

Chem. J. Chinese Universities ›› 2018, Vol. 39 ›› Issue (4): 749.doi: 10.7503/cjcu20170463
• Physical Chemistry • Previous Articles Next Articles
TIAN Linfei1, ZHANG Chunhua2, QU Ning1, BI Yanting1, ZHANG Hongxing3,*( ), PAN Qingjiang1,*(
), PAN Qingjiang1,*( )
)
Received:2017-07-14
Online:2018-04-10
Published:2018-03-16
Contact:
ZHANG Hongxing,PAN Qingjiang
E-mail:zhanghx@jlu.edu.cn;panqjitc@163.com
Supported by:CLC Number:
TrendMD:
TIAN Linfei, ZHANG Chunhua, QU Ning, BI Yanting, ZHANG Hongxing, PAN Qingjiang. Theoretical Studies of Structural Design and Stability of Double-layered Sandwich-like Tetrapyrrolic Uranium Complexes†[J]. Chem. J. Chinese Universities, 2018, 39(4): 749.
| Complex | Config. | ESS | ΔEa/(kJ·mol-1) | Δ | ΔGa/(kJ·mol-1) | ΔHa/(kJ·mol-1) |
|---|---|---|---|---|---|---|
| se | Singlet | 86.6 | 82.2 | 89.5 | 81.4 | |
| Triplet | 11.7 | 11.1 | 12.5 | 11.2 | ||
| Quintet | 0 | 0 | 0 | 0 | ||
| es | Singlet | 92.9 | 91.5 | 99.2 | 90.6 | |
| Tripletb | 23.6 | 16.9 | 21.8 | 14.2 | ||
| Quintet | 0.2 | 2.4 | 6.2 | 1.9 | ||
| se | Singlet | 77.8 | 70.9 | 70.2 | 71.7 | |
| Triplet | 7.9 | 3.5 | 5.4 | 1.5 | ||
| Quintet | 2.9 | -0.2 | 3.2 | -2.0 | ||
| Septet | 10.0 | 9.1 | 5.0 | 9.6 | ||
| es | Singlet | 82.0 | 76.7 | 77.3 | 77.1 | |
| Triplet | 6.7 | 5.0 | 3.9 | 5.4 | ||
| Quintet | 0 | 0 | 0 | 0 | ||
| Septet | 10.0 | 9.2 | 12.0 | 7.1 |
Table 1 Relative energy of Um2Pz3(m=Ⅳ, Ⅲ) complexes in various configurations(staggered-eclipsed and eclipsed-staggered) and electron-spin states(ESS)
| Complex | Config. | ESS | ΔEa/(kJ·mol-1) | Δ | ΔGa/(kJ·mol-1) | ΔHa/(kJ·mol-1) |
|---|---|---|---|---|---|---|
| se | Singlet | 86.6 | 82.2 | 89.5 | 81.4 | |
| Triplet | 11.7 | 11.1 | 12.5 | 11.2 | ||
| Quintet | 0 | 0 | 0 | 0 | ||
| es | Singlet | 92.9 | 91.5 | 99.2 | 90.6 | |
| Tripletb | 23.6 | 16.9 | 21.8 | 14.2 | ||
| Quintet | 0.2 | 2.4 | 6.2 | 1.9 | ||
| se | Singlet | 77.8 | 70.9 | 70.2 | 71.7 | |
| Triplet | 7.9 | 3.5 | 5.4 | 1.5 | ||
| Quintet | 2.9 | -0.2 | 3.2 | -2.0 | ||
| Septet | 10.0 | 9.1 | 5.0 | 9.6 | ||
| es | Singlet | 82.0 | 76.7 | 77.3 | 77.1 | |
| Triplet | 6.7 | 5.0 | 3.9 | 5.4 | ||
| Quintet | 0 | 0 | 0 | 0 | ||
| Septet | 10.0 | 9.2 | 12.0 | 7.1 |
| Species | se | es | Expt.h | |||
|---|---|---|---|---|---|---|
| Singlet | Triplet | Quintet | Singlet | Quintet | ||
