

Chem. J. Chinese Universities ›› 2019, Vol. 40 ›› Issue (3): 508.doi: 10.7503/cjcu20180559
• Physical Chemistry • Previous Articles Next Articles
XU Haiyan1,2, REN Sili3, JIA Weihong1,2, WANG Jinqing1,2,*( )
)
Received:2019-08-10
Online:2019-01-24
Published:2019-01-24
Contact:
WANG Jinqing
E-mail:jqwang@licp.cas.cn
Supported by:CLC Number:
TrendMD:
XU Haiyan,REN Sili,JIA Weihong,WANG Jinqing. Preparation of Magnetically Recyclable Fluorinated Graphene and Its Demulsification Performance for Emulsified Oily Wastewater†[J]. Chem. J. Chinese Universities, 2019, 40(3): 508.
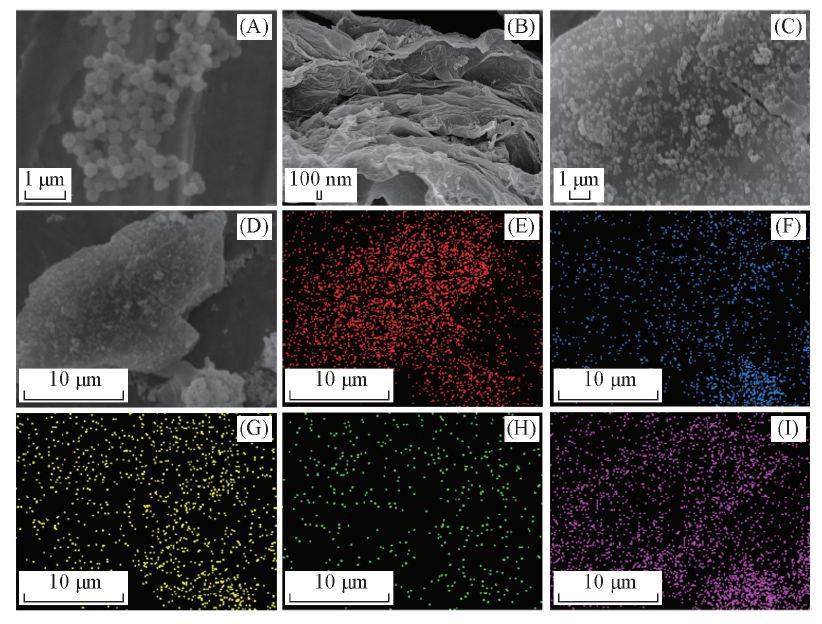
Fig.2 SEM images of Fe3O4 nanoparticles(A), HFG(B), HFG-Fe3O4(C), the element mapping regions of HFG-Fe3O4(D) and the corresponding element mapping images of HFG-Fe3O4(E—I)(E) C; (F) O; (G) F; (H) N; (I) Fe.
| Sample | Atomic ratio(%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | O | F | N | Fe | ||||||
| FG | 54.62 | 1.74 | 43.64 | | | |||||
| HFG | 86.95 | 3.31 | 4.47 | 5.27 | | |||||
| HFG-Fe3O4 | 63.93 | 26.47 | 0.36 | 0.71 | 8.53 | |||||
Table 1 Surface species of FG, HFG and HFG-Fe3O4 samples determined by XPS analysis
| Sample | Atomic ratio(%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | O | F | N | Fe | ||||||
| FG | 54.62 | 1.74 | 43.64 | | | |||||
| HFG | 86.95 | 3.31 | 4.47 | 5.27 | | |||||
| HFG-Fe3O4 | 63.93 | 26.47 | 0.36 | 0.71 | 8.53 | |||||
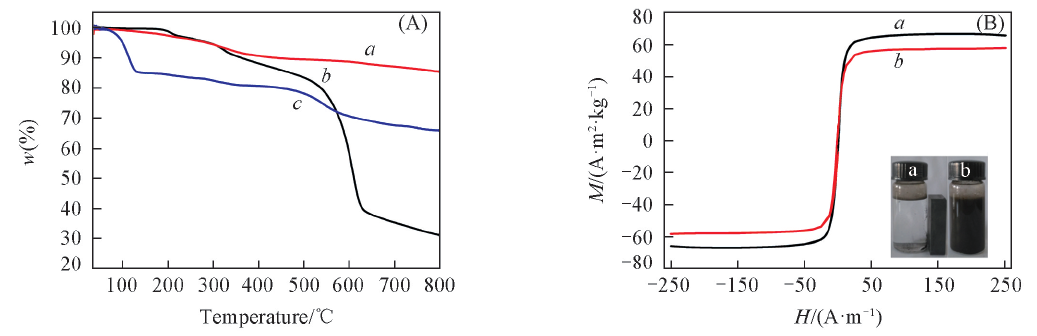
Fig.6 TGA curves of Fe3O4(a), HFG(b) and HFG-Fe3O4(c) samples(A) and magnetic hysteresis loops of Fe3O4(a) and HFG-Fe3O4(b) at room temperature(B)The insets in (B) show the magnetic separation-redispersion results of HFG-Fe3O4(a and b).
