immobilization of Burkholderia Cepacia Lipase on Amine Functionalized Ionic Liquid Modified SBA-15 [J]. Chem. J. Chinese Universities, 2013, 34(5): 1195. |
| [13] | LI Yu, ZHANG Chen, LIU Jian-lin, LI Xiao-Peng, WANG Xia-Jiao. Thermodynamic Characteristics and Mechanisms of Estrogen Hormones Adsorption on Soil Doped with MnO2 [J]. Chem. J. Chinese Universities, 2013, 34(3): 634. |
| [14] | CHEN Pei-Pei, XU Jia, JIANG Yu-Ye, FENG Chen-Chen, GAO Cong-Jie. Preparation and Characterization of a Novel Anti-biofouling Ultrafiltration Membrane with Cu2+ Immobilization [J]. Chem. J. Chinese Universities, 2013, 34(3): 739. |
| [15] | WANG Miao-Miao, LI Qun-Yan, WEI Qi, NIE Zuo-Ren. Studies on Laccase Immobilization of Mesoporous SiO2/Fe3O4 Hollow Microspheres [J]. Chem. J. Chinese Universities, 2013, 34(2): 299. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||