| U1-U2/nm | 0.3513 | 0.3568 | 0.3611 | 0.3499 | 0.3607 | 0.331 |
| U1-N(Pz1 | 0.2326 | 0.2342 | 0.2346 | 0.2348 | 0.2355 | |
| U1-N(Pz2 | 0.2588 | 0.2604 | 0.2619 | 0.2568 | 0.2633 | 0.242-0.248 |
| (U1-N | 0.2457 | 0.2473 | 0.2482 | 0.2458 | 0.2494 | |
| U2-N(Pz2 | 0.2588 | 0.2603 | 0.2619 | 0.2585 | 0.2607 | 0.243-0.253 |
| U2-N(Pz3 | 0.2326 | 0.2342 | 0.2346 | 0.2341 | 0.2350 | |
| (U2-N | 0.2457 | 0.2472 | 0.2482 | 0.2463 | 0.2479 | |
| U1-N4(Pz1)c/nm | 0.1262 | 0.1290 | 0.1291 | 0.1303 | 0.1304 | |
| U1-N4(Pz2)c/nm | 0.1752 | 0.1785 | 0.1806 | 0.1735 | 0.1823 | |
| U1-N | 0.1507 | 0.1538 | 0.1548 | 0.1519 | 0.1563 | |
| U2-N4(Pz2)c/nm | 0.1752 | 0.1784 | 0.1806 | 0.1761 | 0.1785 | |
| U2-N4(Pz3)c/nm | 0.1262 | 0.1290 | 0.1291 | 0.1289 | 0.1298 | |
| U2-N | 0.1507 | 0.1537 | 0.1548 | 0.1525 | 0.1541 | |
| U1-(Pz2)cent. -U2e/(°) | 180.0 | 180.0 | 180.0 | 179.3 | 180.0 | |
| h1f/(°) | 44.9 | 52.7 | 48.9 | 19.0g | -12.8g | |
| h2f/(°) | 0.8 | 0.2 | 7.7 | 42.5 | 30.8 | |
Table 2 Optimized geometry parameters of U2ⅣPz3
| Species | se | es | Expt.h | |||
|---|---|---|---|---|---|---|
| Singlet | Triplet | Quintet | Singlet | Quintet | ||
| U1-U2/nm | 0.3513 | 0.3568 | 0.3611 | 0.3499 | 0.3607 | 0.331 |
| U1-N(Pz1 | 0.2326 | 0.2342 | 0.2346 | 0.2348 | 0.2355 | |
| U1-N(Pz2 | 0.2588 | 0.2604 | 0.2619 | 0.2568 | 0.2633 | 0.242-0.248 |
| (U1-N | 0.2457 | 0.2473 | 0.2482 | 0.2458 | 0.2494 | |
| U2-N(Pz2 | 0.2588 | 0.2603 | 0.2619 | 0.2585 | 0.2607 | 0.243-0.253 |
| U2-N(Pz3 | 0.2326 | 0.2342 | 0.2346 | 0.2341 | 0.2350 | |
| (U2-N | 0.2457 | 0.2472 | 0.2482 | 0.2463 | 0.2479 | |
| U1-N4(Pz1)c/nm | 0.1262 | 0.1290 | 0.1291 | 0.1303 | 0.1304 | |
| U1-N4(Pz2)c/nm | 0.1752 | 0.1785 | 0.1806 | 0.1735 | 0.1823 | |
| U1-N | 0.1507 | 0.1538 | 0.1548 | 0.1519 | 0.1563 | |
| U2-N4(Pz2)c/nm | 0.1752 | 0.1784 | 0.1806 | 0.1761 | 0.1785 | |
| U2-N4(Pz3)c/nm | 0.1262 | 0.1290 | 0.1291 | 0.1289 | 0.1298 | |
| U2-N | 0.1507 | 0.1537 | 0.1548 | 0.1525 | 0.1541 | |
| U1-(Pz2)cent. -U2e/(°) | 180.0 | 180.0 | 180.0 | 179.3 | 180.0 | |
| h1f/(°) | 44.9 | 52.7 | 48.9 | 19.0g | -12.8g | |
| h2f/(°) | 0.8 | 0.2 | 7.7 | 42.5 | 30.8 | |
| Species | se | es | ||||||
|---|---|---|---|---|---|---|---|---|
| Singlet | Triplet | Quintet | Septet | Singlet | Triplet | Quintet | Septet | |
| U1-U2/nm | 0.3400 | 0.3521 | 0.3548 | 0.3548 | 0.3391 | 0.3497 | 0.3504 | 0.3557 |