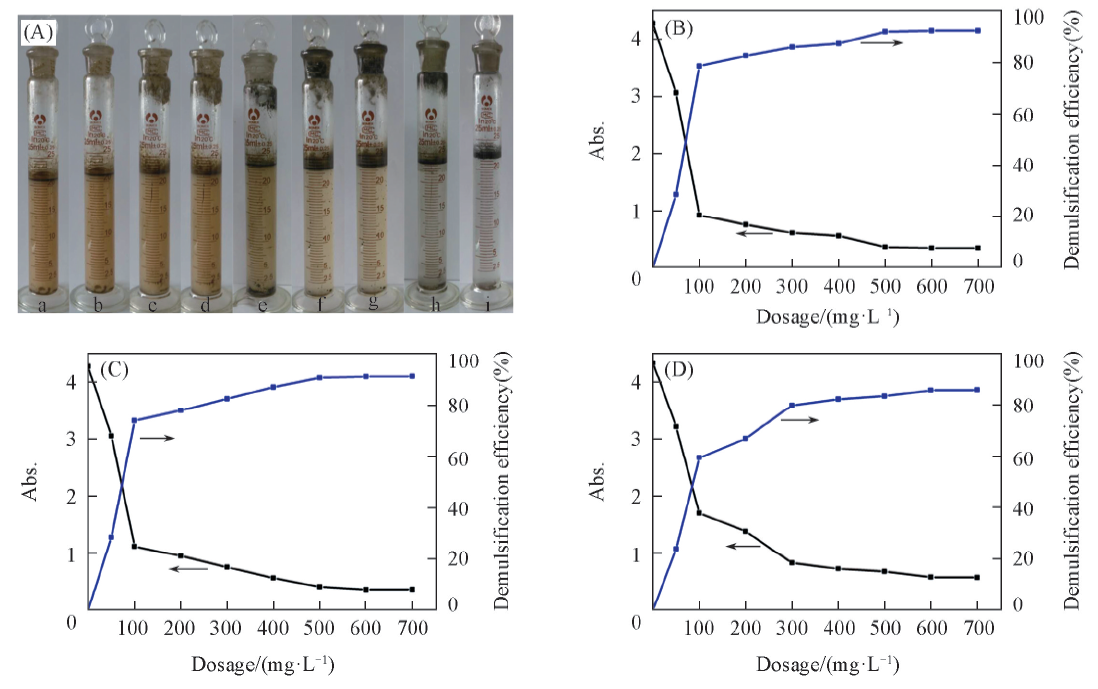
Fig.8 Effects of HFG-Fe3O4 dosage and pH value of oily wastewater on demulsification performance of HFG-Fe3O4(A) pH=2.0, c(HFG-Fe3O4)/(mg·L-1) from a to i: 0, 50, 100, 200, 300, 400, 500, 600,700. (B) pH=2.0; (C) pH=4.0; (D) pH=6.0.
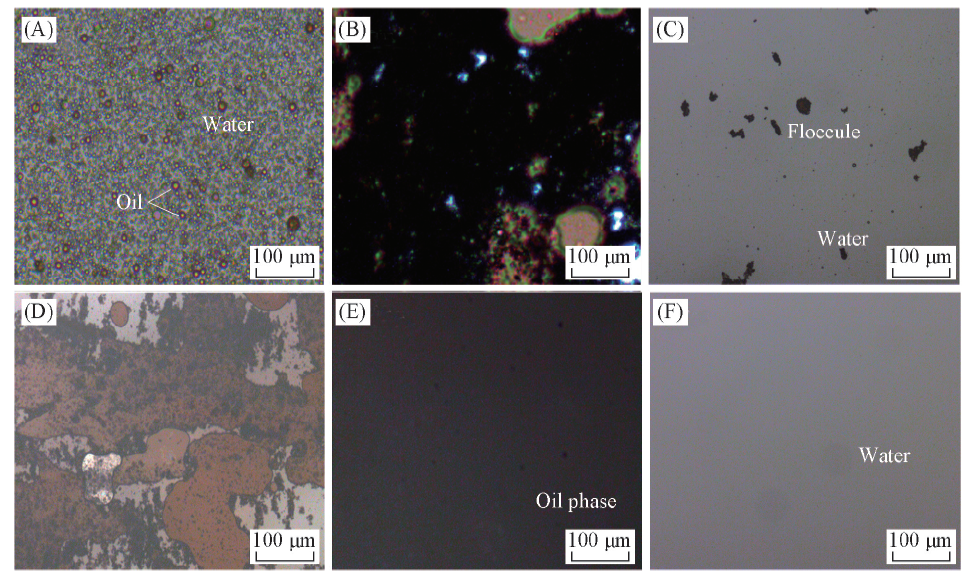
Fig.10 POM images of the emulsified oily wastewater(A), the newly formed floccule(B), the newly formed oil phase(C), the oil phase after magnetic separation and settlement(D), the newly separated water phase(E), and the separated water phase after being treated by a magnet(F)
| [1] | Gao H., Duan Y. Q., Yuan Z. H., Chem. J. Chinese Universities, 2016, 37(6), 1208—1215 |
| (高虹, 段月琴, 袁志好. 高等学校化学学报, 2016, 37(6), 1208—1215) | |
| [2] | Li Q. T., Sun W., Zhang Q., Chem. J. Chinese Universities, 2017, 38(3), 464—470 |
| (李钦涛, 孙文, 张芹. 高等学校化学学报, 2017, 38(3), 464—470) | |
| [3] | Gao R., Li F., Li Y., Wu T., Chem. Eng. J., 2017, 309, 513—521 |
| [4] | Cao Z., Hao T., Wang P., Zhang Y., Cheng B., Chem. Eng. J., 2017, 309, 30—40 |
| [5] | Peng J., Liu Q., Xu Z., Masliyah J., Energ. Fuel., 2012, 26, 2705—2710 |
| [6] | Duong P.H. H.., Chung T. S.,J. Membrane Sci., 2014, 452, 117—126 |
| [7] | Gu J., Xiao P., Chen J., Zhang J., ACS Appl.Mater. Interfaces, 2014, 6, 16204—16209 |
| [8] | Hu G., Li J., Zeng G., J. Hazard. Mater., 2013, 261, 470—490 |
| [9] | Kayvani F. A., Rhadfi T., McKay G., Al-marri M., Chem. Eng. J., 2016, 293, 90—101 |
| [10] | Santander M., Rodrigues R. T., Rubio J., Colloid Surface A, 2011, 375, 237—244 |
| [11] | Chanthamalee J., Wongchitphimon T., Luepromchai E.,Water Air Soil Pollut., 2013, 224, 1601—1613 |
| [12] | Liu J., Zhao Y. P., Hu B., Ren S. L., Chem. Ind. Eng. Process, 2013, 32, 891—897 |
| (刘娟, 赵亚溥, 胡斌, 任嗣利. 化工进展, 2013, 32, 891—897) | |
| [13] | Cao J. P., Zhang S., Han B. L., J. Beijing University Chem. Technol.( Natural Science), 2011, 38, 52—57 |
| (曹建苹, 张胜, 韩宝丽. 北京化工大学学报(自然科学版), 2011, 38, 52—57) | |
| [14] | Cunha R. E. P., Fortuny M., Dariva C., Santos A. F., Ind. Eng. Chem. Res., 2008, 47, 7094—7103 |
| [15] | Fang S., Chen T., Wang R., Xiong Y., Chen B., Duan M., Energ. Fuel., 2016, 30, 3355—3364 |