| U1-N(Pz1 | 0.2338 | 0.2346 | 0.2352 | 0.2378 | 0.2356 | 0.2372 | 0.2374 | 0.2382 |
| U1-N(Pz2 | 0.2554 | 0.2588 | 0.2598 | 0.2603 | 0.2547 | 0.2575 | 0.2573 | 0.2597 |
| (U1-N | 0.2446 | 0.2467 | 0.2475 | 0.2490 | 0.2452 | 0.2474 | 0.2473 | 0.2490 |
| U2-N(Pz2 | 0.2554 | 0.2589 | 0.2598 | 0.2605 | 0.2548 | 0.2593 | 0.2602 | 0.2613 |
| U2-N(Pz3 | 0.2338 | 0.2346 | 0.2352 | 0.2359 | 0.2334 | 0.2351 | 0.2354 | 0.2357 |
| (U2-N | 0.2446 | 0.2467 | 0.2475 | 0.2482 | 0.2441 | 0.2472 | 0.2478 | 0.2485 |
| U1-N4(Pz1)c/nm | 0.1279 | 0.1291 | 0.1298 | 0.1335 | 0.1307 | 0.1328 | 0.1329 | 0.1340 |
| U1-N4(Pz2)c/nm | 0.1700 | 0.1760 | 0.1774 | 0.1773 | 0.1695 | 0.1736 | 0.1731 | 0.1767 |
| U1-N | 0.1490 | 0.1526 | 0.1536 | 0.1554 | 0.1501 | 0.1532 | 0.1530 | 0.1554 |
| U2-N4(Pz2)c/nm | 0.1700 | 0.1761 | 0.1774 | 0.1775 | 0.1696 | 0.1761 | 0.1773 | 0.1790 |
| U2-N4(Pz3)c/nm | 0.1279 | 0.1291 | 0.1297 | 0.1308 | 0.1325 | 0.1298 | 0.1302 | 0.1301 |
| U2-N | 0.1490 | 0.1526 | 0.1536 | 0.1541 | 0.1510 | 0.1530 | 0.1537 | 0.1546 |
| U1-(Pz2)cent.-U2e/(°) | 180.0 | 180.0 | 180.0 | 180.0 | 180.0 | 180.0 | 180.0 | 180.0 |
| h1f/(°) | 45.1 | 45.2 | 49.1 | 50.9 | -0.1 | 0.2 | -0.2 | 0.2 |
| h2f/(°) | 0.8 | -0.7 | 7.4 | 30.5g | 44.8 | 38.8 | 36.9 | 44.2 |
Table 3 Optimized geometry parameters of U2ⅢPz3
| Species | se | es | ||||||
|---|---|---|---|---|---|---|---|---|
| Singlet | Triplet | Quintet | Septet | Singlet | Triplet | Quintet | Septet | |
| U1-U2/nm | 0.3400 | 0.3521 | 0.3548 | 0.3548 | 0.3391 | 0.3497 | 0.3504 | 0.3557 |
| U1-N(Pz1 | 0.2338 | 0.2346 | 0.2352 | 0.2378 | 0.2356 | 0.2372 | 0.2374 | 0.2382 |
| U1-N(Pz2 | 0.2554 | 0.2588 | 0.2598 | 0.2603 | 0.2547 | 0.2575 | 0.2573 | 0.2597 |
| (U1-N | 0.2446 | 0.2467 | 0.2475 | 0.2490 | 0.2452 | 0.2474 | 0.2473 | 0.2490 |
| U2-N(Pz2 | 0.2554 | 0.2589 | 0.2598 | 0.2605 | 0.2548 | 0.2593 | 0.2602 | 0.2613 |
| U2-N(Pz3 | 0.2338 | 0.2346 | 0.2352 | 0.2359 | 0.2334 | 0.2351 | 0.2354 | 0.2357 |
| (U2-N | 0.2446 | 0.2467 | 0.2475 | 0.2482 | 0.2441 | 0.2472 | 0.2478 | 0.2485 |
| U1-N4(Pz1)c/nm | 0.1279 | 0.1291 | 0.1298 | 0.1335 | 0.1307 | 0.1328 | 0.1329 | 0.1340 |
| U1-N4(Pz2)c/nm | 0.1700 | 0.1760 | 0.1774 | 0.1773 | 0.1695 | 0.1736 | 0.1731 | 0.1767 |
| U1-N | 0.1490 | 0.1526 | 0.1536 | 0.1554 | 0.1501 | 0.1532 | 0.1530 | 0.1554 |
| U2-N4(Pz2)c/nm | 0.1700 | 0.1761 | 0.1774 | 0.1775 | 0.1696 | 0.1761 | 0.1773 | 0.1790 |
| U2-N4(Pz3)c/nm | 0.1279 | 0.1291 | 0.1297 | 0.1308 | 0.1325 | 0.1298 | 0.1302 | 0.1301 |
| U2-N | 0.1490 | 0.1526 | 0.1536 | 0.1541 | 0.1510 | 0.1530 | 0.1537 | 0.1546 |
| U1-(Pz2)cent.-U2e/(°) | 180.0 | 180.0 | 180.0 | 180.0 | 180.0 | 180.0 | 180.0 | 180.0 |
| h1f/(°) | 45.1 | 45.2 | 49.1 | 50.9 | -0.1 | 0.2 | -0.2 | 0.2 |
| h2f/(°) | 0.8 | -0.7 | 7.4 | 30.5g | 44.8 | 38.8 | 36.9 | 44.2 |
| Complex | Config. | ESS | QU1/e | QU2/e | SU1/a.u. | SU2/a.u. |
|---|---|---|---|---|---|---|
| se | Singlet | 2.057 | 2.058 | 0 | 0 | |
| Triplet | 2.048 | 2.048 | 1.627 | 1.627 | ||
| Quintet | 2.035 | 2.035 | 1.877 | 1.877 | ||
| es | Singlet | 2.030 | 2.035 | 0 | 0 | |
| Triplet* | 2.043 | 2.031 | 1.470 | 1.741 | ||
| Quintet | 2.028 | 2.007 | 1.879 | 1.995 | ||
| se | Singlet | 2.027 | 2.027 | 0 | 0 | |
| Triplet | 2.024 | 2.024 | 1.747 | 1.749 | ||
| Quintet | 2.013 | 2.013 | 1.924 | 1.926 | ||
| Septet | 1.968 | 1.996 | 2.171 | 2.047 | ||
| es | Singlet | 2.025 | 2.002 | 0 | 0 | |
| Triplet | 2.012 | 1.985 | 1.777 | 1.958 | ||
| Quintet | 2.004 | 1.979 | 1.918 | 2.013 | ||
| Septet | 1.992 | 1.963 | 2.059 | 2.158 |
Table 4 Calculated electron-spin density(SU) and charge(QU) of the uranium atom of Um2Pz3(m=Ⅳ, Ⅲ) complexes
| Complex | Config. | ESS | QU1/e | QU2/e | SU1/a.u. | SU2/a.u. |
|---|---|---|---|---|---|---|
| se | Singlet | 2.057 | 2.058 | 0 | 0 | |
| Triplet | 2.048 | 2.048 | 1.627 | 1.627 | ||
| Quintet | 2.035 | 2.035 | 1.877 | 1.877 | ||
| es | Singlet | 2.030 | 2.035 | 0 | 0 | |
| Triplet* | 2.043 | 2.031 | 1.470 | 1.741 | ||
| Quintet | 2.028 | 2.007 | 1.879 | 1.995 | ||
| se | Singlet | 2.027 | 2.027 | 0 | 0 | |
| Triplet | 2.024 | 2.024 | 1.747 | 1.749 | ||
| Quintet | 2.013 | 2.013 | 1.924 | 1.926 | ||
| Septet | 1.968 | 1.996 | 2.171 | 2.047 | ||
| es | Singlet | 2.025 | 2.002 | 0 | 0 | |
| Triplet | 2.012 | 1.985 | 1.777 | 1.958 | ||
| Quintet | 2.004 | 1.979 | 1.918 | 2.013 | ||
| Septet | 1.992 | 1.963 | 2.059 | 2.158 |
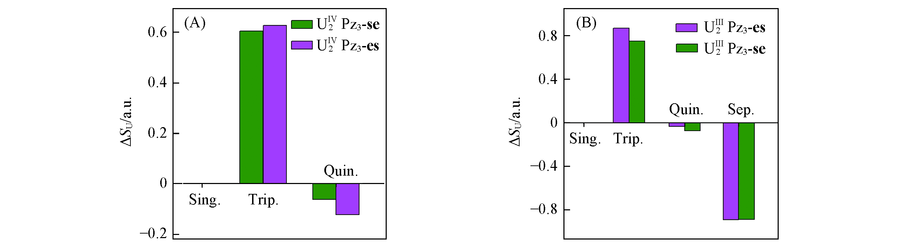
Fig.3 Difference of spin density of uranium atom(ΔSU) of se- and es-configurationUm2Pz3[(m=Ⅳ(A), Ⅲ(B)] in various electron-spin states from the respective expected formal value,i.e., 0, 1, 2 and 3 for the singlet, triplet, quintet and septet states, respectively.