| [16] | Liu J., Li X. C., Jia W. H., Li Z. Y., Zhao Y. P., Ren S. L., Energ. Fuel., 2015, 29, 4644—4653 |
| [17] | Wang H. J., Liu J., Xu H. Y., Ma Z. W., Jia W. H., Ren S. L., RSC Adv., 2016, 6, 106297—106307 |
| [18] | Bettinger H. F., Chem. Phys. Chem., 2003, 4, 1283—1289 |
| [19] | Bai R., Zhao J. P., Li Y., Surf. Technol., 2014, 43, 131—136 |
| (白瑞, 赵九蓬, 李垚. 表面技术, 2014, 43, 131—136) | |
| [20] | Wang X., Shi Y., Graff R. W., Lee D., Gao H., Polymer, 2015, 72, 361—367 |
| [21] | Li S., Li N., Yang S., Liu F., Zhou J., J. Mater. Chem. A, 2014, 2, 94—99 |
| [22] | Lü T., Chen Y., Qi D., Cao Z., Zhang D., Zhao H. T., J. Alloy. Compd., 2017, 696, 1205—1212 |
| [23] | Lü T., Zhang S., Qi D., Zhang D., Vance G. F., Zhao H. T., Appl. Surf. Sci., 2017, 396, 1604—1612 |
| [24] | Hamwi A., Phys. Chem Solids, 1996, 57, 677—688 |
| [25] | Yang T. Z., Shen C. M., Li Z., Zhang H. R., J. Phys. Chem. B, 2005, 109, 23233—23236 |
| [26] | Jian X., Wu B., Wei Y., Dou S. X., ACS Appl. Mater. Interfaces, 2016, 8, 6101—6109 |
| [27] | Ye X. Y., Ma L. M., Yang Z. G., Wang J. Q., Wang H. G., Yang S. R., ACS Appl. Mater. Interfaces, 2016, 8, 7483—7488 |
| [28] | Jiang X., Wang F., Cai W., Zhang X., J. Alloy. Compd., 2015, 636, 34—39 |
| [29] | Wang Y., Lee W. C., Manga K. K., Ang P. K., Lu J., Liu Y. P., Adv. Mater., 2012, 24, 4285—4290 |
| [30] | Bharathidasan T., Narayanan T. N., Sathyanaryanan S., Sreejakumari S. S., Carbon, 2015, 84, 207—213 |
| [31] | Xu H. Y., Jia W. H., Ren S. L., Wang J. Q., Chem. Eng. J., 2019, 337, 10—18 |
| [32] | Liu J., Zhao Y. P., Ren S. L., Energ. Fuel., 2015, 29, 1233—1242 |
| [33] | Yeung A., Dabros T., Masliyah J., Czarnecki J., Colloid Surface A, 2000, 174, 169—181 |
| [34] | Dabros A.Y. T.., Masliyah J.,J. Colloid Inter. Sci., 1999, 210, 222—224 |
| [35] | Costa L. M., Stoyanov S. R., Gusarov S., Tan X., Gray M. R., Energ. Fuel., 2012, 26, 2727—2735 |
| [36] | Bouhadda Y., Bormann D., Sheu E., Bendedouch D., Krallafa A., Daaou M.,Fuel., 2007, 86, 1855—1864 |
| [37] | Pacheco-Sánchez J. H., AÄ lvarez-Ramírez F., Martínez-Magadán J. M., Energ. Fuel., 2004, 18, 1676—1686 |