| BCP | Parameter* | ||||
|---|---|---|---|---|---|
| se | es | se | es | ||
| U1-N(Pz1) | ρ(r)/a.u. | 0.0861 | 0.0853 | 0.0841 | 0.0839 |
| ?2ρ(r)/a.u. | 0.2083 | 0.2047 | 0.2109 | 0.2080 | |
| H(r)/a.u. | -0.0203 | -0.0198 | -0.0190 | -0.0189 | |
| U1-N(Pz2) | ρ(r)/a.u. | 0.0467 | 0.0478 | 0.0499 | 0.0477 |
| ?2ρ(r)/a.u. | 0.1299 | 0.1387 | 0.1324 | 0.1638 | |
| H(r)/a.u. | -0.0030 | -0.0032 | -0.0041 | -0.0027 | |
| U2-N(Pz2) | ρ(r)/a.u. | 0.0468 | 0.0451 | 0.0477 | 0.0497 |
| ?2ρ(r)/a.u. | 0.1301 | 0.1268 | 0.1496 | 0.1301 | |
| H(r)/a.u. | -0.0030 | -0.0029 | -0.0028 | -0.0042 | |
| U2-N(Pz3) | ρ(r)/a.u. | 0.0861 | 0.0831 | 0.0799 | 0.0740 |
| ?2ρ(r)/a.u. | 0.2088 | 0.2055 | 0.2391 | 0.2349 | |
| H(r)/a.u. | -0.0203 | -0.0189 | -0.0162 | -0.0133 | |
Table 5 QTAIM parameters for the U-N bond of ground-state Um2Pz3(m=Ⅳ, Ⅲ) in various steric configurations, including electron density[ρ(r)], Laplacian[?2ρ(r)] and energy density[H(r)] at BCPs
| BCP | Parameter* | ||||
|---|---|---|---|---|---|
| se | es | se | es | ||
| U1-N(Pz1) | ρ(r)/a.u. | 0.0861 | 0.0853 | 0.0841 | 0.0839 |
| ?2ρ(r)/a.u. | 0.2083 | 0.2047 | 0.2109 | 0.2080 | |
| H(r)/a.u. | -0.0203 | -0.0198 | -0.0190 | -0.0189 | |
| U1-N(Pz2) | ρ(r)/a.u. | 0.0467 | 0.0478 | 0.0499 | 0.0477 |
| ?2ρ(r)/a.u. | 0.1299 | 0.1387 | 0.1324 | 0.1638 | |
| H(r)/a.u. | -0.0030 | -0.0032 | -0.0041 | -0.0027 | |
| U2-N(Pz2) | ρ(r)/a.u. | 0.0468 | 0.0451 | 0.0477 | 0.0497 |
| ?2ρ(r)/a.u. | 0.1301 | 0.1268 | 0.1496 | 0.1301 | |
| H(r)/a.u. | -0.0030 | -0.0029 | -0.0028 | -0.0042 | |
| U2-N(Pz3) | ρ(r)/a.u. | 0.0861 | 0.0831 | 0.0799 | 0.0740 |
| ?2ρ(r)/a.u. | 0.2088 | 0.2055 | 0.2391 | 0.2349 | |
| H(r)/a.u. | -0.0203 | -0.0189 | -0.0162 | -0.0133 | |
| Orbital | Energy/eV | U1(%) | U2(%) | Ligand(%) | Assignment(%) | ||
|---|---|---|---|---|---|---|---|
| d | f | d | f | ||||
| L+2 | -5.878 | 1.57 | 43.89 | 1.58 | 43.83 | 5f | |
| L+1 | -5.883 | 1.43 | 44.03 | 1.44 | 43.56 | 5f | |
| LUMO | -5.897 | 37.08 | 37.27 | 5f | |||
| HOMO | -5.912 | 43.58 | 43.68 | 5f | |||
| H-1 | -5.942 | 35.41 | 33.91 | 5f | |||
| H-2 | -5.967 | 22.03 | 22.10 | 26.31 | 5f+L | ||
| H-3 | -6.183 | 2.73 | 44.38 | 2.73 | 44.28 | 5fσ | |
| H-4 | -6.634 | 72.88 | L | ||||
| H-5 | -6.919 | 86.28 | L | ||||
Table 6 Contributions of α-spin orbitals of U2ⅣPz3-se in the quintet(ground) state
| Orbital | Energy/eV | U1(%) | U2(%) | Ligand(%) | Assignment(%) | ||
|---|---|---|---|---|---|---|---|
| d | f | d | f | ||||
| L+2 | -5.878 | 1.57 | 43.89 | 1.58 | 43.83 | 5f | |
| L+1 | -5.883 | 1.43 | 44.03 | 1.44 | 43.56 | 5f | |
| LUMO | -5.897 | 37.08 | 37.27 | 5f | |||
| HOMO | -5.912 | 43.58 | 43.68 | 5f | |||
| H-1 | -5.942 | 35.41 | 33.91 | 5f | |||
| H-2 | -5.967 | 22.03 | 22.10 | 26.31 | 5f+L | ||
| H-3 | -6.183 | 2.73 | 44.38 | 2.73 | 44.28 | 5fσ | |
| H-4 | -6.634 | 72.88 | L | ||||
| H-5 | -6.919 | 86.28 | L | ||||
| [1] | Lu J., Deng Y., Zhang X., Kobayashi N., Jiang J., Inorg. Chem., 2011, 50, 2562-2567 |
| [2] | Gao F., Li Y. Y., Liu C. M., Li Y. Z., Zuo J. L., Dalton Trans., 2013, 42, 11043-11046 |
| [3] | Zhang X., Chen Y., Inorg. Chem. Commun., 2014, 39, 79-82 |
| [4] | Birin K. P., Gorbunova Y. G., Tsivadze A. Y., Dalton Trans., 2012, 41, 9672-9681 |
| [5] | Lu J., Zhang D., Wang H., Jiang J., Zhang X., Inorg. Chem. Commun., 2010, 13, 1144-1147 |
| [6] | Moussavi M., De Cian A., Fischer J., Weiss R., Inorg. Chem., 1986, 25, 2107-2108 |
| [7] | Sun X., Li R., Wang D., Dou J., Zhu P., Lu F., Ma C., Choi C. F., Cheng D. Y. Y., Ng D. K. P., Kobayashi N., Jiang J., Eur. J. Inorg. Chem., 2004, 2004, 3806-3813 |
| [8] | Zhu P., Zhang X., Wang H., Zhang Y., Bian Y., Jiang J., Inorg. Chem., 2012, 51, 5651-5659 |
| [9] | Padmaja K., Youngblood W. J., Wei L., Bocian D. F., Lindsey J. S., Inorg. Chem., 2006, 45, 5479-5492 |
| [10] | Le Borgne T., Lance M., Nierlich M., Ephritikhine M., J. Organomet. Chem., 2000, 598, 313-317 |
| [11] | Evans W. J., Nyce G. W., Ziller J. W., Angew. Chem. Int. Ed., 2000, 39, 240-242 |
| [12] | Zong M. R., He H. C., Dong F. Q., He P., Sun S. Y., Liu M. X., Nie X. Q., Chem. J. Chinese Universities, 2016, 37(9), 1701-1709 |
| (宗美荣, 何辉超, 董发勤, 何平, 孙仕勇, 刘明学, 聂小琴. 高等学校化学学报, 2016, 37(9), 1701-1709.) | |
| [13] | Yue G. Z., Gao R., Zhao P. X., Chu M. F., Shuai M. B., Acta Chim. Sinica, 2016, 74, 657-663 |
| (岳国宗, 高瑞, 赵鹏翔, 褚明福, 帅茂兵. 化学学报, 2016, 74, 657-663) | |
| [14] | Laikov D. N., Ustynyuk Y. A., Russ. Chem. Bull., 2005, 54, 820-826 |
| [15] | Laikov D. N., J. Comput. Chem., 2007, 28, 698-702 |
| [16] | Perdew J. P., Burke K., Ernzerhof M., Phys. Rev. Lett., 1996, 77, 3865-3868 |
| [17] | Bader R. F. W., J. Phys. Chem. A, 1998, 102, 7314-7323 |
| [18] | Bader R. F. W., Chem. Rev., 1991, 91, 893-928 |
| [19] | Frisch M. J., Trucks G. W., Schlegel H. B., Scuseria G. E., Robb M. A., Cheeseman J. R., Scalmani G., Barone V., Mennucci B., Petersson G. A., Nakatsuji H., Caricato M., Li X., Hratchian H. P., Izmaylov A. F., Bloino J., Zheng G., Sonnenberg J. L., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Vreven T., Montgomery J. A. Jr., Peralta J. E., Ogliaro F., Bearpark M., Heyd J. J., Brothers E., Kudin K. N., Staroverov V. N., Kobayashi R., Normand J., Raghavachari K., Rendell A., Burant J. C., Iyengar S. S., Tomasi J., Cossi M., Rega N., Millam N. J., Klene M., Knox J. E., Cross J. B., Bakken V., Adamo C., Jaramillo J., Gomperts R., Stratmann R. E., Yazyev O., Austin A. J., Cammi R., Pomelli C., Ochterski J. W., Martin R. L., Morokuma K., Zakrzewski V. G., Voth G. A., Salvador P., Dannenberg J. J., Dapprich S., Daniels A. D., Farkas Ö., Foresman J. B., Ortiz J. V., Cioslowski J., Fox D. J., Gaussian 09, Revision A. 1, Gaussian Inc., Wallingford CT, 2009 |