| [1] | GAO Yifei, XIAO Changfa, JI Dawei, HUANG Yangzheng. Preparation of PVDF Hollow Fiber Membranes via Melt Spinning-stretching Method and Its Oil-water Separation Performance [J]. Chem. J. Chinese Universities, 2021, 42(6): 2065. |
| [2] | LIU Pengchang, LAI Hua, CHENG Zhongjun, LIU Yuyan. Fabrication of Superwetting Porous Shape Memory Sponge and Its Application in Oil-water Separation [J]. Chem. J. Chinese Universities, 2021, 42(3): 894. |
| [3] | PENG Xinyan, LIU Yunhong, LI Jiawen, FENG Yilong, WANG Hanchun. Preparation and Characterization of Tannin/Zwitterionic Modified Oil-water Separation Membrane † [J]. Chem. J. Chinese Universities, 2020, 41(6): 1337. |
| [4] | GAO Naiwei, MA Qiang, HE Yonglin, WANG Yapei. Green Electronic Devices Based on Ionic Liquids † [J]. Chem. J. Chinese Universities, 2020, 41(5): 901. |
| [5] | LIU Shuaizhuo,ZHANG Qian,LIU Ning,XIAO Wenyan,FAN Leiyi,ZHOU Ying. One-step Synergistic Hydrophobic Modification of Melamine Sponge and Its Application † [J]. Chem. J. Chinese Universities, 2020, 41(3): 521. |
| [6] | ZHANG Jianhui,ZHOU Jinya,LIN Haibo,LI Zhan,FANG Qianrong,XUE Ming,QIU Shilun. MXene-Coated Mesh Membrane with Underwater Superoleophobicity for High-efficiency Oil-water Separation† [J]. Chem. J. Chinese Universities, 2019, 40(4): 624. |
| [7] | GAO Hong, DUAN Yueqin, YUAN Zhihao. Preparation of Superhydrophilic and Underwater Superoleophobic PVDF-g-PAA Porous Membranes and Their Oil-water Separation Performance† [J]. Chem. J. Chinese Universities, 2016, 37(6): 1208. |
| [8] | SHI Yanlong, YANG Wu, FENG Xiaojuan. Fabrication of Superhydrophobic-superoleophilic Cotton Fabric and Its Application in Water-oil Separation† [J]. Chem. J. Chinese Universities, 2015, 36(9): 1724. |
| [9] | PAN Liya, LI Zhifeng, NI Yuxiang, YAO Zhengang, YU Zhiping, WU Wenkang, YING Anguo. Knoevenagel Condensation Catalyzed by Novel Acidic Ionic Liquid [J]. Chem. J. Chinese Universities, 2015, 36(1): 81. |
| [10] | LI Hong-Jian, CAO Yi-Ming, YANG Lin-Song, YUAN Quan . Oil-water Separation Performance of Anti-fouling α-Cellulose Hollow Fiber Ultrafiltration Membrane [J]. Chem. J. Chinese Universities, 2005, 26(10): 1890. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||