| [20] | Cao X., Dolg M., Stoll H., J. Chem. Phys., 2003, 118, 487-496 |
| [21] | Lu T., Chen F., J. Comput. Chem., 2012, 33, 580-592 |
| [22] | Baerends E. J., Ziegler T., Autschbach J., Bashford D., Bérces A., Bickelhaupt F. M., Bo C., Boerrigter P. M., Cavallo L., Chong D. P., Deng L., Dickson R. M., Ellis D. E., van Faassen M., Fan L., Fischer T. H., Fonseca G. C., Franchini M., Ghysels A., Giammona A., van Gisbergen S. J. A., Götz A. W., Groeneveld J. A., Gritsenko O. V., Grüning M., Gusarov S., Harris F. E., van den Hoek P., Jacob C. R., Jacobsen H., Jensen L., Kaminski J. W., van Kessel G., Kootstra F., Kovalenko A., Krykunov M. V., van Lenthe E., McCormack D. A., Michalak A., Mitoraj M., Morton S. M., Neugebauer J., Nicu V. P., Noodleman L., Osinga V. P., Patchkovskii S., Pavanello M., Philipsen P. H. T., Post D., Pye C. C., Ravenek W., Rodríguez J. I., Ros P., Schipper P. R. T., van Schoot H., Schreckenbach G., Seldenthuis J. S., Seth M., Snijders J. G., SolàM., Swart M., Swerhone D., te Velde G., Vernooijs P., Versluis L., Visscher L., Visser O., Wang F., Wesolowski T. A., van Wezenbeek E. M., Wiesenekker G., Wolff S. K., Woo T. K., Yakovlev A. L., ADF2012, SCM, Theoretical Chemistry, Vrije Universiteit, Amsterdam, 2012 |
| [23] | Van Lenthe E., Ehlers A., Baerends E. J., J. Chem. Phys., 1999, 110, 8943-8953 |
| [24] | Klamt A., Jonas V., Burger T., Lohrenz J. C. W., J. Phys. Chem. A, 1998, 102, 5074-5085 |
| [25] | Bao Z., Zhao H. B., Qu N., Schreckenbach G., Pan Q. J., Dalton Trans., 2016, 45, 15970-15982 |
| [26] | Yu L. A., Hu B., Luo M. B., Zhang X., Chen H. W., Chem. J. Chinese Universities, 2009, 30(12), 2460-2463 |
| (于立安, 胡斌, 罗明标, 张燮, 陈焕文. 高等学校化学学报, 2009, 30(12), 2460-2463) | |
| [27] | Wang D., van Gunsteren W. F., Chai Z., Chem. Soc. Rev., 2012, 41, 5836-5865 |
| [28] | Kaltsoyannis N., Inorg. Chem., 2013, 52, 3407-3413 |
| [29] | Huang Q. R., Kingham J. R., Kaltsoyannis N., Dalton Trans., 2015, 44, 2554-2566 |
| [30] | Yao J., Zheng X. J., Pan Q. J., Schreckenbach G., Inorg. Chem., 2015, 54, 5438-5449 |
| [31] | Lewis F. W., Harwood L. M., Hudson M. J., Drew M. G. B., Modolo G., Sypula M., Desreux J. F., Bouslimani N., Vidick G., Dalton Trans., 2010, 39, 5172-5182 |
| [1] | XIA Tian, WAN Jiawei, YU Ranbo. Progress of the Structure-property Correlation of Heteroatomic Coordination Structured Carbon-based Single-atom Electrocatalysts [J]. Chem. J. Chinese Universities, 2022, 43(5): 20220162. |
| [2] | WANG Zumin, MENG Cheng, YU Ranbo. Doping Regulation in Transition Metal Phosphides for Hydrogen Evolution Catalysts [J]. Chem. J. Chinese Universities, 2022, 43(11): 20220544. |
| [3] | ZHUO Zengqing, PAN Feng. Progress of Key Electronic States in Lithium Ion Battery Materials Probed Through Soft X-ray Spectroscopy [J]. Chem. J. Chinese Universities, 2021, 42(8): 2332. |
| [4] | SHI Haihan,WU Xiangping,PENG Xinzhe,YU Guojing,DONG Chaoyang,JI Yaoyao,YANG Siwen,CHEN Junlin,WANG Jin,RAN Xueqin,YANG Lei,XIE Linghai,HUANG Wei. An Effective Method of Reducing the Internal Reorganization Energy Based on Windmill-like Grid Composed of Four Carbazoles† [J]. Chem. J. Chinese Universities, 2020, 41(7): 1670. |
| [5] | SUN Guodong,WANG Xue,JIANG Guoliang,XU Zhiyong,LIU Hongmei. Effects of Gas Adsorption on Two Dimensional Metal-hexaiminobenzene Frameworks† [J]. Chem. J. Chinese Universities, 2019, 40(5): 995. |
| [6] | ZHOU Hegen,JIN Hua,GUO Huirui,LIN Jing,ZHANG Yongfan. Electronic Structures and Optical Properties of Cu-based Semiconductors with Chalcopyrite-type Structure† [J]. Chem. J. Chinese Universities, 2019, 40(3): 518. |
| [7] | ZHANG Zhaoyan,CHEN Hongshan. Theoretical Studies on the Zintl Crystals Assembled by Al6ONa2 Clusters † [J]. Chem. J. Chinese Universities, 2019, 40(11): 2354. |
| [8] |
LI Tan, ZHANG Xiaochao, WANG Kai, LI Rui, FAN Caimei.
First-principle Calculations on Electronic Structures and Optical Properties of α, β, γ, δ, ε, η-Bi2 |
| [9] | CANG Yuping, CHEN Dong, YANG Fan, YANG Huiming. Theoretical Studies on Tetragonal, Monoclinic and Orthorhombic Distortions of Germanium Nitride Polymorphs† [J]. Chem. J. Chinese Universities, 2016, 37(4): 674. |
| [10] | WANG Yan, ZHANG Xiaochao, ZHAO Lijun, ZHAO Xiaoxia, SHI Baoping, FAN Caimei. First Principles Calculations on Electronic Structures and Optical Absorption Properties of Non-metal Doped BiOCl† [J]. Chem. J. Chinese Universities, 2014, 35(12): 2624. |
| [11] | ZHOU Bing, LIU Chuanlin, YANG Jiping, CHEN Gong, HUANG Pengcheng. Electronic Structure and Molecular Packing of Regiosymmetric Oligo(3-methylthiophenes)† [J]. Chem. J. Chinese Universities, 2014, 35(12): 2593. |
| [12] | MENG Su-Ci, YIN Xiu-Lian, MA Jing, XIE Ji-Min. Theoretical Studies on Solvent Effects and Intermolecular Interactions of Organic π-Conjugated Ligand in Solutions [J]. Chem. J. Chinese Universities, 2012, 33(11): 2492. |
| [13] | QIU Yi-Xiang, WANG Shu-Guang. Theoretical Investigations of Phosphine-stabilization on Gold Cluster 3+(R=Me, OMe, H, F, Cl, CN) [J]. Chem. J. Chinese Universities, 2012, 33(11): 2549. |
| [14] | HONG Bo, JIN Dong-Ri, LI Yun, MA Ya-Juan, LU Min, LI Xia, ZHANG Hao-Hao, SUN Yu. Theoretical Investigation of Structure and Properties of Ge@C82 [J]. Chem. J. Chinese Universities, 2012, 33(06): 1259. |
| [15] | JIA Jin-Qian, XIE Xue-Jia, LIANG Zhen-Hai, ZHANG Xiao-Chao, FAN Cai-Mei, HAN Pei-De. First-principles Study of Ti-doped SnO2 Semiconductor Solid Solutions [J]. Chem. J. Chinese Universities, 2012, 33(05): 1050. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||